Structural and Serological Studies of the O6-Related Antigen of Aeromonas veronii bv. sobria Strain K557 Isolated from Cyprinus carpio on a Polish Fish Farm, which Contains l-perosamine (4-amino-4,6-dideoxy-l-mannose), a Unique Sugar Characteristic for Aeromonas Serogroup O6
Abstract
:1. Introduction
2. Results
2.1. Bacterial Cultivation, Isolation of LPS, and SDS-PAGE Study
2.2. Serological Studies of the A. veronii bv. sobria K557 O-antigen
2.3. Chemical and Mass Spectrometry Analyses of LPS
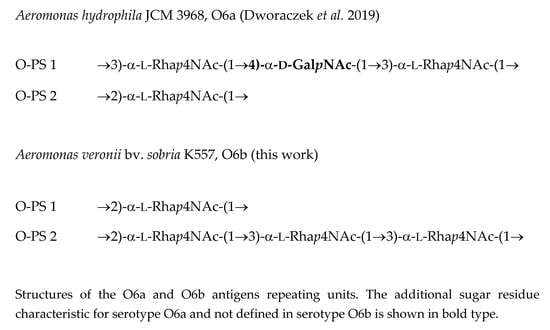
2.4. Structural Studies of O-polysaccharide (O-PS)
3. Discussion
4. Materials and Methods
4.1. Bacterial Strain, Cultivation Conditions, and Isolation of LPS
4.2. Degradation of LPS and Isolation of O-polysaccharide
4.3. Chemical Analyses
4.4. NMR Spectroscopy
4.5. MALDI-TOF Mass Spectrometry (MS)
4.6. SDS-PAGE
4.7. Serological Studies
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Janda, J.M.; Duffy, P.S. Mesophilic aeromonads in human diseases: Current taxonomy, laboratory infection and infectious diseases spectrum. Rev. Infect. Dis. 1988, 10, 980–997. [Google Scholar] [CrossRef] [PubMed]
- Janda, J.M. Recent advances in the study of the taxonomy, pathogenicity and infectious syndromes with the genus Aeromonas. Clin. Microbiol. Rev. 1991, 4, 397–410. [Google Scholar] [CrossRef] [PubMed]
- Martin-Carnahan, A.; Joseph, S.W. Order XII. Aeromonadales ord. nov. In Bergey’s Manual of Systematic Bacteriology, 2nd ed.; Brenner, D.J., Krieg, N.R., Staley, J.T., Eds.; Springer: New York, NY, USA, 2005; Volume 2, pp. 556–578. [Google Scholar]
- Janda, J.M.; Abbott, S. The genus Aeromonas: Taxonomy, pathogenicity, and infection. Clin. Microbiol. Rev. 2010, 23, 35–73. [Google Scholar] [CrossRef] [PubMed]
- Huys, G. The Family Aeromonadaceae. In The Prokaryotes—Gammaproteobacteria; Rosenberg, E., DeLong, E.F., Lory, S., Stackebrandt, E., Thompson, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 27–57. [Google Scholar] [CrossRef]
- Araujo, R.M.; Arribas, R.M.; Pares, R. Distribution of Aeromonas species in waters with different levels of pollution. J. Appl. Bacteriol. 1991, 71, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Cipriano, R.C.; Bullock, G.L. Furunculosis and other diseases caused by Aeromonas salmonicida. Fish. Dis. Leafl. 2001, 66, 1–32. [Google Scholar] [CrossRef]
- Praveen, P.K.; Debnath, C.; Shekhar, S.; Dalai, N.; Ganguly, S. Incidence of Aeromonas spp. infection in fish and chicken meat and its related public health hazards: A review. Vet. World 2016, 9, 6–11. [Google Scholar] [CrossRef]
- Ibrahem, M.D.; Mostafa, M.M.; Arab, R.M.H.; Rezk, M.A.; Elghobashy, H.; Fitzsimmons, K.; Diab, A.S. (Eds.) Prevalence of Aeromonas hydrophila infection in wild and cultured tilapia nilotica (O. niloticus) in Egypt. In Proceedings of the 8th International Symposium on Tilapia in Aquaculture, Cairo, Egypt, 12–14 October 2008; Volume 2, pp. 1257–1270. [Google Scholar]
- Tomas, J.M. The Main Aeromonas Pathogenic Factors. ISRN Microbiol. 2012, 2012, 256261. [Google Scholar] [CrossRef]
- Stratev, D.; Odeyemi, O.A. An overview of motile Aeromonas septicaemia management. Aquacult. Int. 2017, 25, 1095–1105. [Google Scholar] [CrossRef]
- Hoel, S.; Vadstein, O.; Jakobsen, A.N. The significance of mesophilic Aeromonas spp. in minimally processed ready-to-eat seafood. Microorganisms 2019, 7, 91. [Google Scholar] [CrossRef]
- Holmberg, S.D.; Schell, W.L.; Fanning, G.R.; Wachsmuth, I.K.; Blake, P.A.; Brenner, D.J.; Farmer, J.J. Aeromonas intestinal infections in the United States. Ann. Int. Med. 1986, 105, 683–689. [Google Scholar] [CrossRef]
- Figueras, M.J. Clinical relevance of Aeromonas sM503. Rev. Clin. Microbiol. 2005, 16, 145–153. [Google Scholar] [CrossRef]
- Mencacci, A.; Cenci, E.; Mazzolla, R.; Farinella, S.; D’Alo, F.; Vitali, M.; Bistoni, F. Aeromonas veronii biovar veronii septicaemia and acute suppurative cholangitis in a patient with hepatitis B. J. Med. Microbiol. 2003, 52, 727–730. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.T.M.; Enoch, D.A.; Harris, K.A.; Karas, J.A. Aeromonas veronii biovar sobria bacteraemia with septic artritis confirmed by 16S rDNA PCR in an immunocompetent adult. J. Clin. Microbiol. 2006, 55, 241–243. [Google Scholar] [CrossRef]
- Garduño, R.A.; Moore, A.R.; Oliver, G.; Lizama, A.L.; Garduño, E.; Kay, W.W. Host cell invasion and intracellular resistance by Aeromonas salmonicida: Role of the S-layer. J. Clin. Microbiol. 2000, 46, 660–668. [Google Scholar] [CrossRef]
- Merino, S.; Rubires, X.; Aguillar, A.; Guillot, J.F.; Tomas, J.M. The role of the O-antigen lipopolysaccharide on the colonization in vivo of the germfree chicken gut by Aeromonas hydrophila serogroup O:34. Microb. Pathog. 1996, 20, 325–333. [Google Scholar] [CrossRef]
- Rabaan, A.A.; Gryllos, I.; Tomas, J.M.; Shaw, J.G. Motility and polar flagellum are required for Aeromonas caviae adherence to HEp-2 cells. Infect. Immun. 2001, 69, 4257–4267. [Google Scholar] [CrossRef] [PubMed]
- Raetz, C.R.H.; Whitfield, C. Lipopolysaccharide endotoxins. Annu. Rev. Biochem. 2002, 71, 635–700. [Google Scholar] [CrossRef]
- Caroff, M.; Karibian, D. Structure of bacterial lipopolysaccharides. Carbohydr. Res. 2003, 338, 2431–2447. [Google Scholar] [CrossRef]
- Whitfield, C.; Trent, M.S. Biosynthesis and export of bacterial lipopolysaccharides. Annu. Rev. Biochem. 2014, 83, 99–128. [Google Scholar] [CrossRef]
- Maldonado, R.F.; Sá-Correia, I.; Valvano, M.A. Lipopolysaccharide modification in Gram-negative bacteria during chronic infection. FEMS Microbiol. Rev. 2016, 40, 480–493. [Google Scholar] [CrossRef]
- Cao, H.; Wang, M.; Wang, Q.; Xu, T.; Du, Y.; Li, H. Identifying genetic diversity of O antigens in Aeromonas hydrophila for molecular serotype detection. PLoS ONE 2018, 13, e0203445. [Google Scholar] [CrossRef] [PubMed]
- Sakazaki, R.; Shimada, T. O-Serogrouping for mesophilic Aeromonas strains. Jpn. J. Med. Sci. Biol. 1984, 37, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Thomas, L.V.; Gross, R.J.; Cheasty, T.; Rowe, B. Extended serogrouping scheme for motile, mesophilic Aeromonas species. J. Clin. Microbiol. 1990, 28, 980–984. [Google Scholar] [PubMed]
- Kokka, R.P.; Vedros, N.A.; Janda, J.M. Electrophoretic analysis of the surface components of autoagglutinating surface array protein-positive and surface array protein-negative Aeromonas hydrophila and Aeromonas sobria. J. Clin. Microbiol. 1990, 28, 2240–2247. [Google Scholar] [PubMed]
- Kozinska, A.; Pekala, A. Serotyping of Aeromonas species isolated from Polish fish farms in relation to species and virulence phenotype of the bacteria. Bull. Vet. Inst. Pulawy 2010, 54, 315–320. [Google Scholar]
- Esteve, C.; Alcaide, E.; Canals, R.; Merino, S.; Blasco, D.; Figueras, M.J.; Tomas, J.M. Pathogenic Aeromonas hydrophila serogroup O:14 and O:81 strains with S-layer. Appl. Environ. Microbiol. 2004, 70, 5898–5904. [Google Scholar] [CrossRef] [PubMed]
- Hickman-Brenner, F.W.; MacDonald, K.L.; Steigerwalt, A.G.; Fanning, G.R.; Brenner, D.J.; Farmer, J.J., III. Aeromonas veronii, a new ornithine decarboxylase-positive species that may cause diarrhea. J. Clin. Microbiol. 1987, 25, 900–906. [Google Scholar]
- Altwegg, M.; Steigerwalt, A.G.; Altwegg-Bissig, R.; Luthy-Hottenstein, J.; Brenner, D.J. Biochemical identification of Aeromonas genospecies isolated from humans. J. Clin. Microbiol. 1990, 28, 258–264. [Google Scholar]
- Rahman, M.; Colque-Navarro, P.; Kühn, I.; Huys, G.; Swings, J.; Möllby, R. Identification and characterization of pathogenic Aeromonas veronii bv. sobria associated with epizootic ulcerative syndrome in fish in Bangladesh. Appl. Environ. Microbiol. 2002, 68, 650–655. [Google Scholar] [CrossRef]
- Cai, S.-H.; Wu, Z.-H.; Jian, J.-C.; Lu, Y.-S.; Tang, J.F. Characterization of pathogenic Aeromonas veronii bv. veronii associated with ulcerative syndrome from Chinese longsnout catfish (Leiocassis longirostris Günther). Braz. J. Microbiol. 2012, 43, 382–388. [Google Scholar] [CrossRef]
- Turska-Szewczuk, A.; Lindner, B.; Pekala, A.; Palusinska-Szysz, M.; Choma, A.; Russa, R.; Holst, O. Structural analysis of the O-specific polysaccharide from the lipopolysaccharide of Aeromonas veronii bv. sobria strain K49. Carbohydr. Res. 2012, 353, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Turska-Szewczuk, A.; Pietras, H.; Duda, K.A.; Kozińska, A.; Pękala, A.; Holst, O. Structure of the O-specific polysaccharide from the lipopolysaccharide of Aeromonas sobria strain Pt312. Carbohydr. Res. 2015, 403, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Kozińska, A. Genotypic and Serological Analysis of Domestic Mesophilic Isolates Aeromonas sp. in Terms of Pathogenicity and the Type of Disease Symptoms Caused by Them in Fish. Habilitation Thesis, The National Veterinary Institute—The National Research Institute, Puławy, Poland, 2009. [Google Scholar]
- Dworaczek, K.; Kurzylewska, M.; Karaś, M.A.; Janczarek, M.; Pękala-Safińska, A.; Turska-Szewczuk, A. A unique sugar L-perosamine (4-amino-4,6-dideoxy-L-mannose) is a compound building two O-chain polysaccharides in the lipopolysaccharide of Aeromonas hydrophila strain JCM 3968, serogroup O6. Mar. Drugs 2019, 17, 254. [Google Scholar] [CrossRef] [PubMed]
- Westphal, O.; Jann, K. Bacterial lipopolysaccharide. Extraction with phenol-water and further applications of the procedure. Meth. Carbohydr. Chem. 1965, 5, 83–91. [Google Scholar]
- Knirel, Y.A.; Vinogradov, E.; Jimenez, N.; Merino, S.; Tomas, J.M. Structural studies on the R-type lipopolysaccharide of Aeromonas hydrophila. Carbohydr. Res. 2004, 339, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Pieretti, G.; Corsaro, M.M.; Lanzetta, R.; Parrilli, M.; Nicolaus, B.; Gambacorta, A.; Lindner, B.; Holst, O. Structural characterization of the core region of the lipopolysaccharide from the haloalkaliphilic Halomonas pantelleriensis: Identification of the biological O-antigen repeating unit. Eur. J. Org. Chem. 2008, 2008, 721–728. [Google Scholar] [CrossRef]
- Domon, B.; Costello, C.E. A systamatic nomenclature for carbohydrate fragmentations in FAB MS/MS spectra of glycoconjugates. Glycoconj. J. 1988, 5, 397–409. [Google Scholar] [CrossRef]
- Leontein, K.; Lindberg, B.; Lönngren, J. Assignment of absolute configuration of sugars by GLC of their acetylated glycosides formed from chiral alcohols. Carbohydr. Res. 1978, 62, 359–362. [Google Scholar] [CrossRef]
- Lipiński, T.; Zatonsky, G.V.; Kocharova, N.A.; Jaquinod, M.; Forest, E.; Shashkov, A.S.; Gamian, A.; Knirel, Y.A. Structures of two O-chain polysaccharides of Citrobacter gillenii O9a,9b lipopolysaccharide. A new homopolymer of 4-amino-4,6-dideoxy-D-mannose (perosamine). Eur. J. Biochem. 2002, 269, 93–99. [Google Scholar] [CrossRef]
- Lipkind, G.M.; Shashkov, A.S.; Knirel, Y.A.; Vinogradov, E.V.; Kochetkov, N.K. A computer-assisted structural analysis of regular polysaccharides on the basis of 13C-n.m.r. data. Carbohydr. Res. 1988, 175, 59–75. [Google Scholar] [CrossRef]
- Ovchinnikova, O.G.; Kocharova, N.A.; Katzenellenbogen, E.; Zatonsky, G.V.; Shashkov, A.S.; Knirel, Y.A.; Lipiński, T.; Gamian, A. Structures of two O-polysaccharides of the lipopolysaccharide of Citrobacter youngae PCM 1538 (serogroup O9). Carbohydr. Res. 2004, 339, 881–884. [Google Scholar] [CrossRef] [PubMed]
- Jansson, P.E.; Kenne, L.; Widmalm, G. Computer-assisted structural analysis of polysaccharides with an extended version of CASPER using 1H- and 13C-NMR data. Carbohydr. Res. 1989, 188, 169–191. [Google Scholar] [CrossRef]
- Knirel, Y.A.; Ovod, V.V.; Paramonov, N.A.; Krohn, K.J. Structural heterogeneity in the O polysaccharide of Pseudomonas syringe pv. coriandricola GSPB 2028 (NCPPB 3780, W-43). Eur. J. Biochem. 1998, 258, 716–721. [Google Scholar] [CrossRef] [PubMed]
- Pękala-Safińska, A. Contemporary threats of bacterial infections in freshwater fish. J. Vet. Res. 2018, 62, 261–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lirski, A.; Myszkowski, L. Polish aquaculture in 2016 based on the analysis of questionnaire RRW-22. Part 1. Komun. Ryb. 2017, 6, 20–27. [Google Scholar]
- Terech-Majewska, E. Improving disease prevention and treatment in controlled fish culture. Arch. Pol. Fish. 2016, 24, 115–165. [Google Scholar] [CrossRef] [Green Version]
- Kozińska, A.; Pękala, A. Characteristics of disease spectrum in relation to species, serogroups, and adhesion ability of motile aeromonads in fish. Sci. World J. 2012. [Google Scholar] [CrossRef] [PubMed]
- Grudniewska, J.; Dobosz, S.; Terech-Majewska, E.; Zalewski, T.; Siwicki, A.K. Economic and health aspects of vaccinating against furunculosis and yersiniosis in rainbow trout culture. Komun. Ryb. 2010, 1, 18–21. [Google Scholar]
- Siwicki, A.K.; Baranowski, P.; Dobosz, S.; Kuźmiński, H.; Grudniewska, J.; Kazun, K.; Głąbski, E.; Kazuń, B.; Terech-Majewska, E.; Trapkowska, S. Using new generation vaccines administered per os in granulate as prophylaxis against furunculosis and yersiniosis in salmonids. In Current Challenges in Fish Disease Prevention and Treatment; Siwicki, A.K., Ed.; Publisher IRS: Olsztyn, Poland, 2004; pp. 117–122. [Google Scholar]
- Sukenda, S.; Romadhona, E.I.; Yuhana, M.; Pasaribu, W.; Hidayatullah, D. Efficacy of whole-cell and lipopolysaccharide vaccine of Aeromonas hydrophila on juvenile tilapia Oreochromis niloticus against motile aeromonad septicemia. AACL Bioflux 2018, 11, 1456–1466. [Google Scholar]
- Dehghani, S.; Akhlaghi, M.; Dehghani, M. Efficacy of formalin-killed, heat-killed and lipopolysaccharide vaccines against motile aeromonads infection in rainbow trout (Oncorhynchus mykiss). Glob. Veterin. 2012, 9, 409–415. [Google Scholar] [CrossRef]
- Fernandez, J.B.; Yambot, A.V.; Almeria, O. Vaccination of Nile tilapia (Oreochromis niloticus) using lipopolysaccharide (LPS) prepared from Aeromonas hydrophila. Int. J. Fauna Biol. Stud. 2014, 1, 1–3. [Google Scholar]
- Pajdak, J.; Terech-Majewska, E.; Platt-Samoraj, A.; Schulz, P.; Siwicki, A.K.; Szweda, W. Characterization of pathogenic Yersinia ruckeri strains and their importance in rainbow trout immunoprophylaxis. Med. Weter 2017, 73, 579–584. [Google Scholar] [CrossRef]
- Osawa, K.; Shigemura, K.; Iguchi, A.; Shirai, H.; Imayama, T.; Seto, K.; Raharjo, D.; Fujisawa, M.; Osawa, R.; Shirakawa, T. Modulation of the O-antigen chain length by the wzz gene in Escherichia coli O157 influences its sensitivities to serum complement. Microbiol. Immunol. 2013, 57, 616–623. [Google Scholar] [PubMed]
- Turska-Szewczuk, A.; Lindner, B.; Komaniecka, I.; Kozinska, A.; Pekala, A.; Choma, A.; Holst, O. Structural and immunochemical studies of the lipopolysaccharide from the fish pathogen, Aeromonas bestiarum strain K296, serotype O18. Mar. Drugs 2013, 11, 1235–1255. [Google Scholar] [CrossRef] [PubMed]
- Turska-Szewczuk, A.; Duda, K.A.; Schwudke, D.; Pekala, A.; Kozinska, A.; Holst, O. Structural studies of the lipopolysaccharide from the fish pathogen, Aeromonas veronii strain Bs19, serotype O16. Mar. Drugs 2014, 12, 1298–1316. [Google Scholar] [CrossRef] [PubMed]
- Russa, R.; Urbanik-Sypniewska, T.; Lindström, K.; Mayer, H. Chemical characterization of two lipopolysaccharide species isolated from Rhizobium loti NZP2213. Arch. Microbiol. 1995, 163, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Ciucanu, I.; Kerek, F. A simple and rapid method for the permethylation of carbohydrates. Carbohydr. Res. 1984, 131, 209–217. [Google Scholar] [CrossRef]
- Pieretti, G.; Corsaro, M.M.; Lanzetta, R.; Parrilli, M.; Vilches, S.; Merino, S.; Tomas, J.M. Structure of the core region from the lipopolysaccharide of Plesiomonas shigelloides strain 302-73 (serotype O1). Eur. J. Org. Chem. 2009, 2009, 1365–1371. [Google Scholar] [CrossRef]
- Komaniecka, I.; Choma, A.; Lindner, B.; Holst, O. The structure of a novel lipid A from the lipopolysaccharide of Bradyrhizobium elkanii containing three mannose units in the backbone. Chem. Eur. J. 2010, 16, 2922–2929. [Google Scholar] [CrossRef]
- Silipo, A.; Molinaro, A.; Sturiale, L.; Dow, J.M.; Erbs, G.; Lanzetta, R.; Newman, M.A.; Parrilli, M. The elicitation of plant innate immunity by lipooligosaccharide of Xanthomonas campestris. J. Biol. Chem. 2005, 280, 33660–33668. [Google Scholar] [CrossRef]
- Tsai, C.M.; Frasch, C.E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal. Biochem. 1982, 119, 115–119. [Google Scholar] [CrossRef]
- Turska-Szewczuk, A.; Pietras, H.; Borucki, W.; Russa, R. Alteration of O-specific polysaccharide structure of symbiotically defective Mesorhizobium loti mutant 2213.1 derived from strain NZP2213. Acta Biochim. Pol. 2008, 55, 191–199. [Google Scholar] [PubMed]
- Sidorczyk, Z.; Zych, K.; Toukach, F.V.; Arbatsky, N.P.; Zabłotni, A.; Shashkov, A.S.; Knirel, Y.A. Structure of the O-polysaccharide and classification of Proteus mirabilis G1 in Proteus serogroup O3. Eur. J. Biochem. 2002, 269, 1406–1412. [Google Scholar] [CrossRef] [PubMed]
- Drzewiecka, D.; Arbatsky, N.P.; Shashkov, A.S.; Stączek, P.; Knirel, Y.A.; Sidorczyk, Z. Structure and serological properties of the O antigen of two clinical Proteus mirabilis strains classified into a new Proteus O77 serogroup. FEMS Immunol. Med. Microbiol. 2008, 54, 185–194. [Google Scholar] [CrossRef] [PubMed]









| Serum Specific to Strain | JCM 3968 LPS | K557 LPS | |
|---|---|---|---|
| JCM 3968 | intact | 1,024,000 | 64,000 |
| K557 adsorbed | 256,000 | <1000 | |
| K557 | intact | 64,000 | 64,000 |
| JCM 3968 adsorbed | <1000 | <1000 | |
| [M − H]− Observed | [M − H]− Calculated | M Monoisotopic | Assigned Composition |
|---|---|---|---|
| 1768.178 | 1768.181 | 1769.188 | HexN2P2[14:0(3-OH)]4(12:0)2 |
| 1796.194 | 1796.139 | 1797.146 | HexN2P2[14:0(3-OH)]3[i15:0(3-OH)](12:0)2 |
| 1824.224 | 1824.243 | 1825.250 | HexN2P2[14:0(3-OH)]4(14:0)2 |
| 1830.535 | 1830.626 | 1831.633 | Hep6Hex2HexN1Kdo-COO |
| 1874.542 | 1874.616 | 1875.624 | Hep6Hex2HexN1Kdo |
| 1910.519 | 1910.593 | 1911.600 | [Hep6Hex2HexN1KdoP]-COO |
| 1954.556 | 1954.583 | 1955.599 | Hep6Hex2HexN1KdoP |
| Sugar Residue | Chemical Shifts (δ, ppm) | ||||||
|---|---|---|---|---|---|---|---|
| H-1 C-1 | H-2 C-2 | H-3 C-3 | H-4 C-4 | H-5 C-5 | H-6 C-6 | ||
| →2)-α-l-Rhap4NAc-(1→ | A | 5.18 | 4.16 | 4.07 | 3.93 | 3.85 | 1.21 |
| 101.7 | 78.3 | 69.2 | 54.3 | 69.7 | 18.2 | ||
| →2)-α-l-Rhap4NAc-(1→ | B | 5.04 | 3.81 | 4.01 | 3.87 | 3.91 | 1.22 |
| 102.1 | 79.8 | 69.2 | 54.3 | 69.6 | 18.2 | ||
| →3)-α-l-RhapNAc-(1→ | C | 5.00 | 3.87 | 3.93 | 4.00 | 3.92 | 1.24 |
| 103.3 | 70.7 | 78.3 | 53.2 | 69.5 | 18.2 | ||
| →3)-α-l-Rhap4NAc-(1→ | D | 4.97 | 4.20 | 3.99 | 3.94 | 3.92 | 1.24 |
| 103.5 | 70.2 | 78.4 | 53.2 | 69.5 | 18.2 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dworaczek, K.; Drzewiecka, D.; Pękala-Safińska, A.; Turska-Szewczuk, A. Structural and Serological Studies of the O6-Related Antigen of Aeromonas veronii bv. sobria Strain K557 Isolated from Cyprinus carpio on a Polish Fish Farm, which Contains l-perosamine (4-amino-4,6-dideoxy-l-mannose), a Unique Sugar Characteristic for Aeromonas Serogroup O6. Mar. Drugs 2019, 17, 399. https://doi.org/10.3390/md17070399
Dworaczek K, Drzewiecka D, Pękala-Safińska A, Turska-Szewczuk A. Structural and Serological Studies of the O6-Related Antigen of Aeromonas veronii bv. sobria Strain K557 Isolated from Cyprinus carpio on a Polish Fish Farm, which Contains l-perosamine (4-amino-4,6-dideoxy-l-mannose), a Unique Sugar Characteristic for Aeromonas Serogroup O6. Marine Drugs. 2019; 17(7):399. https://doi.org/10.3390/md17070399
Chicago/Turabian StyleDworaczek, Katarzyna, Dominika Drzewiecka, Agnieszka Pękala-Safińska, and Anna Turska-Szewczuk. 2019. "Structural and Serological Studies of the O6-Related Antigen of Aeromonas veronii bv. sobria Strain K557 Isolated from Cyprinus carpio on a Polish Fish Farm, which Contains l-perosamine (4-amino-4,6-dideoxy-l-mannose), a Unique Sugar Characteristic for Aeromonas Serogroup O6" Marine Drugs 17, no. 7: 399. https://doi.org/10.3390/md17070399
APA StyleDworaczek, K., Drzewiecka, D., Pękala-Safińska, A., & Turska-Szewczuk, A. (2019). Structural and Serological Studies of the O6-Related Antigen of Aeromonas veronii bv. sobria Strain K557 Isolated from Cyprinus carpio on a Polish Fish Farm, which Contains l-perosamine (4-amino-4,6-dideoxy-l-mannose), a Unique Sugar Characteristic for Aeromonas Serogroup O6. Marine Drugs, 17(7), 399. https://doi.org/10.3390/md17070399