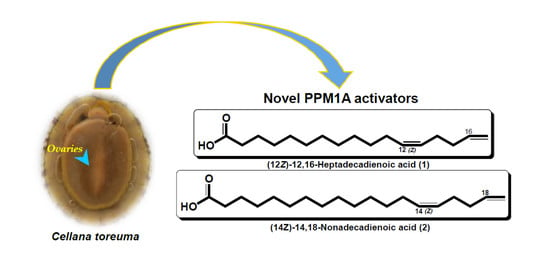
Identification and Total Synthesis of Two Previously Unreported Odd-Chain Bis-Methylene-Interrupted Fatty Acids with a Terminal Olefin that Activate Protein Phosphatase, Mg2+/Mn2+-Dependent 1A (PPM1A) in Ovaries of the Limpet Cellana toreuma
Abstract
:1. Introduction
2. Results and Discussion
2.1. Identification of 1 and 2 in Ovaries of the Limpet Cellana toreuma
2.2. Total Synthesis of 1 and 2
2.3. Biological Activity of 1 and 2
3. Experimental Section
3.1. General Experimental Procedures
3.2. Collection of Biological Materials, Extraction, Fractionation, and Structural Determination of NMI FAs
3.3. Identification of 1 and 2 by GC-MS of their Methyl Esters and 3-Pyridylcarbinol Derivatives
3.3.1. General Procedure for the Monoprotection of 3a and 3b
14-(Methoxymethoxy)tetradecan-1-ol (4b)
3.3.2. General Procedure for the PCC Oxidation of 4a and 4b
3.3.3. General Procedure for the Wittig Reaction of 5a and 5b
(Z)-17-(Methoxymethoxy)heptadeca-1,5-diene (6a)
(Z)-19-(Methoxymethoxy)nonadeca-1,5-diene (6b)
3.3.4. General Procedure for the Synthesis of 1 and 2
(Z)-Heptadeca-12,16-Dienoic Acid (1)
(Z)-Nonadeca-14,18-Dienoic Acid (2)
3.4. PPM1A Activation Activity of 1 and 2
3.5. Cytotoxic Activity of 1 and 2 against HL60 Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Barnathan, G. Non-methylene-interrupted fatty acids from marine invertebrate: Occurrence, characterization and biological properties. Biochimie 2009, 91, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Carballeria, N.M. New advances in fatty acids as antimalarial, antimycobacterial and antifungal agents. Prog. Lipid Res. 2008, 47, 50–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolff, R.L.; Deluc, L.G.; Marpeau, A.M. Conifer seeds: Oil content and fatty acid composition. J. Am. Oil Chem. Soc. 1996, 73, 765–771. [Google Scholar] [CrossRef]
- Wolff, R.L.; Lavialle, O.; Pédrono, F.; Pasquier, E.; Deluc, L.G.; Marpeau, A.; Aitzetmüller, K. Fatty acid composition of pinaceae as taxonomic markers. Lipids 2001, 36, 439–451. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, H. Unusual minor nonmethylene-interrupted di-, tri-, and tetraenoic fatty acids in limpet gonads. Lipids 2005, 40, 627–630. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, H.; Ohnishi, M. Occurrence of novel nonmethylene-interrupted C24 polyenoic fatty acids in female gonad lipids of the limpet Cellana grata. Biosci. Biotechnol. Biochem. 2006, 70, 2575–2578. [Google Scholar] [CrossRef]
- Kawashima, H.; Ohnishi, M.; Ogawa, S.; Matsui, K. Unusual fatty acid isomers of triacylglycerols and polar lipids in female limpet gonads of Cellana grata. Lipids 2008, 43, 559–567. [Google Scholar] [CrossRef]
- Kawashima, H.; Ohnishi, M. Novel heneicosadienoic and tricosadienoic acid isomers in ovaries of marine archaeogastropods. Lipids 2012, 47, 827–833. [Google Scholar] [CrossRef]
- Kawashima, H.; Ohnishi, M. An unprecedented occurrence of Δ5, 9-and Δ9, 15-dienoic fatty acids in ovaries of the archaeogastropod limpet Cellana toreuma. Lipids 2016, 51, 257–262. [Google Scholar] [CrossRef]
- Yoshida, J.; Uesugi, S.; Kawamura, T.; Kimura, K.; Hu, D.; Xia, S.; Toyooka, N.; Ohnishi, M.; Kawashima, H. (4Z, 15Z)-Octadecadienoic acid inhibits glycogen synthase kinase-3β and glucose production in H4IIE cells. Lipids 2017, 52, 295–301. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Yamakawa, Y.; Matsuya, Y.; Toyooka, N.; Tohda, C.; Awale, S.; Li, F.; Kadota, S.; Tezuka, Y. Synthesis of long-chain fatty acid derivatives as a novel anti-Alzheimer’s agent. Bioorg. Med. Chem. Lett. 2014, 24, 604–608. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, H.; Ohnishi, M. Novel odd-chain fatty acids with a terminal double bond in ovaries of the limpet Cellana toreuma. Lipids 2017, 52, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, H. Diverse odd-chain monoenoic fatty acids and novel non-methylene-interrupted heptadecadienoic acids in ovaries of the limpet Cellana toreuma. Lipids 2018, 53, 841–847. [Google Scholar] [CrossRef]
- Shimada, K.; Sugawara, A.; Korenaga, T.; Kawashima, H. Total synthesis and structural elucidation of two unusual non-methylene-interrupted fatty acids in ovaries of the limpet Cellana Toreuma. Lipids 2017, 52, 1019–1032. [Google Scholar] [CrossRef] [PubMed]
- Klumpp, S.; Thissen, M.-C.; Krieglstein, J. Protein phosphatases types 2Cα and 2Cβ in apoptosis. Biochem. Soc. Trans. 2006, 34, 1370–1375. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Duan, X.; Liang, Y.Y.; Su, Y.; Wrighton, K.H.; Long, J.; Hu, M.; Davis, C.M.; Wang, J.; Brunicardi, F.C.; et al. PPM1A functions as a Smad phosphatase to terminate TGFbeta signaling. Cell 2006, 125, 915–928. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Gifford, C.C.; Samarakoon, R.; Higgins, P.J. Deregulation of negative controls on TGF-β1 signaling in tumor progression. Cancers Basel 2018, 10, 159. [Google Scholar] [CrossRef]
- Wang, L.; Wang, X.; Chen, J.; Yang, Z.; Yu, L.; Hu, L.; Shen, X. Activation of protein serine/threonine phosphatase PP2Cα efficiently prevents liver fibrosis. PLoS ONE 2010, 5, e14230. [Google Scholar] [CrossRef]
- Klumpp, S.; Selke, D.; Hermensmeier, J. Protein Phospatase type 2C active at physiological Mg2+: Stimulation by unsaturated fatty acids. FEBS Lett. 1998, 437, 229–232. [Google Scholar] [CrossRef]
- Klumpp, S.; Selke, D.; Ahlemeyer, B.; Schaper, C.; Krieglstein, J. Relationship between protein phosphatase type-2C activity and induction of apoptosis in cultured neuronal cells. Neurochem. Int. 2002, 41, 251–259. [Google Scholar] [CrossRef]
- Schwarz, S.; Hufnagel, B.; Dworak, M.; Klumpp, S.; Krieglstein, J. Protein phosphatases types 2Cα and 2Cβ are involved in fatty acid-induced apoptosis of neuronal and endothelial cells. Apoptosis 2006, 11, 1111–1119. [Google Scholar] [CrossRef] [PubMed]
- Wolff, R.L.; Christie, W.W. Structures, practical sources (gymnosperm seeds), gas-liquid chromatographic data (equivalent chain lengths), and mass spectrometric characteristics of all-cis Δ5-olefinic acids. Eur. J. Lipid Sci. Technol. 2002, 104, 234–244. [Google Scholar] [CrossRef]
- Schlosser, M.; Schaub, B. Cis selectivity of salt-free Wittig reactions: A “Leeward Approach” of the aldehyde at the origin? J. Am. Chem. Soc. 1982, 104, 5821–5823. [Google Scholar] [CrossRef]
- Nguyen, H.D.; Okada, T.; Kitamura, S.; Yamaoka, S.; Horaguchi, Y.; Kasanami, Y.; Sekiguchi, F.; Tsubota, M.; Yoshida, S.; Nishikawa, H.; et al. Design and synthesis of novel anti-hyperalgesic agents based on 6-prenylnaringenin as the T-type calcium channel blockers. Bioorg. Med. Chem. 2018, 26, 4410–4427. [Google Scholar] [CrossRef] [PubMed]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, H.; Sasaki, K.; Sekiya, J.; Maeda, Y.; Amagai, A.; Kubohara, Y.; Oshima, Y. Structural requirements of dictyopyrones isolated from Dictyostelium Spp. in the regulation of Dictyostelium development and in anti-leukemic activity. Bioorg. Med. Chem. 2004, 12, 3203–3214. [Google Scholar] [CrossRef]
- Aburai, N.; Yoshida, M.; Ohnishi, M.; Kimura, K. Pisiferdiol and Pisiferic acid isolated from Chamaecyparis pisifera activate protein phosphatase 2C in vitro and induce caspase-3/7-dependent apoptosis via dephosphorylation of Bad in HL60 cells. Phytomedicine 2010, 17, 782–788. [Google Scholar] [CrossRef]
- Kusakabe, K.; Honmura, Y.; Uesugi, S.; Tonouchi, A.; Maeda, H.; Kimura, K.; Koshino, H.; Hashimoto, M. Neomacrophorin X, a [4.4.3] propellane-type meroterpenoid from Trichoderma sp. 1212-03. J. Nat. Prod. 2017, 80, 1484–1492. [Google Scholar] [CrossRef]








| FA methyl Ester | ECL 1 | Calculated ECL 2 | Source |
|---|---|---|---|
| 17:0 | 17.00 | - | C. toreuma |
| 17:1Δ12Z (17:1n-5) | 17.39 | - | C. toreuma |
| 17:1Δ16 | 17.53 | - | C. toreuma |
| Naturally occurring 1 | 17.93 | 17.92 | C. toreuma |
| 1 (obtained by synthesis) | 17.93 | - | - |
| 19:0 | 19.00 | - | Commercial reagent 3 |
| 19:1Δ14Z (19:1n-5) | 19.38 | - | C. toreuma |
| 19:1Δ18 | 19.54 | - | C. toreuma |
| Naturally occurring 2 | 19.93 | 19.92 | C. toreuma |
| 2 (obtained by synthesis) | 19.93 | - | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawashima, H.; Toyooka, N.; Okada, T.; Nguyen, H.D.; Nishikawa, Y.; Miura, Y.; Inoue, N.; Kimura, K.-i. Identification and Total Synthesis of Two Previously Unreported Odd-Chain Bis-Methylene-Interrupted Fatty Acids with a Terminal Olefin that Activate Protein Phosphatase, Mg2+/Mn2+-Dependent 1A (PPM1A) in Ovaries of the Limpet Cellana toreuma. Mar. Drugs 2019, 17, 410. https://doi.org/10.3390/md17070410
Kawashima H, Toyooka N, Okada T, Nguyen HD, Nishikawa Y, Miura Y, Inoue N, Kimura K-i. Identification and Total Synthesis of Two Previously Unreported Odd-Chain Bis-Methylene-Interrupted Fatty Acids with a Terminal Olefin that Activate Protein Phosphatase, Mg2+/Mn2+-Dependent 1A (PPM1A) in Ovaries of the Limpet Cellana toreuma. Marine Drugs. 2019; 17(7):410. https://doi.org/10.3390/md17070410
Chicago/Turabian StyleKawashima, Hideki, Naoki Toyooka, Takuya Okada, Huy Du Nguyen, Yuya Nishikawa, Yuka Miura, Nana Inoue, and Ken-ichi Kimura. 2019. "Identification and Total Synthesis of Two Previously Unreported Odd-Chain Bis-Methylene-Interrupted Fatty Acids with a Terminal Olefin that Activate Protein Phosphatase, Mg2+/Mn2+-Dependent 1A (PPM1A) in Ovaries of the Limpet Cellana toreuma" Marine Drugs 17, no. 7: 410. https://doi.org/10.3390/md17070410
APA StyleKawashima, H., Toyooka, N., Okada, T., Nguyen, H. D., Nishikawa, Y., Miura, Y., Inoue, N., & Kimura, K. -i. (2019). Identification and Total Synthesis of Two Previously Unreported Odd-Chain Bis-Methylene-Interrupted Fatty Acids with a Terminal Olefin that Activate Protein Phosphatase, Mg2+/Mn2+-Dependent 1A (PPM1A) in Ovaries of the Limpet Cellana toreuma. Marine Drugs, 17(7), 410. https://doi.org/10.3390/md17070410