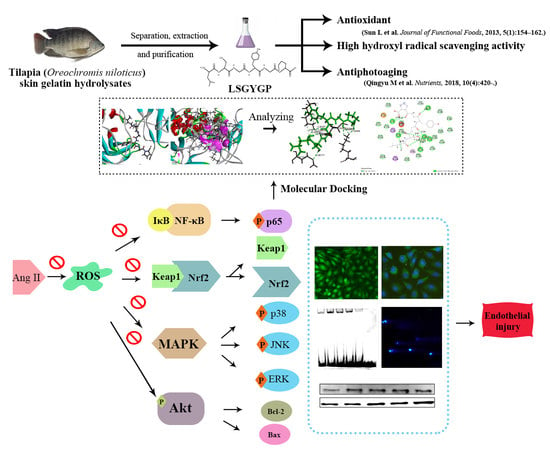
In Vitro Vascular-Protective Effects of a Tilapia By-Product Oligopeptide on Angiotensin II-Induced Hypertensive Endothelial Injury in HUVEC by Nrf2/NF-κB Pathways
Abstract
:1. Introduction
2. Results
2.1. Cytoprotective Effect of LSGYGP on Ang II-Stimulated HUVEC
2.2. LSGYGP against NO and ROS Production
2.3. Effect of LSGYGP on the Nrf2 Pathway
2.4. Effect of LSGYGP on MAPK Pathway
2.5. Effect of LSGYGP on Inflammatory Factor and NF-κB Pathway
2.6. Effect of LSGYGP on the Akt Pathway
2.7. DNA Damage in Comet Assay
2.8. Docking Results of LSGYGP with NF-κB
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture
4.3. Cell Viability Assay
4.4. Determination of NO and ROS
4.5. Western Blot
4.6. Immunocytochemistry
4.7. EMSA Assay
4.8. Comet Assay
4.9. Molecular Docking
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Montezano, A.C.; Nguyen Dinh Cat, A.; Rios, F.J.; Touyz, R.M. Angiotensin II and vascular injury. Curr. Hypertens. Rep. 2014, 16, 431. [Google Scholar] [CrossRef] [PubMed]
- Davignon, J. Role of endothelial dysfunction in atherosclerosis. Circulation 2004, 109, III27–III32. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, M.; Xue, S.J.; Liu, D.H.; Tang, Y.B. Simvastatin ameliorates Angiotensin II-induced endothelial dysfunction through restoration of Rho-BH4-eNOS-NO pathway. Cardiovasc. Drugs Ther. 2012, 26, 31–40. [Google Scholar] [CrossRef]
- Xia, F.; Wang, C.; Jin, Y.; Liu, Q.; Meng, Q.; Liu, K.; Sun, H. Luteolin protects HUVECs from TNF-α-induced oxidative stress and inflammation via its effects on the Nox4/ROS-NF-κB and MAPK pathways. J. Atheroscler. Thromb. 2014, 21, 768–783. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Qu, Z.; Zhao, Y.; Yang, L.; Gao, P. Orientin ameliorates LPS-induced inflammatory responses through the inhibitory of the NF- κ B pathway and NLRP3 inflammasome. Evid. Based Complement Altern. Med. 2017, 2017, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Gu, X.; Xu, E.; Ren, S.; Zhang, L.; Liu, W.; Lin, X.; Yang, J.; Chen, C. Osthole protects against Ang II-induced endotheliocyte death by targeting NF-κB pathway and Keap-1/Nrf2 pathway. Am. J. Transl. Res. 2019, 11, 142–159. [Google Scholar] [PubMed]
- Mehta, P.K.; Griendling, K.K. Angiotensin II cell signaling: Physiological and pathological effects in the cardiovascular system. Am. J. Physiol. Cell Physiol. 2007, 292, C82–C97. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Galicia, M.; Maier, K.G.; Greene, A.S.; Cowley, A.W.; Roman, R.J. Role of 20-hydroxyeicosatetraenoic acid in the renal and vasoconstrictor actions of angiotensin II. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002, 283, R60–R68. [Google Scholar] [CrossRef] [Green Version]
- Gragasin, F.S.; Xu, Y.; Arenas, I.A.; Kainth, N.; Davidge, S.T. Estrogen reduces angiotensin II–induced nitric oxide synthase and NAD(P)H oxidase expression in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 38–44. [Google Scholar] [CrossRef]
- Hahn, A.W.; Resink, T.J.; Scott-Burden, T.; Powell, J.; Dohi, Y.; Bühler, F.R. Stimulation of endothelin mRNA and secretion in rat vascular smooth muscle cells: A novel autocrine function. Cell Regul. 1990, 1, 649–659. [Google Scholar] [CrossRef]
- Bendall, J.K.; Rinze, R.; Adlam, D.; Tatham, A.L.; de Bono, J.; Channon, K.M. Endothelial Nox2 overexpression potentiates vascular oxidative stress and hemodynamic response to Angiotensin II: Studies in endothelial-targeted Nox2 transgenic mice. Circ. Res. 2007, 100, 1016–1025. [Google Scholar] [CrossRef] [PubMed]
- Sprague, A.H.; Khalil, R.A. Inflammatory cytokines in vascular dysfunction and vascular disease. Biochem. Pharmacol. 2009, 78, 539–552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mueller, C.F.H.; Becher, M.U.; Zimmer, S.; Wassmann, S.; Keuler, B.; Nickenig, G. Angiotensin II triggers release of leukotriene C4 in vascular smooth muscle cells via the multidrug resistance-related protein 1. Mol. Cell. Biochem. 2010, 333, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.-J.; Vapaatalo, H.; Mervaala, E. Angiotensin II and vascular inflammation. Med. Sci. Monit. 2005, 11, RA194. [Google Scholar] [CrossRef] [PubMed]
- Brasier, A.-R. The nuclear factor-κb-interleukin-6 signalling pathway mediating vascular inflammation. Cardiovasc. Res. 2010, 86, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Xiong, D.; Hu, W.; Ye, S.T.; Tan, Y.S. Isoliquiritigenin alleviated the Ang II-induced hypertensive renal injury through suppressing inflammation cytokines and oxidative stress-induced apoptosis via Nrf2 and NF-κB pathways. Biochem. Biophys. Res. Commun. 2018, 506, 161–168. [Google Scholar] [CrossRef]
- Li, W.; Kong, A.N. Molecular mechanisms of Nrf2-mediated antioxidant response. Mol. Carcinog. 2009, 48, 91–104. [Google Scholar] [CrossRef]
- Kim, Y.M.; Pae, H.O.; Park, J.E.; Lee, Y.C.; Woo, J.M.; Kim, N.-H.; Choi, Y.K.; Lee, B.-S.; Kim, S.R.; Chung, H.-T. Heme oxygenase in the regulation of vascular biology: From molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 2011, 14, 137–167. [Google Scholar] [CrossRef]
- Li, Z.X.; Chen, J.W.; Yuan, F.; Huang, Y.Y.; Zhao, L.Y.; Li, J.; Su, H.X.; Liu, J.; Pang, J.Y.; Lin, Y.C.; et al. Xyloketal B exhibits its antioxidant activity through induction of ho-1 in vascular endothelial cells and zebrafish. Mar. Drugs 2013, 11, 504–522. [Google Scholar] [CrossRef]
- Walensky, L.D. BCL-2 in the crosshairs: Tipping the balance of life and death. Cell. Death Differ. 2006, 13, 1339–1350. [Google Scholar] [CrossRef]
- Li, M.; Liu, X.; He, Y.; Zheng, Q.; Wang, M.; Wu, Y.; Zhang, Y.; Wang, C. Celastrol attenuates angiotensin II mediated human umbilical vein endothelial cells damage through activation of Nrf2/ERK1/2/Nox2 signal pathway. Eur. J. Pharmacol. 2017, 797, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Sivasinprasasn, S.; Pantan, R.; Thummayot, S.; Tocharus, J.; Suksamrarn, A.; Tocharus, C. Cyanidin-3-glucoside attenuates angiotensin II-induced oxidative stress and inflammation in vascular endothelial cells. Chem. Biol. Interact. 2016, 260, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Sable, R.; Parajuli, P.; Jois, S. Peptides, peptidomimetics, and polypeptides from marine sources: A wealth of natural sources for pharmaceutical applications. Mar. Drugs 2017, 15, 124. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.B.; Lin, H.C.; Chang, Y.W. Analysis of proteins and potential bioactive peptides from tilapia (Oreochromis spp.) processing co-products using proteomic techniques coupled with BIOPEP database. J. Funct. Food 2015, 19, 629–640. [Google Scholar] [CrossRef]
- Zhuang, Y.; Hou, H.; Zhao, X.; Zhang, Z.; Li, B. Effects of collagen and collagen hydrolysate from jellyfish (Rhopilema esculentum) on mice skin photoaging induced by UV irradiation. J. Food Sci. 2009, 74, H183–H188. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Zhang, Y.; Zhuang, Y. Antiphotoaging effect and purification of an antioxidant peptide from tilapia (Oreochromis niloticus) gelatin peptides. J. Funct. Food. 2013, 5, 154–162. [Google Scholar] [CrossRef]
- Ma, Q.; Liu, Q.; Yuan, L.; Zhuang, Y. Protective EFFECTS of LSGYGP from fish skin gelatin hydrolysates on UVB-Induced MEFs by regulation of oxidative stress and matrix metalloproteinase activity. Nutrients 2018, 10, 420. [Google Scholar] [CrossRef]
- Gu, L.; Bai, W.; Li, S.; Zhang, Y.; Han, Y.; Gu, Y.; Meng, G.; Xie, L.; Wang, J.; Xiao, Y.; et al. Celastrol prevents atherosclerosis via inhibiting LOX-1 and oxidative stress. PLoS ONE 2013, 8, e65477. [Google Scholar] [CrossRef]
- Bermudez, E.A.; Rifai, N.; Buring, J.; Manson, J.E.; Ridker, P.M. Interrelationships among circulating interleukin-6, c-reactive protein, and traditional cardiovascular risk factors in women. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 1668–1673. [Google Scholar] [CrossRef]
- Guzik, T.J.; West, N.E.J.; Black, E.; McDonald, D.; Ratnatunga, C.; Pillai, R.; Channon, K.M. Vascular superoxide production by NAD(P)H oxidase: Association with endothelial dysfunction and clinical risk factors. Circ. Res. 2000, 86. [Google Scholar] [CrossRef]
- Cao, Y.; Zheng, L.; Liu, S.; Peng, Z.; Zhang, S. Total flavonoids from Plumula Nelumbinis suppress angiotensin II-induced fractalkine production by inhibiting the ROS/NF-κB pathway in human umbilical vein endothelial cells. Exp. Ther. Med. 2014, 7, 1187–1192. [Google Scholar] [CrossRef] [PubMed]
- Guzik, T.J.; Hoch, N.E.; Brown, K.A.; McCann, L.A.; Rahman, A.; Dikalov, S.; Goronzy, J.; Weyand, C.; Harrison, D.G. Role of the T cell in the genesis of angiotensin II–induced hypertension and vascular dysfunction. J. Exp. Med. 2007, 204, 2449–2460. [Google Scholar] [CrossRef] [PubMed]
- Griendling, K.K.; Sorescu, D.; Ushio-Fukai, M. NAD(P)H oxidase: Role in cardiovascular biology and disease. Circ. Res. 2000, 86, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Rajagopalan, S.; Kurz, S.; Münzel, T.; Tarpey, M.; Freeman, B.A.; Griendling, K.K.; Harrison, D.G. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J. Clin. Investig. 1996, 97, 1916–1923. [Google Scholar] [CrossRef]
- Kalinowski, L.; Malinski, T. Endothelial NADH/NADPH-dependent enzymatic sources of superoxide production: Relationship to endothelial dysfunction. Acta Biochim. Pol. 2004, 51, 459. [Google Scholar]
- Harrison, D.; Griendling, K.K.; Landmesser, U.; Hornig, B.; Drexler, H. Role of oxidative stress in atherosclerosis. Am. J. Cardiol. 2003, 91, 7–11. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, J.; Pang, X.; Wang, S.; Wu, D.; Zhang, X.; Feng, L. Angiotensin II induces c-reactive protein expression via AT1-ROS-MAPK-NF-κB signal pathway in hepatocytes. Cell. Physiol. Biochem. 2013, 32, 569–580. [Google Scholar] [CrossRef]
- Murdoch, C.E.; Chaubey, S.; Zeng, L.; Yu, B.; Ivetic, A.; Walker, S.J.; Vanhoutte, D.; Heymans, S.; Grieve, D.J.; Cave, A.C.; et al. Endothelial NADPH Oxidase-2 promotes interstitial cardiac fibrosis and diastolic dysfunction through proinflammatory effects and endothelial-mesenchymal transition. J. Am. Coll. Cardiol. 2014, 63, 2734–2741. [Google Scholar] [CrossRef]
- Bharadwaj, A.S.; Schewitz-Bowers, L.P.; Wei, L.; Lee, R.W.J.; Smith, J.R. Intercellular adhesion molecule 1 mediates migration of Th1 and Th17 cells across human retinal vascular endothelium. Investig. Ophtalmol. Vis. Sci. 2013, 54, 6917. [Google Scholar] [CrossRef]
- Gyeong-Jin, Y.; Il-Whan, C.; Gi-Young, K.; Byung-Woo, K.; Cheol, P.; Su-Hyun, H.; Sung-Kwon, M.; Hee-Jae, C.; Young-Chae, C.; Kee Yoeup, P.; et al. Anti-inflammatory potential of saponins derived from cultured wild ginseng roots in lipopolysaccharide-stimulated RAW 264.7 macrophages. Int. J. Mol. Med. 2015, 35, 1690–1698. [Google Scholar] [CrossRef]
- Peng, S.; Hou, Y.; Yao, J.; Fang, J. Activation of Nrf2-driven antioxidant enzymes by cardamonin confers neuroprotection of PC12 cells against oxidative damage. Food Funct. 2017, 8, 997–1007. [Google Scholar] [CrossRef] [PubMed]
- Daniels, D.; Yee, D.K.; Faulconbridge, L.F.; Fluharty, S.J. Divergent behavioral roles of angiotensin receptor intracellular signaling cascades. Endocrinology 2005, 146, 5552–5560. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Huang, X.R.; Canlas, E.; Oka, K.; Truong, L.D.; Deng, C.; Bhowmick, N.A.; Ju, W.; Bottinger, E.P.; Lan, H.Y. Essential role of Smad3 in angiotensin II–induced vascular fibrosis. Circ. Res. 2006, 98, 1032–1039. [Google Scholar] [CrossRef] [PubMed]
- Hansson, G.K.; Hermansson, A. The immune system in atherosclerosis. Nat. Immunol. 2011, 12, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Ruiz-Ortega, M.; Lorenzo, O.; Ruperez, M.; Esteban, V.; Egido, J. Inflammation and angiotensin II. Int. J. Biochem. Cell Biol. 2003, 35, 881–900. [Google Scholar] [CrossRef]
- Shan, H.; Zhang, S.; Wei, X.; Li, X.; Qi, H.; He, Y.; Liu, A.; Luo, D.; Yu, X. Protection of endothelial cells against Ang II-induced impairment: Involvement of both PPARα and PPARγ via PI3K/Akt pathway. Clin. Exp. Hypertens. 2016, 38, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Manimaran, D.; Ghanendra, S.; Sridhar, M.; Kaliaperumal, J.; Muniyaraj, S.; Mohankumar, T.; Vijayakumar, R.; Manigandan, K.; Balakrishnan, R.; Elangovan, N. Molecular insights of newly identified potential peptide inhibitors of hypoxia inducible factor 1α causing breast cancer. J. Mol. Struct. 2019, 1177, 558–563. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, Y.; Yang, C. Evaluating in vitro DNA damage using comet assay. Jove-J. Vis. Exp. 2017, 128, e56450. [Google Scholar] [CrossRef]










| Number | Interacting Atoms | Distance (Å) | Interaction Force |
|---|---|---|---|
| 1 | Lys79 | 5.42 | Conventional Hydrogen Bond |
| 2 | 6.11 | ||
| 3 | Gln220 | 4.83 | |
| 4 | Gln29 | 5.35 | |
| 5 | 4.74 | ||
| 6 | Met279 | 4.61 | |
| 7 | Glu282 | 3.57 | |
| 8 | Lys221 | 5.24 | |
| 9 | 5.81 | ||
| 10 | 5.46 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Gong, F.; Chen, M.-F.; Li, C.; Hong, P.; Sun, S.; Zhou, C.; Qian, Z.-J. In Vitro Vascular-Protective Effects of a Tilapia By-Product Oligopeptide on Angiotensin II-Induced Hypertensive Endothelial Injury in HUVEC by Nrf2/NF-κB Pathways. Mar. Drugs 2019, 17, 431. https://doi.org/10.3390/md17070431
Chen J, Gong F, Chen M-F, Li C, Hong P, Sun S, Zhou C, Qian Z-J. In Vitro Vascular-Protective Effects of a Tilapia By-Product Oligopeptide on Angiotensin II-Induced Hypertensive Endothelial Injury in HUVEC by Nrf2/NF-κB Pathways. Marine Drugs. 2019; 17(7):431. https://doi.org/10.3390/md17070431
Chicago/Turabian StyleChen, Jiali, Fang Gong, Mei-Fang Chen, Chengyong Li, Pengzhi Hong, Shengli Sun, Chunxia Zhou, and Zhong-Ji Qian. 2019. "In Vitro Vascular-Protective Effects of a Tilapia By-Product Oligopeptide on Angiotensin II-Induced Hypertensive Endothelial Injury in HUVEC by Nrf2/NF-κB Pathways" Marine Drugs 17, no. 7: 431. https://doi.org/10.3390/md17070431
APA StyleChen, J., Gong, F., Chen, M. -F., Li, C., Hong, P., Sun, S., Zhou, C., & Qian, Z. -J. (2019). In Vitro Vascular-Protective Effects of a Tilapia By-Product Oligopeptide on Angiotensin II-Induced Hypertensive Endothelial Injury in HUVEC by Nrf2/NF-κB Pathways. Marine Drugs, 17(7), 431. https://doi.org/10.3390/md17070431