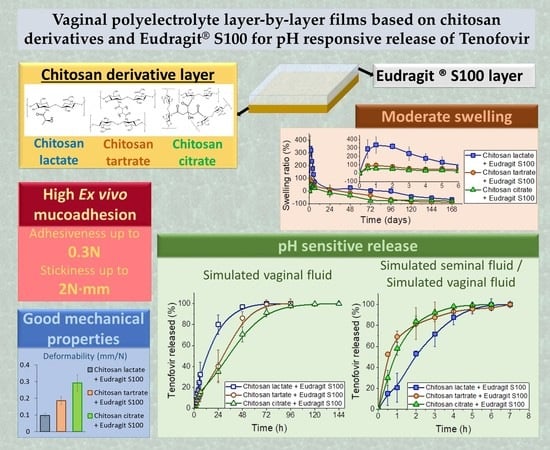
Vaginal Polyelectrolyte Layer-by-Layer Films Based on Chitosan Derivatives and Eudragit® S100 for pH Responsive Release of Tenofovir
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Chitosan Gels
2.2. Chitosan Derivative-Based Films
2.2.1. Attenuated Total Reflection Fourier Transform Infrared (FTIR-ATR) Spectroscopy
2.2.2. Appearance and Mechanical Properties
2.2.3. Drug Release Assessment
2.3. Layer-by-Layer Films
2.3.1. Texture Analysis
2.3.2. Swelling Behaviour
2.3.3. Ex Vivo Mucoadhesion
2.3.4. Drug Release
2.4. Material Cytotoxicity
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Manufacture and Characterization of Chitosan Gels
3.2.2. Chitosan Derivative-Based Films
Film Manufacture
Attenuated Total Reflection Fourier Transform Infrared (FTIR-ATR) Spectroscopy
Drug Release
3.2.3. Layer-by-Layer Films
Manufacture
SEM Microscopy
Texture Analysis
Swelling Behaviour
Ex Vivo Mucoadhesion
Drug Release
3.2.4. Material Cytotoxicity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- United Nations Joint Programme on HIV/AIDS (UNAIDS). On International Women’s Day, UNAIDS Calls for Greater Action to Protect Young Women and Adolescent Girls; United Nations Joint Programme on HIV/AIDS (UNAIDS): Geneva, Switzerland, 2019. [Google Scholar]
- Stankevitz, K.; Schwartz, K.; Hoke, T.; Li, Y.; Lanham, M.; Mahaka, I.; Mullick, S. Reaching at-risk women for PrEP delivery: What can we learn from clinical trials in sub-Saharan Africa? PLoS ONE 2019, 14, e0218556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.; Li, J.; Patel, S.K.; Palmer, K.E.; Devlin, B.; Rohan, L.C. Design of Poly(lactic-co-glycolic Acid) (PLGA) Nanoparticles for Vaginal Co-Delivery of Griffithsin and Dapivirine and Their Synergistic Effect for HIV Prophylaxis. Pharmaceutics 2019, 11, 184. [Google Scholar] [CrossRef] [Green Version]
- McConville, C.; Boyd, P.; Major, I. Efficacy of Tenofovir 1% Vaginal Gel in Reducing the Risk of HIV-1 and HSV-2 Infection. Clin. Med. Insights Women’s Heal. 2014, 7, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Thurman, A.R.; Schwartz, J.L.; Brache, V.; Clark, M.R.; McCormick, T.; Chandra, N.; Marzinke, M.A.; Stanczyk, F.Z.; Dezzutti, C.S.; Hillier, S.L.; et al. Randomized, placebo controlled phase I trial of safety, pharmacokinetics, pharmacodynamics and acceptability of tenofovir and tenofovir plus levonorgestrel vaginal rings in women. PLoS ONE 2018, 13, e0199778. [Google Scholar] [CrossRef]
- Delany-Moretlwe, S.; Lombard, C.; Baron, D.; Bekker, L.-G.; Nkala, B.; Ahmed, K.; Sebe, M.; Brumskine, W.; Nchabeleng, M.; Palanee-Philips, T.; et al. Tenofovir 1% vaginal gel for prevention of HIV-1 infection in women in South Africa (FACTS-001): A phase 3, randomised, double-blind, placebo-controlled trial. Lancet Infect. Dis. 2018, 18, 1241–1250. [Google Scholar] [CrossRef]
- Marzinke, M.A.; Moncla, B.J.; Hendrix, C.W.; Richardson-Harman, N.; Dezzutti, C.S.; Schwartz, J.L.; Spiegel, H.M.L.; Hillier, S.L.; Bunge, K.E.; Meyn, L.A.; et al. FAME-04: A Phase 1 trial to assess the safety, acceptability, pharmacokinetics and pharmacodynamics of film and gel formulations of tenofovir. J. Int. AIDS Soc. 2018, 21, e25156. [Google Scholar]
- Jalil, A.; Asim, M.H.; Le, N.-M.N.; Laffleur, F.; Matuszczak, B.; Tribus, M.; Bernkop–Schnürch, A. S-protected gellan gum: Decisive approach towards mucoadhesive antimicrobial vaginal films. Int. J. Biol. Macromol. 2019, 130, 148–157. [Google Scholar] [CrossRef]
- Machado, R.M.; Palmeira-De-Oliveira, A.; Martinez-De-Oliveira, J.; Palmeira-De-Oliveira, R. Vaginal films for drug delivery. J. Pharm. Sci. 2013, 102, 2069–2081. [Google Scholar] [CrossRef]
- Guzmán, E.; Mateos-Maroto, A.; Ruano, M.; Ortega, F.; Rubio, R.G. Layer-by-Layer polyelectrolyte assemblies for encapsulation and release of active compounds. Adv. Colloid Interface Sci. 2017, 249, 290–307. [Google Scholar] [CrossRef]
- Notario-Pérez, F.; Martín-Illana, A.; Cazorla-Luna, R.; Ruiz-Caro, R.; Peña, J.; Veiga, M.D. Improvement of Tenofovir vaginal release from hydrophilic matrices through drug granulation with hydrophobic polymers. Eur. J. Pharm. Sci. 2018, 117, 204–215. [Google Scholar] [CrossRef]
- Melegari, C.; Bertoni, S.; Genovesi, A.; Hughes, K.; Rajabi-Siahboomi, A.R.; Passerini, N.; Albertini, B. Ethylcellulose film coating of guaifenesin-loaded pellets: A comprehensive evaluation of the manufacturing process to prevent drug migration. Eur. J. Pharm. Biopharm. 2016, 100, 15–26. [Google Scholar] [CrossRef]
- Volodkin, D.; von Klitzing, R. Competing mechanisms in polyelectrolyte multilayer formation and swelling: Polycation–polyanion pairing vs. polyelectrolyte–ion pairing. Curr. Opin. Colloid Interface Sci. 2014, 19, 25–31. [Google Scholar] [CrossRef]
- Jeganathan, B.; Prakya, V.; Deshmukh, A. Preparation and Evaluation of Diclofenac Sodium Tablet Coated with Polyelectrolyte Multilayer Film Using Hypromellose Acetate Succinate and Polymethacrylates for pH-Dependent, Modified Release Drug Delivery. AAPS PharmSciTech 2016, 17, 578–587. [Google Scholar] [CrossRef] [Green Version]
- Lokova, A.Y.; Zaborova, O.V. Modification of fliposomes with a polycation can enhance the control of pH-induced release. Int. J. Nanomed. 2019, 14, 1039–1049. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.-Q.; Zhang, Q. pH-sensitive polymeric nanoparticles to improve oral bioavailability of peptide/protein drugs and poorly water-soluble drugs. Eur. J. Pharm. Biopharm. 2012, 82, 219–229. [Google Scholar] [CrossRef]
- Martín-Illana, A.; Notario-Pérez, F.; Cazorla-Luna, R.; Ruiz-Caro, R.; Veiga, M.D. Smart Freeze-Dried Bigels for the Prevention of the Sexual Transmission of HIV by Accelerating the Vaginal Release of Tenofovir during Intercourse. Pharmaceutics 2019, 11, 232. [Google Scholar] [CrossRef] [Green Version]
- Cazorla-Luna, R.; Notario-Pérez, F.; Martín-Illana, A.; Tamayo, A.; Rubio, J.; Ruiz-Caro, R.; Veiga, M.D. Chitosan-Based Mucoadhesive Vaginal Tablets for Controlled Release of the Anti-HIV Drug Tenofovir. Pharmaceutics 2019, 11, 20. [Google Scholar] [CrossRef] [Green Version]
- Frank, L.A.; Sandri, G.; D’Autilia, F.; Contri, R.V.; Bonferoni, M.C.; Caramella, C.; Frank, A.G.; Pohlmann, A.R.; Guterres, S.S. Chitosan gel containing polymeric nanocapsules: A new formulation for vaginal drug delivery. Int. J. Nanomedicine 2014, 9, 3151. [Google Scholar] [PubMed] [Green Version]
- Kilicarslan, M.; Ilhan, M.; Inal, O.; Orhan, K. Preparation and evaluation of clindamycin phosphate loaded chitosan/alginate polyelectrolyte complex film as mucoadhesive drug delivery system for periodontal therapy. Eur. J. Pharm. Sci. 2018, 123, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Layek, B.; Rahman Nirzhor, S.S.; Rathi, S.; Kandimalla, K.K.; Wiedmann, T.S.; Prabha, S. Design, Development, and Characterization of Imiquimod-Loaded Chitosan Films for Topical Delivery. AAPS PharmSciTech 2019, 20, 58. [Google Scholar] [CrossRef]
- Ali, A.; Ahmed, S. A review on chitosan and its nanocomposites in drug delivery. Int. J. Biol. Macromol. 2018, 109, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Valenta, C. The use of mucoadhesive polymers in vaginal delivery. Adv. Drug Deliv. Rev. 2005, 57, 1692–1712. [Google Scholar] [CrossRef] [PubMed]
- Cheung, R.C.F.; Ng, T.B.; Wong, J.H.; Chan, W.Y. Chitosan: An Update on Potential Biomedical and Pharmaceutical Applications. Mar. Drugs 2015, 13, 5156–5186. [Google Scholar] [CrossRef] [PubMed]
- Furuike, T.; Komoto, D.; Hashimoto, H.; Tamura, H. Preparation of chitosan hydrogel and its solubility in organic acids. Int. J. Biol. Macromol. 2017, 104, 1620–1625. [Google Scholar] [CrossRef]
- Soares, L.S.; Perim, R.B.; de Alvarenga, E.S.; Guimarães, L.M.; Teixeira, A.V.N.C.; Coimbra, J.S.D.R.; de Oliveira, E.B. Insights on physicochemical aspects of chitosan dispersion in aqueous solutions of acetic, glycolic, propionic or lactic acid. Int. J. Biol. Macromol. 2019, 128, 140–148. [Google Scholar] [CrossRef]
- Tronci, G.; Ajiro, H.; Russell, S.J.; Wood, D.J.; Akashi, M. Tunable drug-loading capability of chitosan hydrogels with varied network architectures. Acta Biomater. 2014, 10, 821–830. [Google Scholar] [CrossRef]
- Libio, I.C.; Demori, R.; Ferrão, M.F.; Lionzo, M.I.Z.; da Silveira, N.P. Films based on neutralized chitosan citrate as innovative composition for cosmetic application. Mater. Sci. Eng. C 2016, 67, 115–124. [Google Scholar] [CrossRef]
- Stie, M.B.; Jones, M.; Sørensen, H.O.; Jacobsen, J.; Chronakis, I.S.; Nielsen, H.M. Acids ‘generally recognized as safe’ affect morphology and biocompatibility of electrospun chitosan/polyethylene oxide nanofibers. Carbohydr. Polym. 2019, 215, 253–262. [Google Scholar] [CrossRef] [Green Version]
- Benucci, I.; Liburdi, K.; Cacciotti, I.; Lombardelli, C.; Zappino, M.; Nanni, F.; Esti, M. Chitosan/clay nanocomposite films as supports for enzyme immobilization: An innovative green approach for winemaking applications. Food Hydrocoll. 2018, 74, 124–131. [Google Scholar] [CrossRef]
- Cacciotti, I.; Lombardelli, C.; Benucci, I.; Esti, M. Clay/chitosan biocomposite systems as novel green carriers for covalent immobilization of food enzymes. J. Mater. Res. Technol. 2019, 8, 3644–3652. [Google Scholar] [CrossRef]
- Notario-Pérez, F.; Cazorla-Luna, R.; Martín-Illana, A.; Ruiz-Caro, R.; Tamayo, A.; Rubio, J.; Veiga, M.D. Optimization of tenofovir release from mucoadhesive vaginal tablets by polymer combination to prevent sexual transmission of HIV. Carbohydr. Polym. 2018, 179, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Kyzioł, A.; Mazgała, A.; Michna, J.; Regiel-Futyra, A.; Sebastian, V. Preparation and characterization of alginate/chitosan formulations for ciprofloxacin-controlled delivery. J. Biomater. Appl. 2017, 32, 162–174. [Google Scholar] [CrossRef]
- Ali, A.; Shahid, M.A.; Hossain, M.D.; Islam, M.N. Antibacterial bi-layered polyvinyl alcohol (PVA)-chitosan blend nanofibrous mat loaded with Azadirachta indica (neem) extract. Int. J. Biol. Macromol. 2019, 138, 13–20. [Google Scholar] [CrossRef]
- Ubaid, M.; Shah, S.N.H.; Khan, S.A.; Murtaza, G. Synthesis and Characterization of pH-Sensitive Genipin Cross-Linked Chitosan/Eudragit® L100 Hydrogel for Metformin Release Study Using Response Surface Methodology. Curr. Drug Deliv. 2018, 15, 1343–1358. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Guo, F.; Deng, T.; Zhu, S.; Liu, W.; Zhong, H.; Yu, H.; Luo, R.; Deng, Z. Eudragit S100-Coated Chitosan Nanoparticles Co-loading Tat for Enhanced Oral Colon Absorption of Insulin. AAPS PharmSciTech 2017, 18, 1277–1287. [Google Scholar] [CrossRef] [PubMed]
- Ansari, F.; Pourjafar, H.; Jodat, V.; Sahebi, J.; Ataei, A. Effect of Eudragit S100 nanoparticles and alginate chitosan encapsulation on the viability of Lactobacillus acidophilus and Lactobacillus rhamnosus. AMB Express 2017, 7, 144. [Google Scholar] [CrossRef] [Green Version]
- Qindeel, M.; Ahmed, N.; Sabir, F.; Khan, S.; Ur-Rehman, A. Development of novel pH-sensitive nanoparticles loaded hydrogel for transdermal drug delivery. Drug Dev. Ind. Pharm. 2019, 45, 629–641. [Google Scholar] [CrossRef]
- Prasad, S.; Dangi, J.S. Development and characterization of pH responsive polymeric nanoparticles of SN-38 for colon cancer. Artif. Cells Nanomed.Biotechnol. 2016, 44, 1824–1834. [Google Scholar] [CrossRef]
- Solanki, A.; Thakore, S. Cellulose crosslinked pH-responsive polyurethanes for drug delivery: α-hydroxy acids as drug release modifiers. Int. J. Biol. Macromol. 2015, 80, 683–691. [Google Scholar] [CrossRef]
- Martín-illana, A.; Cazorla-luna, R.; Notario-pérez, F.; Bedoya, L.M.; Ruiz-caro, R.; Dolores, M. Freeze-dried bioadhesive vaginal bigels for controlled release of Tenofovir. Eur. J. Pharm. Sci. 2019, 127, 38–51. [Google Scholar] [CrossRef]
- Nelson, A.L. An overview of properties of Amphora (Acidform) contraceptive vaginal gel. Expert Opin. Drug Saf. 2018, 17, 935–943. [Google Scholar] [CrossRef] [PubMed]
- Notario-Pérez, F.; Ruiz-Caro, R.; Veiga-Ochoa, M.D. Historical development of vaginal microbicides to prevent sexual transmission of HIV in women: From past failures to future hopes. Drug Des. Devel. Ther. 2017, 11, 1767–1787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benucci, I.; Lombardelli, C.; Cacciotti, I.; Liburdi, K.; Nanni, F.; Esti, M. Chitosan beads from microbial and animal sources as enzyme supports for wine application. Food Hydrocoll. 2016, 61, 191–200. [Google Scholar] [CrossRef]
- Kowalczyk, D.; Kordowska-Wiater, M.; Nowak, J.; Baraniak, B. Characterization of films based on chitosan lactate and its blends with oxidized starch and gelatin. Int. J. Biol. Macromol. 2015, 77, 350–359. [Google Scholar] [CrossRef]
- Izutsu, H.; Mizukami, F.; Kiyozumi, Y.; Maeda, K. Preparation and characterization ofL-tartaric acid–silica composites recognizing molecular asymmetry. J. Mater. Chem. 1997, 7, 1519–1525. [Google Scholar] [CrossRef]
- Lin, H.; Su, J.; Kankala, R.K.; Zeng, M.; Zhou, S.-F.; Lin, X. Using pH-Activable Carbon Nanoparticles as Cell Imaging Probes. Micromachines 2019, 10, 568. [Google Scholar] [CrossRef] [Green Version]
- Bhattarai, N.; Ramay, H.R.; Chou, S.H.; Zhang, M. Chitosan and lactic acid-grafted chitosan nanoparticles as carriers for prolonged drug delivery. Int. J. Nanomed. 2006, 1, 181–187. [Google Scholar] [CrossRef]
- Basumallick, S.; Gabriela Nogueira Campos, M.; Richardson, D.; Gesquiere, A.; Santra, S. Hydrothermally treated chitosan spontaneously forms water-soluble spherical particles stable at a wide pH range. Int. J. Polym. Mater. Polym. Biomater. 2016, 65, 751–758. [Google Scholar] [CrossRef]
- Gylienė, O.; Nivinskienė, O.; Vengris, T. Sorption of tartrate, citrate, and EDTA onto chitosan and its regeneration applying electrolysis. Carbohydr. Res. 2008, 343, 1324–1332. [Google Scholar] [CrossRef]
- Bagheri, M.; Younesi, H.; Hajati, S.; Borghei, S.M. Application of chitosan-citric acid nanoparticles for removal of chromium (VI). Int. J. Biol. Macromol. 2015, 80, 431–444. [Google Scholar] [CrossRef]
- Miles, K.B.; Ball, R.L.; Matthew, H.W.T. Chitosan films with improved tensile strength and toughness from N-acetyl-cysteine mediated disulfide bonds. Carbohydr. Polym. 2016, 139, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Li, H.; Ding, H.; Fan, Z.; Pi, P.; Cheng, J.; Wen, X. Allylated chitosan-poly(N-isopropylacrylamide) hydrogel based on a functionalized double network for controlled drug release. Carbohydr. Polym. 2019, 214, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Cazorla-Luna, R.; Martín-Illana, A.; Notario-Pérez, F.; Bedoya, L.-M.; Bermejo, P.; Ruiz-Caro, R.; Veiga, M.-D. Dapivirine Bioadhesive Vaginal Tablets Based on Natural Polymers for the Prevention of Sexual Transmission of HIV. Polymers (Basel) 2019, 11, 483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehta, R.; Chawla, A.; Sharma, P.; Pawar, P. Formulation and in vitro evaluation of Eudragit S-100 coated naproxen matrix tablets for colon-targeted drug delivery system. J. Adv. Pharm. Technol. Res. 2019, 4, 31–41. [Google Scholar]
- Wilkinson, D.; Ramjee, G.; Tholandi, M.; Rutherford, G.W. Nonoxynol-9 for preventing vaginal acquisition of HIV infection by women from men. Cochrane Database Syst. Rev. 2002, 4, CD003939. [Google Scholar] [CrossRef]
- Ai, Z.; Jiang, Z.; Li, L.; Deng, W.; Kusakabe, I.; Li, H. Immobilization of Streptomyces olivaceoviridis E-86 xylanase on Eudragit S-100 for xylo-oligosaccharide production. Process Biochem. 2005, 40, 2707–2714. [Google Scholar] [CrossRef]
- Moss, J.A.; Malone, A.M.; Smith, T.J.; Butkyavichene, I.; Cortez, C.; Gilman, J.; Kennedy, S.; Kopin, E.; Nguyen, C.; Sinha, P.; et al. Safety and pharmacokinetics of intravaginal rings delivering tenofovir in pig-tailed macaques. Antimicrob. Agents Chemother. 2012, 56, 5952–5960. [Google Scholar] [CrossRef] [Green Version]
- Notario-Pérez, F.; Martín-Illana, A.; Cazorla-Luna, R.; Ruiz-Caro, R.; Bedoya, L.M.; Tamayo, A.; Rubio, J.; Veiga, M.D. Influence of chitosan swelling behaviour on controlled release of tenofovir from mucoadhesive vaginal systems for prevention of sexual transmission of HIV. Mar. Drugs 2017, 15, 50. [Google Scholar] [CrossRef] [Green Version]
- Bermejo, P.; Rubio, J.; Martín-Illana, A.; Sánchez-Sánchez, M.-P.; Bedoya, L.-M.; Otero-Espinar, F.; Fernández-Ferreiro, A.; Carro, R.; Veiga, M.-D.; Ruiz-Caro, R.; et al. Chitosan and Kappa-Carrageenan Vaginal Acyclovir Formulations for Prevention of Genital Herpes. In Vitro and Ex Vivo Evaluation. Mar. Drugs 2015, 13, 5976–5992. [Google Scholar]
- Notario-Pérez, F.; Martín-Illana, A.; Cazorla-Luna, R.; Ruiz-Caro, R.; Bedoya, L.-M.; Peña, J.; Veiga, M.-D. Development of mucoadhesive vaginal films based on HPMC and zein as novel formulations to prevent sexual transmission of HIV. Int. J. Pharm. 2019, 570, 118643. [Google Scholar] [CrossRef]
- Mamani, P.L.; Ruiz-Caro, R.; Veiga, M.D. Matrix Tablets: The Effect of Hydroxypropyl Methylcellulose/Anhydrous Dibasic Calcium Phosphate Ratio on the Release Rate of a Water-Soluble Drug Through the Gastrointestinal Tract I. In Vitro Tests. AAPS PharmSciTech 2012, 13, 1073–1083. [Google Scholar] [CrossRef] [Green Version]
- Owen, D.H.; Katz, D.F. A review of the physical and chemical properties of human semen and the formulation of a semen simulant. J. Androl. 2005, 26, 459–469. [Google Scholar] [CrossRef] [Green Version]
- Shah, V.P.; Tsong, Y.; Sathe, P.; Liu, J.P. In vitro dissolution profile comparison- Statistics and analysis of the similarity factor, f2. Pharm. Res. 1998, 15, 889–896. [Google Scholar] [CrossRef]
- Harada, S.; Koyanagi, Y.; Yamamoto, N. Infection of HTLV-III/LAV in HTLV-I-carrying cells MT-2 and MT-4 and application in a plaque assay. Science 1985, 229, 563–566. [Google Scholar] [CrossRef]
- Krug, H.F. Handbook Standard Procedures for Nanoparticle Testing; Comprehensive Assessment of Hazardous Effects of Engineering Nanomaterials on the Immune System Quality; EMPA: Dübendorf, Switzerland, 2011; p. 225. [Google Scholar]















| Batch | Pliability | Organoleptic Characteristics | Comments |
|---|---|---|---|
| ChL-a | ✔ | Homogeneous. Translucent, yellowish and shiny. Odourless. Soft touch. | Although somewhat flexible, it breaks if folded in half. |
| ChL-b | ✔✔ | Homogeneous. Translucent, yellowish and shiny. Odourless. Soft touch. | |
| ChL-c | ✖ | Homogeneous. Translucent, yellowish and shiny. Odourless. Sticky touch. | The film can barely be handled due to its stickiness. |
| ChT-a | ✔✔ | Homogeneous. Translucent, yellowish and shiny. Odourless. Soft touch. | |
| ChT-b | ✔✔✔ | Homogeneous. Translucent, yellowish and shiny. Odourless. Soft touch. | |
| ChT-c | ✖ | Heterogeneous. Opaque, yellowish and matt. Odourless. Rough touch. | Completely rigid, it breaks when even slight force is applied. |
| ChC-a | ✔✔ | Homogeneous. Translucent, yellowish and shiny. Odourless. Soft touch. | |
| ChC-b | ✔✔✔ | Homogeneous. Translucent, yellowish and shiny. Odourless. Soft touch. | |
| ChC-c | ✖ | Heterogeneous. Opaque, yellowish and matt. Odourless. Rough touch. | Completely rigid, it breaks when even slight force is applied. |
| ChL/E-a SVF | ChL/E-b SVF/SSF | |
| ChL/E-a SVF/SSF | 19.68 | 59.96 |
| ChL/E-b SVF | 73.63 | 21.21 |
| ChT/E-a SVF | ChT/E-b SVF/SSF | |
| ChT/E-a SVF/SSF | 14.34 | 34.43 |
| ChT/E-b SVF | 64.99 | 9.72 |
| ChC/E-a SVF | ChC/E-b SVF/SSF | |
| ChC/E-a SVF/SSF | 13.00 | 50.80 |
| ChC/E-b SVF | 43.01 | 11.71 |
| CC50 µg/mL (CI95%; R2) | |||
|---|---|---|---|
| HEC-1A | THP1 | MT-2 | |
| Lactic acid | >1000 | >1000 | >1000 |
| Tartaric acid | ≈200 | >1000 | >1000 |
| Citric acid | >1000 | ≈1000 | >1000 |
| TEC | >1000 | >1000 | ≈1000 |
| ES100 | >1000 | >1000 | >1000 |
| Batch | Chitosan (%) | Lactic Acid (M) | Tartaric Acid (M) | Citric Acid (M) |
|---|---|---|---|---|
| gChL-a | 3 | 0.25 | ||
| gChL-b | 3 | 0.5 | ||
| gChL-c | 3 | 1 | ||
| gChT-a | 3 | 0.25 | ||
| gChT-b | 3 | 0.5 | ||
| gChT-c | 3 | 1 | ||
| gChC-a | 3 | 0.25 | ||
| gChC-b | 3 | 0.5 | ||
| gChC-c | 3 | 1 |
| Batch | Chitosan (mg) | Lactic Acid (mg) | Tartaric Acid (mg) | Citric Acid (mg) | TFV (mg) |
|---|---|---|---|---|---|
| ChL-a | 150 | 112.5 (0.25 M) | |||
| ChL-b | 150 | 225 (0.5 M) | |||
| ChL-c | 150 | 450 (1 M) | |||
| ChT-a | 150 | 187.5 (0.25 M) | |||
| ChT-b | 150 | 375 (0.5 M) | |||
| ChT-c | 150 | 750 (1 M) | |||
| ChC-a | 150 | 240 (0.25 M) | |||
| ChC-b | 150 | 480 (0.5 M) | |||
| ChC-c | 150 | 960 (1 M) | |||
| ChL-TFV | 150 | 225 (0.5 M) | 30 | ||
| ChT-TFV | 150 | 375 (0.5 M) | 30 | ||
| ChC-TFV | 150 | 480 (0.5 M) | 30 |
| ChL/E-a | ChL/E-b | ChT/E-a | ChT/E-b | ChC/E-a | ChC/E-b | ||
|---|---|---|---|---|---|---|---|
| CHITOSAN DERIVATIVE-BASED LAYER | Chitosan (mg) | 150 | 150 | 150 | 150 | 150 | 150 |
| Lactic acid (mg) | 225 | 225 | |||||
| Tartaric acid (mg) | 375 | 375 | |||||
| Citric acid (mg) | 480 | 480 | |||||
| Tenofovir (mg) | 30 | 30 | 30 | 30 | 30 | 30 | |
| EUDRAGIT® S100-BASED LAYER | Eudragit® S100 (mg) | 75 | 150 | 75 | 150 | 75 | 150 |
| Triethylcitrate (mg) | 37.5 | 75 | 37.5 | 75 | 37.5 | 75 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cazorla-Luna, R.; Martín-Illana, A.; Notario-Pérez, F.; Bedoya, L.M.; Tamayo, A.; Ruiz-Caro, R.; Rubio, J.; Veiga, M.-D. Vaginal Polyelectrolyte Layer-by-Layer Films Based on Chitosan Derivatives and Eudragit® S100 for pH Responsive Release of Tenofovir. Mar. Drugs 2020, 18, 44. https://doi.org/10.3390/md18010044
Cazorla-Luna R, Martín-Illana A, Notario-Pérez F, Bedoya LM, Tamayo A, Ruiz-Caro R, Rubio J, Veiga M-D. Vaginal Polyelectrolyte Layer-by-Layer Films Based on Chitosan Derivatives and Eudragit® S100 for pH Responsive Release of Tenofovir. Marine Drugs. 2020; 18(1):44. https://doi.org/10.3390/md18010044
Chicago/Turabian StyleCazorla-Luna, Raúl, Araceli Martín-Illana, Fernando Notario-Pérez, Luis Miguel Bedoya, Aitana Tamayo, Roberto Ruiz-Caro, Juan Rubio, and María-Dolores Veiga. 2020. "Vaginal Polyelectrolyte Layer-by-Layer Films Based on Chitosan Derivatives and Eudragit® S100 for pH Responsive Release of Tenofovir" Marine Drugs 18, no. 1: 44. https://doi.org/10.3390/md18010044
APA StyleCazorla-Luna, R., Martín-Illana, A., Notario-Pérez, F., Bedoya, L. M., Tamayo, A., Ruiz-Caro, R., Rubio, J., & Veiga, M. -D. (2020). Vaginal Polyelectrolyte Layer-by-Layer Films Based on Chitosan Derivatives and Eudragit® S100 for pH Responsive Release of Tenofovir. Marine Drugs, 18(1), 44. https://doi.org/10.3390/md18010044