Marine Seaweed Polysaccharides-Based Engineered Cues for the Modern Biomedical Sector
Abstract
:1. Introduction
2. Methodological Approach—Inclusion/Exclusion Criteria
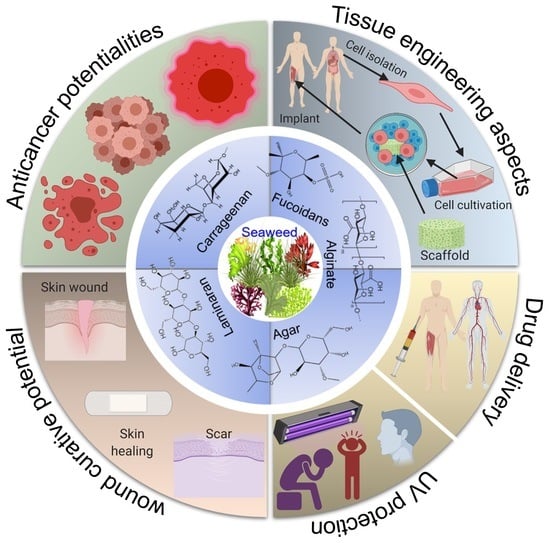
3. Marine Seaweed Polysaccharides
3.1. Carrageenan—Structural and Functional Entities
3.2. Agar and Agarose—Structural and Functional Entities
3.3. Alginate—Structural and Functional Entities
3.4. Fucoidan—Structural and Functional Entities
3.5. Laminaran—Structural and Functional Entities
3.6. Ulvan—Structural and Functional Entities
3.7. Furcellaran—Structural and Functional Entities
4. Biomedical Values of Engineered Cues
4.1. Targeted Drug Delivery
4.2. Wound-Curative Potential
4.3. Anticancer Potentialities
4.4. Tissue-Engineering Aspects
4.5. Ultraviolet (UV) Protectant Potential
5. Current Challenges and Literature Gaps
6. Concluding Remarks and Outlook
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tiwari, G.; Tiwari, R.; Sriwastawa, B.; Bhati, L.; Pandey, S.; Pandey, P.; Bannerjee, S.K. Drug delivery systems: An updated review. Int. J. Pharm. Investig. 2012, 2, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bilal, M.; Rasheed, T.; Ahmed, I.; Iqbal, H.M.N. High-value compounds from microalgae with industrial exploitability—A review. Front. Biosci. Sch. 2017, 9, 319–342. [Google Scholar]
- Sosa-Hernández, J.E.; Escobedo-Avellaneda, Z.; Iqbal, H.; Welti-Chanes, J. State-of-the-art extraction methodologies for bioactive compounds from algal biome to meet bio-economy challenges and opportunities. Molecules 2018, 23, 2953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albinali, K.E.; Zagho, M.M.; Deng, Y.; Elzatahry, A.A. A perspective on magnetic core–shell carriers for responsive and targeted drug delivery systems. Int. J. Nanomed. 2019, 14, 1707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasheed, T.; Nabeel, F.; Raza, A.; Bilal, M.; Iqbal, H.M.N. Biomimetic nanostructures/cues as drug delivery systems: A review. Mater. Today Chem. 2019, 13, 147–157. [Google Scholar] [CrossRef]
- Raza, A.; Rasheed, T.; Nabeel, F.; Hayat, U.; Bilal, M.; Iqbal, H. Endogenous and exogenous stimuli-responsive drug delivery systems for programmed site-specific release. Molecules 2019, 24, 1117. [Google Scholar] [CrossRef] [Green Version]
- Du, Y.; Chen, B. Combination of drugs and carriers in drug delivery technology and its development. Drug Des. Dev. Ther. 2019, 13, 1401. [Google Scholar] [CrossRef] [Green Version]
- Craigie, J.S. Cell walls. In Biology of the Red Algae; Cole, K.M., Sheath, R.G., Eds.; Cambridge University Press: Cambridge, UK, 1990; pp. 221–257. [Google Scholar]
- Kraan, S. Algal polysaccharides, novel applications and outlook. In Carbohydrates—Comprehensive Studies on Glycobiology and Glycotechnology; Chang, C.F., Ed.; InTech: Rijeka, Croatia, 2012; pp. 489–532. [Google Scholar]
- Iqbal, H.M.; Rasheed, T.; Bilal, M. Design and processing aspects of polymer and composite materials. Green Sustain. Adv. Mater. Process. Charact. 2018, 1, 155–189. [Google Scholar]
- Cardozo, K.H.; Guaratini, T.; Barros, M.P.; Falc˜ao, V.R.; Tonon, A.P.; Lopes, N.P.; Pinto, E. Metabolites from algae with economical impact. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2007, 146, 60–78. [Google Scholar] [CrossRef]
- Cunha, L.; Grenha, A. Sulfated seaweed polysaccharides as multifunctional materials in drug delivery applications. Mar. Drugs 2016, 14, E42. [Google Scholar] [CrossRef]
- Campo, V.L.; Kawano, D.F.; da Silva, D.B.; Carvalho, I., Jr. Carrageenans: Biological properties, chemical modifications and structural analysis—A review. Carbohydr. Polym. 2009, 77, 167–180. [Google Scholar] [CrossRef]
- Synytsya, A.; Čopíková, J.; Kim, W.J.; Park, Y.I. Cell wall polysaccharides of marine algae. In Springer Handbook of Marine Biotechnology; Kim, S.K., Ed.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 543–590. [Google Scholar]
- Venugopal, V. Sulfated and Non-Sulfated Polysaccharides from Seaweeds and their Uses: An Overview. EC Nutr. 2019, 14, 126–141. [Google Scholar]
- Venkatesan, J.; Bhatnagar, I.; Manivasagan, P.; Kang, K.H.; Kim, S.K. Alginate composites for bone tissue engineering: A review. Int. J. Biol. Macromol. 2015, 72, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rehm, B.H. Alginate production: Precursor biosynthesis, polymerization and secretion. In Alginates: Biology and Applications; Springer: Berlin/Heidelberg, Germany, 2009; pp. 55–71. [Google Scholar]
- Rupérez, P.; Gómez-Ordóñez, E.; Jiménez-Escrig, A. Biological activity of algal sulfated and nonsulfated polysaccharides. Bioact. Compd. Mar. Foods Plant Anim. Sources 2013, Ch11, 219–247. [Google Scholar] [CrossRef]
- Zia, K.M.; Zia, F.; Zuber, M.; Rehman, S.; Ahmad, M.N. Alginate-based polyurethanes: A review of recent advances and perspective. Int. J. Biol. Macromol. 2015, 79, 377–387. [Google Scholar] [CrossRef]
- Smidsrod, O.; Draget, K.I. Chemistry and physical properties of alginates. Carbohydrate 1999, 14, 7–12. [Google Scholar]
- Clementi, F.; Crudele, M.A.; Parente, E.; Mancini, M.; Moresi, M. Production and characterisation of alginate from Azotobacter vinelandii. J. Sci. Food Agric. 1999, 79, 602–610. [Google Scholar] [CrossRef]
- Berteau, O.; Mulloy, B. Sulfated fucans, fresh perspectives: Structures, functions, and biological properties of sulfated fucans and an overview of enzymes active toward this class of polysaccharide. Glycobiology 2003, 13, 29R–40R. [Google Scholar] [CrossRef] [Green Version]
- Athukorala, Y.; Jung, W.K.; Vasanthan, T.; Jeon, Y.J. An anticoagulative polysaccharide from an enzymatic hydrolysate of Ecklonia cava. Carbohydr. Polym. 2006, 66, 184–191. [Google Scholar] [CrossRef]
- Bixler, H.J.; Porse, H. A decade of change in the seaweed hydrocolloids industry. J. Appl. Phycol. 2011, 23, 321–335. [Google Scholar] [CrossRef]
- Kadam, S.U.; Tiwari, B.K.; O’donnell, C.P. Extraction, structure and biofunctional activities of laminarin from brown algae. Int. J. Food Sci. Technol. 2015, 50, 24–31. [Google Scholar] [CrossRef]
- Rioux, L.E.; Turgeon, S.L.; Beaulieu, M. Characterization of polysaccharides extracted from brown seaweeds. Carbohydr. Polym. 2007, 69, 530–537. [Google Scholar] [CrossRef]
- Lahaye, M.; Robic, A. Structure and functional properties of ulvan, a polysaccharide from green seaweeds. Biomacromolecules 2007, 8, 1765–1774. [Google Scholar] [CrossRef] [PubMed]
- Barsanti, L.; Gualtieri, P. Algae: Anatomy, Biochemistry, and Biotechnology, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Hinou, H.; Kurosawa, H.; Matsuoka, K.; Terunuma, D.; Kuzuhara, H. Novel synthesis of L-iduronic acid using trehalose as the disaccharidic starting material. Tetrahedron Lett. 1999, 40, 1501–1504. [Google Scholar] [CrossRef]
- Hernández-Garibay, E.; Zertuche-González, J.A.; Pacheco-Ruíz, I. Isolation and chemical characterization of algal polysaccharides from the green seaweed Ulva clathrata (Roth) C. Agardh. J. Appl. Phycol. 2011, 23, 537–542. [Google Scholar] [CrossRef]
- Imeson, S.P. Carrageenan and furcelleran. In Handbook of Hydrocolloids, 2nd Ed.; Phillips, G.O., Williams, P.A., Eds.; CRC Press: Boca Raton, FL, USA, 2010; pp. 164–184. [Google Scholar]
- Grenha, A.; Gomes, M.E.; Rodrigues, M.; Santo, V.E.; Mano, J.F.; Neves, N.M.; Reis, R.L. Development of new chitosan/carrageenan nanoparticles for drug delivery applications. J. Biomed. Mater. Res. Part A 2010, 92, 1265–1272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Li, X.; Zhong, N.; Huang, Y.; He, K.; Ye, X. Injectable in situ dual-crosslinking hyaluronic acid and sodium alginate based hydrogels for drug release. J. Biomater. Sci. Polym. Ed. 2019, 1–10. [Google Scholar] [CrossRef]
- Bi, Y.G.; Lin, Z.T.; Deng, S.T. Fabrication and characterization of hydroxyapatite/sodium alginate/chitosan composite microspheres for drug delivery and bone tissue engineering. Mater. Sci. Eng. C 2019, 100, 576–583. [Google Scholar] [CrossRef]
- Youssouf, L.; Bhaw-Luximon, A.; Diotel, N.; Catan, A.; Giraud, P.; Gimié, F.; Lallemand, L. Enhanced effects of curcumin encapsulated in polycaprolactone-grafted oligocarrageenan nanomicelles, a novel nanoparticle drug delivery system. Carbohydr. Polym. 2019, 217, 35–45. [Google Scholar] [CrossRef]
- Pozharitskaya, O.; Shikov, A.; Faustova, N.; Obluchinskaya, E.; Kosman, V.; Vuorela, H.; Makarov, V. Pharmacokinetic and tissue distribution of fucoidan from Fucus vesiculosus after oral administration to rats. Mar. Drugs 2018, 16, 132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhivya, S.; Padma, V.V.; Santhini, E. Wound dressings—A review. BioMedicine 2015, 5, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Pozharitskaya, O.N.; Shikov, A.N.; Obluchinskaya, E.D.; Vuorela, H. The pharmacokinetics of fucoidan after topical application to rats. Mar. Drugs 2019, 17, 687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Leary, R.; Rerek, M.; Wood, E.J. Fucoidan modulates the effect of transforming growth factor (TGF)-β1 on fibroblast proliferation and wound repopulation in in vitro models of dermal wound repair. Biol. Pharm. Bull. 2004, 27, 266–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feki, A.; Bardaa, S.; Hajji, S.; Ktari, N.; Hamdi, M.; Chabchoub, N.; Amara, I.B. Falkenbergia rufolanosa polysaccharide-poly (vinyl alcohol) composite films: A promising wound healing agent against dermal laser burns in rats. Int. J. Biol. Macromol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Slima, S.B.; Trabelsi, I.; Ktari, N.; Bardaa, S.; Elkaroui, K.; Bouaziz, M.; Salah, R.B. Novel Sorghum bicolor (L.) seed polysaccharide structure, hemolytic and antioxidant activities, and laser burn wound healing effect. Int. J. Biol. Macromol. 2019, 132, 87–96. [Google Scholar] [CrossRef]
- Janarthanan, M.; Senthil Kumar, M. Extraction of alginate from brown seaweeds and evolution of bioactive alginate film coated textile fabrics for wound healing application. J. Ind. Text. 2019, 49, 328–351. [Google Scholar] [CrossRef]
- Sellimi, S.; Maalej, H.; Rekik, D.M.; Benslima, A.; Ksouda, G.; Hamdi, M.; Hajji, M. Antioxidant, antibacterial and in vivo wound healing properties of laminaran purified from Cystoseira barbata seaweed. Int. J. Biol. Macromol. 2018, 119, 633–644. [Google Scholar] [CrossRef]
- Obluchinsksya, E.D.; Makarova, M.N.; Pozharitskaya, O.N.; Shikov, A.N. Effects of ultrasound treatment on the chemical composition and anticoagulant properties of dry fucus extract. Pharm. Chem. J. 2015, 49, 183–186. [Google Scholar] [CrossRef]
- Chen, X.; Song, L.; Wang, H.; Liu, S.; Yu, H.; Wang, X.; Li, P. Partial characterization, the immune modulation and anticancer activities of sulfated polysaccharides from filamentous microalgae Tribonema sp. Molecules 2019, 24, 322. [Google Scholar] [CrossRef] [Green Version]
- Alboofetileh, M.; Rezaei, M.; Tabarsa, M. Enzyme-assisted extraction of Nizamuddinia zanardinii for the recovery of sulfated polysaccharides with anticancer and immune-enhancing activities. J. Appl. Phycol. 2019, 31, 1391–1402. [Google Scholar] [CrossRef]
- Prabhu, R.; Mohammed, M.A.; Anjali, R.; Archunan, G.; Prabhu, N.M.; Pugazhendhi, A.; Suganthy, N. Ecofriendly one pot fabrication of methyl gallate@ ZIF-L nanoscale hybrid as pH responsive drug delivery system for lung cancer therapy. Process Biochem. 2019, 84, 39–52. [Google Scholar]
- Ale, M.T.; Maruyama, H.; Tamauchi, H.; Mikkelsen, J.D.; Meyer, A.S. Fucose-containing sulfated polysaccharides from brown seaweeds inhibit proliferation of melanoma cells and induce apoptosis by activation of caspase-3 in vitro. Mar. Drugs 2011, 9, 2605–2621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kyung, J.; Kim, D.; Park, D.; Yang, Y.H.; Choi, E.K.; Lee, S.P.; Kim, Y.B. Synergistic anti-inflammatory effects of Laminaria japonica fucoidan and Cistanche tubulosa extract. Lab. Anim. Res. 2012, 28, 91–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senthilkumar, K.; Manivasagan, P.; Venkatesan, J.; Kim, S.K. Brown seaweed fucoidan: Biological activity and apoptosis, growth signaling mechanism in cancer. Int. J. Biol. Macromol. 2013, 60, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Abudabbus, A.; Badmus, J.A.; Shalaweh, S.; Bauer, R.; Hiss, D. Effects of fucoidan and chemotherapeutic agent combinations on malignant and non-malignant breast cell lines. Curr. Pharm. Biotechnol. 2017, 18, 748–757. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, C.; Tang, S.; Sun, Y.; Zhao, D.; Zhang, S.; Xiao, X. TNFR1/TNF-α and mitochondria interrelated signaling pathway mediates quinocetone-induced apoptosis in HepG2 cells. Food Chem. Toxicol. 2013, 62, 825–838. [Google Scholar] [CrossRef]
- Arumugam, P.; Arunkumar, K.; Sivakumar, L.; Murugan, M.; Murugan, K. Anticancer effect of fucoidan on cell proliferation, cell cycle progression, genetic damage and apoptotic cell death in HepG2 cancer cells. Toxicol. Rep. 2019, 6, 556–563. [Google Scholar]
- Vaikundamoorthy, R.; Krishnamoorthy, V.; Vilwanathan, R.; Rajendran, R. Structural characterization and anticancer activity (MCF7 and MDA-MB-231) of polysaccharides fractionated from brown seaweed Sargassum wightii. Int. J. Biol. Macromol. 2018, 111, 1229–1237. [Google Scholar] [CrossRef]
- Schmidt, C.E.; Leach, J.B. Neural tissue engineering: Strategies for repair and regeneration. Annu. Rev. Biomed. Eng. 2003, 5, 293–347. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.; Bellamkonda, R.V. Tissue-engineered scaffolds are effective alternatives to autografts for bridging peripheral nerve gaps. Tissue Eng. 2003, 9, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Ahmad, S.R. Polysaccharides and their derivatives for versatile tissue engineering application. Macromol. Biosci. 2013, 13, 395–421. [Google Scholar] [CrossRef] [PubMed]
- Lalzawmliana, V.; Anand, A.; Mukherjee, P.; Chaudhuri, S.; Kundu, B.; Nandi, S.K.; Thakur, N.L. Marine organisms as a source of natural matrix for bone tissue engineering. Ceram. Int. 2019, 45, 1469–1481. [Google Scholar] [CrossRef]
- Cho, Y.S.; Jung, W.K.; Kim, J.A.; Choi, I.W.; Kim, S.K. Beneficial effects of fucoidan on osteoblastic MG-63 cell differentiation. Food Chem. 2009, 116, 990–994. [Google Scholar] [CrossRef]
- Park, S.B.; Chun, K.R.; Kim, J.K.; Suk, K.; Jung, Y.M.; Lee, W.H. The differential effect of high and low molecular weight fucoidans on the severity of collagen-induced arthritis in mice. Phytother. Res. 2010, 24, 1384–1391. [Google Scholar] [CrossRef]
- Irhimeh, M.R.; Fitton, J.H.; Lowenthal, R.M. Fucoidan ingestion increases the expression of CXCR4 on human CD34+ cells. Exp. Hematol. 2007, 35, 989–994. [Google Scholar] [CrossRef]
- Changotade, S.I.T.; Korb, G.; Bassil, J.; Barroukh, B.; Willig, C.; Colliec-Jouault, S.; Senni, K. Potential effects of a low-molecular-weight fucoidan extracted from brown algae on bone biomaterial osteoconductive properties. J. Biomed. Mater. Res. Part A 2008, 87, 666–675. [Google Scholar] [CrossRef] [Green Version]
- Jin, G.; Kim, G.H. Rapid-prototyped PCL/fucoidan composite scaffolds for bone tissue regeneration: Design, fabrication, and physical/biological properties. J. Mater. Chem. 2011, 21, 17710–17718. [Google Scholar] [CrossRef]
- Lee, J.S.; Jin, G.H.; Yeo, M.G.; Jang, C.H.; Lee, H.; Kim, G.H. Fabrication of electrospun biocomposites comprising polycaprolactone/fucoidan for tissue regeneration. Carbohydr. Polym. 2012, 90, 181–188. [Google Scholar] [CrossRef]
- Barralet, J.E.; Wang, L.; Lawson, M.; Triffitt, J.T.; Cooper, P.R.; Shelton, R.M. Comparison of bone marrow cell growth on 2D and 3D alginate hydrogels. J. Mater. Sci. Mater. Med. 2005, 16, 515–519. [Google Scholar] [CrossRef] [Green Version]
- Turco, G.; Marsich, E.; Bellomo, F.; Semeraro, S.; Donati, I.; Brun, F.; Paoletti, S. Alginate/hydroxyapatite biocomposite for bone ingrowth: A trabecular structure with high and isotropic connectivity. Biomacromolecules 2009, 10, 1575–1583. [Google Scholar] [CrossRef] [PubMed]
- Park, D.J.; Choi, B.H.; Zhu, S.J.; Huh, J.Y.; Kim, B.Y.; Lee, S.H. Injectable bone using chitosan-alginate gel/mesenchymal stem cells/BMP-2 composites. J. Cranio Maxillofac. Surg. 2005, 33, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ramay, H.R.; Hauch, K.D.; Xiao, D.; Zhang, M. Chitosan–alginate hybrid scaffolds for bone tissue engineering. Biomaterials 2005, 26, 3919–3928. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.S.; Park, J.H.; Shin, U.S.; Kim, H.W. Direct deposited porous scaffolds of calcium phosphate cement with alginate for drug delivery and bone tissue engineering. Acta Biomater. 2011, 7, 3178–3186. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.C.; Chang, J.J.; Lee, Y.H.; Lin, Y.C.; Wu, M.H.; Yang, M.C.; Chien, C.T. Electrospun scaffolds composing of alginate, chitosan, collagen and hydroxyapatite for applying in bone tissue engineering. Mater. Lett. 2013, 93, 133–136. [Google Scholar] [CrossRef]
- Nakaoka, R.; Hirano, Y.; Mooney, D.J.; Tsuchiya, T.; Matsuoka, A. Study on the potential of RGD-and PHSRN-modified alginates as artificial extracellular matrices for engineering bone. J. Artif. Organs 2013, 16, 284–293. [Google Scholar] [CrossRef]
- Venkatesan, J.; Bhatnagar, I.; Kim, S.K. Chitosan-alginate biocomposite containing fucoidan for bone tissue engineering. Mar. Drugs 2014, 12, 300–316. [Google Scholar] [CrossRef]
- Singh, D.; Zo, S.M.; Singh, D.; Han, S.S. Interpenetrating alginate on gelatin–poly (2-hydroxyethyl methacrylate) as a functional polymeric matrix for cartilage tissue engineering. Int. J. Polym. Mater. Polym. Biomater. 2019, 68, 551–563. [Google Scholar] [CrossRef]
- Ayoub, A.; Pereira, J.M.; Rioux, L.E.; Turgeon, S.L.; Beaulieu, M.; Moulin, V.J. Role of seaweed laminaran from Saccharina longicruris on matrix deposition during dermal tissue-engineered production. Int. J. Biol. Macromol. 2015, 75, 13–20. [Google Scholar] [CrossRef]
- Bhadja, P.; Tan, C.Y.; Ouyang, J.M.; Yu, K. Repair effect of seaweed polysaccharides with different contents of sulfate group and molecular weights on damaged HK-2 cells. Polymers 2016, 8, 188. [Google Scholar] [CrossRef]
- Jung, S.M.; Kim, S.H.; Min, S.K.; Shin, H.S. Controlled activity of mouse astrocytes on electrospun PCL nanofiber containing polysaccharides from brown seaweed. Vitro Cell. Dev. Biol. Anim. 2012, 48, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Popa, E.G.; Caridade, S.G.; Mano, J.F.; Reis, R.L.; Gomes, M.E. Chondrogenic potential of injectable κ-carrageenan hydrogel with encapsulated adipose stem cells for cartilage tissue-engineering applications. J. Tissue Eng. Regen. Med. 2015, 9, 550–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farage, M.A.; Miller, K.W.; Elsner, P.; Maibach, H.I. Intrinsic and extrinsic factors in skin ageing: A review. Int. J. Cosmet. Sci. 2008, 30, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, Y.; Ananthaswamy, H.N. Toxic effects of ultraviolet radiation on the skin. Toxicol. Appl. Pharmacol. 2004, 195, 298–308. [Google Scholar] [CrossRef]
- Moon, H.J.; Lee, S.H.; Ku, M.J.; Yu, B.C.; Jeon, M.J.; Jeong, S.H.; Lee, Y.H. Fucoidan inhibits UVB-induced MMP-1 promoter expression and down regulation of type I procollagen synthesis in human skin fibroblasts. Eur. J. Dermatol. 2009, 19, 129–134. [Google Scholar] [CrossRef] [Green Version]
- Polefka, T.G.; Meyer, T.A.; Agin, P.P.; Bianchini, R.J. Effects of solar radiation on the skin. J. Cosmet. Dermatol. 2012, 11, 134–143. [Google Scholar] [CrossRef]
- Fuentealba, D.; Galvez, M.; Alarcon, E.; Lissi, E.; Silva, E. Photosensitizing activity of advanced glycation endproducts on tryptophan, glucose 6-phosphate dehydrogenase, human serum albumin and ascorbic acid evaluated at low oxygen pressure. Photochem. Photobiol. 2007, 83, 563–569. [Google Scholar] [CrossRef]
- Priyan Shanura Fernando, I.; Kim, K.N.; Kim, D.; Jeon, Y.J. Algal polysaccharides: Potential bioactive substances for cosmeceutical applications. Crit. Rev. Biotechnol. 2019, 39, 99–113. [Google Scholar] [CrossRef]
- Shah, H.; Rawal Mahajan, S. Photoaging: New insights into its stimulators, complications, biochemical changes and therapeutic interventions. Biomed. Aging Pathol. 2013, 3, 161–169. [Google Scholar] [CrossRef]
- Kim, J.A.; Ahn, B.N.; Kong, C.S.; Kim, S.K. The chromene sargachromanol E inhibits ultraviolet A-induced ageing of skin in human dermal fibroblasts. Br. J. Dermatol. 2013, 168, 968–976. [Google Scholar] [CrossRef]
- Shao, P.; Chen, X.; Sun, P. Improvement of antioxidant and moisture-preserving activities of Sargassum horneri polysaccharide enzymatic hydrolyzates. Int. J. Biol. Macromol. 2015, 74, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Y.; Yu, C.H.; Lin, Y.T.; Su, H.L.; Kan, K.W.; Liu, F.C.; Lin, Y.H. The potential application of spring Sargassum glaucescens extracts in the moisture-retention of keratinocytes and dermal fibroblast regeneration after UVA-irradiation. Cosmetics 2019, 6, 17. [Google Scholar] [CrossRef] [Green Version]
- Ye, Y.; Ji, D.; You, L.; Zhou, L.; Zhao, Z.; Brennan, C. Structural properties and protective effect of Sargassum fusiforme polysaccharides against ultraviolet B radiation in hairless Kun Ming mice. J. Funct. Foods 2018, 43, 8–16. [Google Scholar] [CrossRef]
- Moon, H.J.; Park, K.S.; Ku, M.J.; Lee, M.S.; Jeong, S.H.; Imbs, T.I.; Lee, Y.H. Effect of Costaria costata fucoidan on expression of matrix metalloproteinase-1 promoter, mRNA, and protein. J. Nat. Prod. 2009, 72, 1731–1734. [Google Scholar] [CrossRef]
- Fitton, J.; Dell’Acqua, G.; Gardiner, V.A.; Karpiniec, S.; Stringer, D.; Davis, E. Topical benefits of two fucoidan-rich extracts from marine macroalgae. Cosmetics 2015, 2, 66–81. [Google Scholar] [CrossRef] [Green Version]








| Search Terms | Total Articles | Number of Articles Published in the Last Five Years Filtered with Best Match Term on | |||||
|---|---|---|---|---|---|---|---|
| 2019 | 2018 | 2017 | 2016 | 2015 | All Past Years | ||
| Marine seaweed polysaccharides | 384 | 40 | 63 | 42 | 48 | 25 | 166 |
| Drug delivery using polysaccharides | 23,946 | 928 | 2030 | 1910 | 1880 | 1810 | 15,388 |
| Wound healing using polysaccharides | 6855 | 209 | 482 | 444 | 391 | 356 | 4973 |
| Anticancer activities of polysaccharides | 2280 | 170 | 272 | 256 | 232 | 233 | 1117 |
| Polysaccharides based engineered carriers | 1114 | 94 | 173 | 166 | 120 | 105 | 456 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bilal, M.; Iqbal, H.M.N. Marine Seaweed Polysaccharides-Based Engineered Cues for the Modern Biomedical Sector. Mar. Drugs 2020, 18, 7. https://doi.org/10.3390/md18010007
Bilal M, Iqbal HMN. Marine Seaweed Polysaccharides-Based Engineered Cues for the Modern Biomedical Sector. Marine Drugs. 2020; 18(1):7. https://doi.org/10.3390/md18010007
Chicago/Turabian StyleBilal, Muhammad, and Hafiz M. N. Iqbal. 2020. "Marine Seaweed Polysaccharides-Based Engineered Cues for the Modern Biomedical Sector" Marine Drugs 18, no. 1: 7. https://doi.org/10.3390/md18010007
APA StyleBilal, M., & Iqbal, H. M. N. (2020). Marine Seaweed Polysaccharides-Based Engineered Cues for the Modern Biomedical Sector. Marine Drugs, 18(1), 7. https://doi.org/10.3390/md18010007