Chemically Diverse and Biologically Active Secondary Metabolites from Marine Phylum chlorophyta
Abstract
:1. Introduction
2. Methods
3. Green Algae, a Source of Bioactive Secondary Metabolites
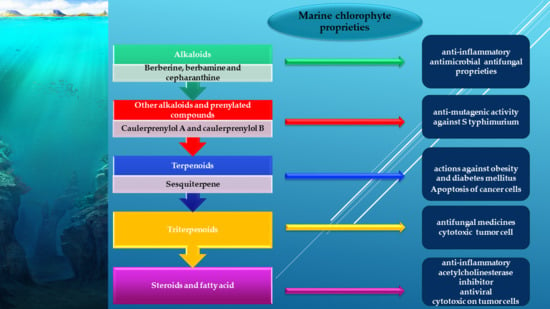
3.1. Alkaloids from Green Algae
3.1.1. Bisindole Alkaloids
Mode of Action
3.1.2. Other Alkaloids and Prenylated Compounds
3.2. Terpenes from Green Algae
3.2.1. Sesquiterpenes
3.2.2. Mode of Action of PTPs Inhibitor
3.3. Diterpenoids
3.4. Triterpenoids
3.5. Steroids and Fatty Acid
- -
- Hexadeca-4,7,10,13-tetraenoic acid (HDTA) (57);
- -
- Octadeca-6,9,12,15-tetraenoic acid (ODTA) (58);
- -
- α-linolenic acid (59), with strong algicidal activity against Heterosigma akashiwo (LC50 1.35, 0.83 and 1.13 µg/mL for HDTA, ODTA and α-linolenic acid, respectively). It has to be mentioned that α-linolenic acid was isolated before and β-sitosterol (60) was isolated from Caulerpa racemosa [59].
3.6. Glycerol and Lipids
3.7. Polysaccharides (Ulvans) from Green Algae
4. Applications of Green Algae
4.1. Chlorophyte as a Spring of Pharmaceuticals and Nutraceuticals
4.1.1. Antioxidants
4.1.2. Cosmetics
4.1.3. Antibacterial
4.1.4. Anti-SARS-CoV-2 (COVID-19)
4.1.5. Anticancer
5. Algae as Nutraceuticals
5.1. Astaxanthin
5.2. Omega 3 Polyunsaturated Fatty Acids
6. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Shams, S.; Ishaq, M.; Zhang, W.; Jin, H. An Overview of the Mechanisms of Marine Fungi-Derived Anti-Inflammatory and Anti-Tumor Agents and their Novel Role in Drug Targeting. Curr. Pharm. Des. 2020, 26. [Google Scholar] [CrossRef]
- Xie, Y.G.; Zhao, X.C.; ul Hassan, S.S.; Zhen, X.Y.; Muhammad, I.; Yan, S. kai; Yuan, X.; Li, H. liang; Jin, H. zi One new sesquiterpene and one new iridoid derivative from Valeriana amurensis. Phytochem. Lett. 2019, 32, 6–9. [Google Scholar] [CrossRef]
- ul Hassan, S.S.; Jin, H.Z.; Abu-Izneid, T.; Rauf, A.; Ishaq, M.; Suleria, H.A.R. Stress-driven discovery in the natural products: A gateway towards new drugs. Biomed. Pharmacother. 2019, 109, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.S.; Shah, S.A.A.; Pan, C.; Fu, L.; Cao, X.; Shi, Y.; Wu, X.; Wang, K.; Wu, B. Production of an antibiotic enterocin from a marine actinobacteria strain H1003 by metal-stress technique with enhanced enrichment using response surface methodology. Pak. J. Pharm. Sci. 2017, 30, 313–324. [Google Scholar] [PubMed]
- Anjum, K.; Abbas, S.Q.; Akhter, N.; Shagufta, B.I.; Shah, S.A.A.; Hassan, S.S. ul Emerging Biopharmaceuticals from Bioactive Peptides derived from Marine Organisms. Chem. Biol. Drug Des. 2016. [Google Scholar] [CrossRef]
- Hassan, S.S. ul; Shaikh, A.L. Marine actinobacteria as a drug treasure house. Biomed. Pharmacother. 2017, 87, 46–57. [Google Scholar] [CrossRef]
- Lever, J.; Brkljaca, R.; Kraft, G.; Urban, S. Natural products of marine macroalgae from South Eastern Australia, with emphasis on the Port Phillip bay and heads regions of Victoria. Mar. Drugs 2020, 18, 142. [Google Scholar] [CrossRef] [Green Version]
- Jesus, A.; Correia-da-Silva, M.; Afonso, C.; Pinto, M.; Cidade, H. Isolation and potential biological applications of haloaryl secondary metabolites from macroalgae. Mar. Drugs 2019, 17, 73. [Google Scholar] [CrossRef] [Green Version]
- Guiry, M.D. How many species of algae are there? J. Phycol. 2012, 1057–1063. [Google Scholar] [CrossRef]
- Ji, X.; Li, H.; Zhang, J.; Saiyin, H.; Zheng, Z. The collaborative effect of Chlorella vulgaris-Bacillus licheniformis consortia on the treatment of municipal water. J. Hazard. Mater. 2019, 365, 483–493. [Google Scholar] [CrossRef]
- Trivedi, J.; Aila, M.; Bangwal, D.P.; Kaul, S.; Garg, M.O. Algae based biorefinery—How to make sense? Renew. Sustain. Energy Rev. 2015, 295–307. [Google Scholar] [CrossRef]
- Dahms, H.U.; Dobretsov, S. Antifouling compounds from marine macroalgae. Mar. Drugs 2017, 15, 265. [Google Scholar] [CrossRef] [PubMed]
- Levasseur, W.; Perré, P.; Pozzobon, V. A review of high value-added molecules production by microalgae in light of the classification. Biotechnol. Adv. 2020, 41, 107545. [Google Scholar] [CrossRef] [PubMed]
- Mehra, R.; Bhushan, S.; Bast, F.; Singh, S. Marine macroalga Caulerpa: Role of its metabolites in modulating cancer signaling. Mol. Biol. Rep. 2019, 46, 3545–3555. [Google Scholar] [CrossRef]
- Leal, M.C.; Munro, M.H.G.; Blunt, J.W.; Puga, J.; Jesus, B.; Calado, R.; Rosa, R.; Madeira, C. Biogeography and biodiscovery hotspots of macroalgal marine natural products. Nat. Prod. Rep. 2013, 30, 1380–1390. [Google Scholar] [CrossRef]
- Blunt, J.W.; Copp, B.R.; Munro, M.H.G.; Northcote, P.T.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2006, 23, 26–78. [Google Scholar] [CrossRef]
- Handley, J.T.; Blackman, A.J. Secondary metabolites from the marine alga caulerpa brownii (chlorophyta). Aust. J. Chem. 2005, 58, 39–46. [Google Scholar] [CrossRef]
- Awad, N.E. Biologically active steroid from the green alga Ulva lactuca. Phytother. Res. 2000, 14, 641–643. [Google Scholar] [CrossRef]
- Lunagariya, J.; Bhadja, P.; Zhong, S.; Vekariya, R.; Xu, S. Marine Natural Product Bis-indole Alkaloid Caulerpin: Chemistry and Biology. Mini-Rev. Med. Chem. 2017, 19, 751–761. [Google Scholar] [CrossRef]
- Vairappan, C.S. Antibacterial activity of major secondary metabolites found in four species of edible green macroalgae genus Caulerpa. Asian J. Microbiol. Biotechnol. Environ. Sci. 2004, 6, 197–201. [Google Scholar]
- Mao, S.C.; Guo, Y.W.; Shen, X. Two novel aromatic valerenane-type sesquiterpenes from the Chinese green alga Caulerpa taxifolia. Bioorg. Med. Chem. Lett. 2006, 16, 2947–2950. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Morgan, J.B.; Coothankandaswamy, V.; Liu, R.; Jekabsons, M.B.; Mahdi, F.; Nagle, D.G.; Zhou, Y.D. The Caulerpa pigment caulerpin inhibits HIF-1 activation and mitochondrial respiration. J. Nat. Prod. 2009, 72, 2104–2109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macedo, N.R.P.V.; Ribeiro, M.S.; Villaça, R.C.; Ferreira, W.; Pinto, A.M.; Teixeira, V.L.; Cirne-Santos, C.; Paixão, I.C.N.P.; Giongo, V. Caulerpin as a potential antiviral drug against herpes simplex virus type 1. Braz. J. Pharmacogn. 2012, 22, 861–867. [Google Scholar] [CrossRef]
- De Souza, É.T.; De Lira, D.P.; De Queiroz, A.C.; Da Silva, D.J.C.; De Aquino, A.B.; Campessato Mella, E.A.; Lorenzo, V.P.; De Miranda, G.E.C.; De Araújo-Júnior, J.X.; De Oliveira Chaves, M.C.; et al. The antinociceptive and anti-inflammatory activities of caulerpin, a bisindole alkaloid isolated from seaweeds of the genus Caulerpa. Mar. Drugs 2009, 7, 689–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pelletier, S.W. (Ed.) The nature and definition of an alkaloid. In Alkaloids: Chemical and Biological Perspectives; Springer: New York, NY, USA, 1983; pp. 1–31. [Google Scholar]
- Liu, A.H.; Liu, D.Q.; Liang, T.J.; Yu, X.Q.; Feng, M.T.; Yao, L.G.; Fang, Y.; Wang, B.; Feng, L.H.; Zhang, M.X.; et al. Caulerprenylols A and B, two rare antifungal prenylated para-xylenes from the green alga Caulerpa racemosa. Bioorganic Med. Chem. Lett. 2013, 23, 2491–2494. [Google Scholar] [CrossRef]
- Yang, H.; Liu, D.Q.; Liang, T.J.; Li, J.; Liu, A.H.; Yang, P.; Lin, K.; Yu, X.Q.; Guo, Y.W.; Mao, S.C.; et al. Racemosin C, a novel minor bisindole alkaloid with protein tyrosine phosphatase-1B inhibitory activity from the green alga Caulerpa racemosa. J. Asian Nat. Prod. Res. 2014, 16, 1158–1165. [Google Scholar] [CrossRef]
- Ornano, L.; Donno, Y.; Sanna, C.; Ballero, M.; Serafini, M.; Bianco, A. Phytochemical study of Caulerpa racemosa (Forsk.) J. Agarth, an invading alga in the habitat of la Maddalena archipelago. Nat. Prod. Res. 2014, 28, 1795–1799. [Google Scholar] [CrossRef]
- Cavalcante-Silva, L.H.A.; De Carvalho Correia, A.C.; Barbosa-Filho, J.M.; Da Silva, B.A.; De Oliveira Santos, B.V.; De Lira, D.P.; Sousa, J.C.F.; De Miranda, G.E.C.; Cavalcante, F.A.; Alexandre-Moreira, M.S. Spasmolytic effect of caulerpine involves blockade of Ca2+ influx on guinea pig ileum. Mar. Drugs 2013, 11, 1553–1564. [Google Scholar] [CrossRef] [Green Version]
- Güven, K.C.; Percot, A.; Sezik, E. Alkaloids in marine algae. Mar. Drugs 2010, 8, 269–284. [Google Scholar]
- Liu, D.Q.; Mao, S.C.; Yu, X.Q.; Feng, L.H.; Lai, X.P. Caulerchlorin, a novel chlorinated bisindole alkaloid with antifungal activity from the Chinese green alga Caulerpa racemosa. Heterocycles 2012, 85, 661–666. [Google Scholar] [CrossRef]
- Souto, A.L.; Tavares, J.F.; Da Silva, M.S.; De Diniz, M.F.F.M.; De Athayde-Filho, P.F.; Barbosa Filho, J.M. Anti-inflammatory activity of alkaloids: An update from 2000 to 2010. Molecules 2011, 16, 8515–8534. [Google Scholar] [CrossRef] [PubMed]
- Anjum, K.; Abbas, S.Q.; Shah, S.A.A.; Akhter, N.; Batool, S.; Hassan, S.S.U. Marine Sponges as a Drug Treasure. Biomol. Ther. 2016, 24, 347–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anjum, K.; Shagufta, B.I.; Abbas, S.Q.; Patel, S.; Khan, I.; Shah, S.A.A.; Akhter, N.; Hassan, S.S. ul Current status and future therapeutic perspectives of glioblastoma multiforme (GBM) therapy: A review. Biomed. Pharmacother. 2017. [Google Scholar] [CrossRef]
- Barbosa-Filho, J.M.; Piuvezam, M.R.; Moura, M.D.; Silva, M.S.; Lima, K.V.B.; Da-Cunha, E.V.L.; Fechine, I.M.; Takemura, O.S. Anti-inflammatory activity of alkaloids: A twenty-century review. Rev. Bras. Farmacogn. 2006, 16, 109–139. [Google Scholar] [CrossRef] [Green Version]
- Qing, Z.-X.; Huang, J.-L.; Yang, X.-Y.; Liu, J.-H.; Cao, H.-L.; Xiang, F.; Cheng, P.; Zeng, J.-G. Anticancer and Reversing Multidrug Resistance Activities of Natural Isoquinoline Alkaloids and their Structure-activity Relationship. Curr. Med. Chem. 2017, 25, 5088–5114. [Google Scholar] [CrossRef]
- González-Barnadas, A.; Camps-Font, O.; Martín-Fatás, P.; Figueiredo, R.; Gay-Escoda, C.; Valmaseda-Castellón, E. Efficacy and safety of selective COX-2 inhibitors for pain management after third molar removal: A meta-analysis of randomized clinical trials. Clin. Oral Investig. 2020, 24, 79–96. [Google Scholar] [CrossRef]
- Sales, T.A.; Marcussi, S.; Ramalho, T.C. Current Anti-Inflammatory Therapies and the Potential of Secretory Phospholipase A2 Inhibitors in the Design of New Anti-Inflammatory Drugs: A Review of 2012–2018. Curr. Med. Chem. 2019, 27, 477–497. [Google Scholar] [CrossRef]
- Netz, N.; Opatz, T. Marine indole alkaloids. Mar. Drugs 2015, 13, 4814–4914. [Google Scholar] [CrossRef] [Green Version]
- Jiang, H.P.; Gao, B.B.; Li, W.H.; Zhu, M.; Zheng, C.F.; Zheng, Q.S.; Wang, C.H. Physiological and biochemical responses of Ulva prolifera and Ulva linza to cadmium stress. Sci. World J. 2013, 289537. [Google Scholar] [CrossRef] [Green Version]
- Dorta, E.; Darias, J.; San Martín, A.; Cueto, M. New prenylated bromoquinols from the green alga Cymopolia barbata. J. Nat. Prod. 2002, 65, 329–333. [Google Scholar] [CrossRef]
- Gallimore, W.A.; Sambo, T.; Campbell, T. Debromocymopolone from the green alga, Cymopolia barbata. J. Chem. Res. 2009, 3, 160–161. [Google Scholar] [CrossRef]
- Balboa, E.M.; Conde, E.; Moure, A.; Falqué, E.; Domínguez, H. In vitro antioxidant properties of crude extracts and compounds from brown algae. Food Chem. 2013, 138, 1764–1785. [Google Scholar] [CrossRef] [PubMed]
- Tonks, N.K. Protein tyrosine phosphatases: From genes, to function, to disease. Nat. Rev. Mol. Cell Biol. 2006, 7, 833–846. [Google Scholar] [CrossRef] [PubMed]
- Traurig, M.; Hanson, R.L.; Kobes, S.; Bogardus, C.; Baier, L.J. Protein tyrosine phosphatase 1B is not a major susceptibility gene for type 2 diabetes mellitus or obesity among Pima Indians. Diabetologia 2007, 50, 985–989. [Google Scholar] [CrossRef]
- Stuible, M.; Doody, K.M.; Tremblay, M.L. PTP1B and TC-PTP: Regulators of transformation and tumorigenesis. Cancer Metastasis Rev. 2008, 27, 215–230. [Google Scholar] [CrossRef]
- Dubé, N.; Tremblay, M.L. Involvement of the small protein tyrosine phosphatases TC-PTP and PTP1B in signal transduction and diseases: From diabetes, obesity to cell cycle, and cancer. Biochim. Biophys. Acta Proteins Proteom. 2005, 1754, 108–117. [Google Scholar] [CrossRef]
- Tonks, N.K.; Muthuswamy, S.K. A Brake Becomes an Accelerator: PTP1B-A New Therapeutic Target for Breast Cancer. Cancer Cell 2007, 11, 214–216. [Google Scholar] [CrossRef] [Green Version]
- Shams, S.; Zhang, W.; Jin, H.; Basha, S.H.; Priya, S.V.S.S. In-silico anti-inflammatory potential of guaiane dimers from Xylopia vielana targeting COX-2. J. Biomol. Struct. Dyn. 2020. [Google Scholar] [CrossRef]
- Bollu, L.R.; Mazumdar, A.; Savage, M.I.; Brown, P.H. Molecular pathways: Targeting protein tyrosine phosphatases in cancer. Clin. Cancer Res. 2017, 23, 2136–2142. [Google Scholar] [CrossRef] [Green Version]
- Huang, T.T.; Su, J.C.; Liu, C.Y.; Shiau, C.W.; Chen, K.F. Alteration of SHP-1/p-STAT3 signaling: A potential target for anticancer therapy. Int. J. Mol. Sci. 2017, 18, 1234. [Google Scholar] [CrossRef] [Green Version]
- Smyrniotopoulos, V.; Abatis, D.; Tziveleka, L.A.; Tsitsimpikou, C.; Roussis, V.; Loukis, A.; Vagias, C. Acetylene sesquiterpenoid esters from the green alga Caulerpa prolifera. J. Nat. Prod. 2003, 66, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, K.; Lipton, A.P.; Paulraj, R.; Chakraborty, R.D. Guaiane sesquiterpenes from seaweed Ulva fasciata Delile and their antibacterial properties. Eur. J. Med. Chem. 2010, 45, 2237–2244. [Google Scholar] [CrossRef] [PubMed]
- Cengiz, S.; Cavas, L.; Yurdakoc, K.; Pohnert, G. The Sesquiterpene Caulerpenyne from Caulerpa spp. is a Lipoxygenase Inhibitor. Mar. Biotechnol. 2011, 13, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Máximo, P.; Ferreira, L.M.; Branco, P.; Lima, P.; Lourenço, A. Secondary metabolites and biological activity of invasive macroalgae of southern Europe. Mar. Drugs 2018, 16, 265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, P.; Liu, D.Q.; Liang, T.J.; Li, J.; Zhang, H.Y.; Liu, A.H.; Guo, Y.W.; Mao, S.C. Bioactive constituents from the green alga Caulerpa racemosa. Bioorganic Med. Chem. 2015, 23, 38–45. [Google Scholar] [CrossRef]
- Jiang, R.W.; Hay, M.E.; Fairchild, C.R.; Prudhomme, J.; Roch, K. Le; Aalbersberg, W.; Kubanek, J. Antineoplastic unsaturated fatty acids from Fijian macroalgae. Phytochemistry 2008, 69, 2495–2500. [Google Scholar] [CrossRef] [Green Version]
- Puglisi, M.P.; Tan, L.T.; Jensen, P.R.; Fenical, W. Capisterones A and B from the tropical green alga Penicillus capitatus: Unexpected anti-fungal defenses targeting the marine pathogen Lindra thallasiae. Tetrahedron 2004, 60, 7035–7039. [Google Scholar] [CrossRef]
- Ragasa, C.Y.; Ebajo, V.D.; Lazaro-Llanos, N.; Brkljaca, R.; Urban, S. Secondary metabolites from Caulerpa racemosa. Der Pharm. Lett. 2015, 7, 122–125. [Google Scholar]
- Ali, L.; Khan, A.L.; Al-Kharusi, L.; Hussain, J.; Al-Harrasi, A. New α-glucosidase inhibitory triterpenic acid from marine macro green alga codium dwarkense Boergs. Mar. Drugs 2015, 13, 4344–4356. [Google Scholar] [CrossRef]
- Sun, Y. ying; Wang, H.; Guo, G. lin; Pu, Y. fang; Yan, B. lun; Wang, C. hai Isolation, purification, and identification of antialgal substances in green alga Ulva prolifera for antialgal activity against the common harmful red tide microalgae. Environ. Sci. Pollut. Res. 2016, 23, 1449–1459. [Google Scholar] [CrossRef]
- Salehi, B.; Sharifi-rad, J.; Seca, A.M.L.; Pinto, D.C.G.A. Current Trends on Seaweeds: Looking at Chemical. Molecules 2019, 24, 4182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao, S.C.; Liu, D.Q.; Yu, X.Q.; Lai, X.P. A new polyacetylenic fatty acid and other secondary metabolites from the Chinese green alga Caulerpa racemosa (Caulerpaceae) and their chemotaxonomic significance. Biochem. Syst. Ecol. 2011, 39, 253–257. [Google Scholar] [CrossRef]
- Alamsjah, M.A.; Hirao, S.; Ishibashi, F.; Fujita, Y. Isolation and structure determination of algicidal compounds from Ulva fasciata. Biosci. Biotechnol. Biochem. 2005, 69, 2186–2192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, M.S.; Saleem, M.; Yamdagni, R.; Ali, M.A. Steroid and antibacterial steroidal glycosides from marine green alga Codium iyengarii borgesen. Nat. Prod. Lett. 2002, 16, 407–413. [Google Scholar] [CrossRef]
- Li, G.L.; Guo, W.J.; Wang, G.B.; Wang, R.R.; Hou, Y.X.; Liu, K.; Liu, Y.; Wang, W. Sterols from the green alga ulva Australis. Mar. Drugs 2017, 15, 299. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Paul, V.J.; Luesch, H. Seaweed extracts and unsaturated fatty acid constituents from the green alga Ulva lactuca as activators of the cytoprotective Nrf2-ARE pathway. Free Radic. Biol. Med. 2013, 57, 141–153. [Google Scholar] [CrossRef] [Green Version]
- Fang, Z.; Jeong, S.Y.; Jung, H.A.; Choi, J.S.; Min, B.S.; Woo, M.H. Capsofulvesins A-C, cholinesterase inhibitors from Capsosiphon fulvescens. Chem. Pharm. Bull. 2012, 60, 1351–1358. [Google Scholar] [CrossRef] [Green Version]
- Williams, D.E.; Sturgeon, C.M.; Roberge, M.; Andersen, R.J. Nigricanosides A and B, antimitotic glycolipids isolated from the green alga Avrainvillea nigricans collected in Dominica. J. Am. Chem. Soc. 2007, 129, 5822–5823. [Google Scholar] [CrossRef]
- Ishibashi, Y.; Nagamatsu, Y.; Miyamoto, T.; Matsunaga, N.; Okino, N.; Yamaguchi, K.; Ito, M. A novel ether-linked phytol-containing digalactosylglycerolipid in the marine green alga, Ulva pertusa. Biochem. Biophys. Res. Commun. 2014, 452, 873–880. [Google Scholar] [CrossRef]
- Wang, H.; Li, Y.L.; Shen, W.Z.; Rui, W.; Ma, X.J.; Cen, Y.Z. Antiviral activity of a sulfoquinovosyldiacylglycerol (SQDG) compound isolated from the green alga Caulerpa racemosa. Bot. Mar. 2007, 50, 185–190. [Google Scholar] [CrossRef]
- Esteves, P.O.; de Oliveira, M.C.; de Souza Barros, C.; Cirne-Santos, C.C.; Laneuvlille, V.T.; Palmer Paixão, I.C. Antiviral Effect of Caulerpin Against Chikungunya. Nat. Prod. Commun. 2019, 14, 1–6. [Google Scholar] [CrossRef]
- Andersen, R.J.; Taglialatela-Scafati, O. Avrainvilloside, a 6-deoxy-6-aminoglucoglycerolipid from the green alga Avrainvillea nigricans. J. Nat. Prod. 2005, 68, 1428–1430. [Google Scholar] [CrossRef] [PubMed]
- Lahaye, M.; Robic, A. Structure and function properties of Ulvan, a polysaccharide from green seaweeds. Biomacromolecules 2007, 1765–1774. [Google Scholar] [CrossRef] [PubMed]
- Vera, J.; Castro, J.; Gonzalez, A.; Moenne, A. Seaweed polysaccharides and derived oligosaccharides stimulate defense responses and protection against pathogens in plants. Mar. Drugs 2011, 9, 2514–2525. [Google Scholar] [CrossRef]
- Ray, B.; Lahaye, M. Cell-wall polysaccharides from the marine green alga Ulva “rigida” (ulvales, chlorophyta). Extraction and chemical composition. Carbohydr. Res. 1995, 283, 161–173. [Google Scholar] [CrossRef]
- Pengzhan, Y.; Quanbin, Z.; Ning, L.; Zuhong, X.; Yanmei, W.; Zhi’en, L. Polysaccharides from Ulva pertusa (Chlorophyta) and preliminary studies on their antihyperlipidemia activity. J. Appl. Phycol. 2003, 15, 21–27. [Google Scholar] [CrossRef]
- Kloareg, B.; Quatrano, R.S. Structure of the cell walls of marine algae and ecophysiological functions of the matrix polysaccharides. Annu. Rev. 1988, 26, 259–315. [Google Scholar]
- He, J.; Xu, Y.; Chen, H.; Sun, P. Extraction, structural characterization, and potential antioxidant activity of the polysaccharides from four seaweeds. Int. J. Mol. Sci. 2016, 17, 1988. [Google Scholar] [CrossRef]
- El Azm, N.A.; Fleita, D.; Rifaat, D.; Mpingirika, E.Z.; Amleh, A.; El-Sayed, M.M.H. Production of bioactive compounds from the sulfated polysaccharides extracts of ulva lactuca: Post-extraction enzymatic hydrolysis followed by ion-exchange chromatographic fractionation. Molecules 2019, 24, 2132. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Xu, H.; Wang, X.; Wan, Y.; Jiang, N.; Qi, H.; Liu, X. Antioxidant and antihyperlipidemic activities of high sulfate content purified polysaccharide from Ulva pertusa. Int. J. Biol. Macromol. 2020, 146, 756–762. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Sun, Y. Antioxidant activity of high sulfate content derivative of ulvan in hyperlipidemic rats. Int. J. Biol. Macromol. 2015, 76, 326–329. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.H.; Chan, Y.L.; Li, T.L.; Wu, C.J. Inhibition of Japanese Encephalitis Virus Infection by the Sulfated Polysaccharide Extracts from Ulva lactuca. Mar. Biotechnol. 2012, 14, 468–478. [Google Scholar] [CrossRef] [PubMed]
- Sathivel, A.; Balavinayagamani; Hanumantha Rao, B.R.; Devaki, T. Sulfated polysaccharide isolated from Ulva lactuca attenuates D-galactosamine induced DNA fragmentation and necrosis during liver damage in rats. Pharm. Biol. 2014, 52, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Sathivel, A.; Raghavendran, H.R.B.; Srinivasan, P.; Devaki, T. Anti-peroxidative and anti-hyperlipidemic nature of Ulva lactuca crude polysaccharide on d-Galactosamine induced hepatitis in rats. Food Chem. Toxicol. 2008, 46, 3262–3267. [Google Scholar] [CrossRef]
- Rosa, G.P.; Tavares, W.R.; Sousa, P.M.C.; Pagès, A.K.; Seca, A.M.L.; Pinto, D.C.G.A. Seaweed secondary metabolites with beneficial health effects: An overview of successes in in vivo studies and clinical trials. Mar. Drugs 2020, 18, 8. [Google Scholar] [CrossRef] [Green Version]
- Najdenski, H.M.; Gigova, L.G.; Iliev, I.I.; Pilarski, P.S.; Lukavský, J.; Tsvetkova, I.V.; Ninova, M.S.; Kussovski, V.K. Antibacterial and antifungal activities of selected microalgae and cyanobacteria. Int. J. Food Sci. Technol. 2013, 48, 1533–1540. [Google Scholar] [CrossRef]
- Xu, N.; Fan, X.; Yan, X.; Tseng, C.K. Screening marine algae from China for their antitumor activities. J. Appl. Phycol. 2004, 16, 451–456. [Google Scholar] [CrossRef]
- Olasehinde, T.A.; Olaniran, A.O.; Okoh, A.I. Macroalgae as a valuable source of naturally occurring bioactive compounds for the treatment of Alzheimer’s disease. Mar. Drugs 2019, 17, 609. [Google Scholar] [CrossRef] [Green Version]
- Allmendinger, A.; Spavieri, J.; Kaiser, M.; Casey, R.; Hingley-Wilson, S.; Lalvani, A.; Guiry, M.; Blunden, G.; Tasdemir, D. Antiprotozoal, antimycobacterial and cytotoxic potential of twenty-three British and Irish red algae. Phytother. Res. 2010, 24, 1099–1103. [Google Scholar] [CrossRef]
- Koishi, A.C.; Zanello, P.R.; Bianco, É.M.; Bordignon, J.; Nunes Duarte dos Santos, C. Screening of Dengue Virus Antiviral Activity of Marine Seaweeds by an In Situ Enzyme-Linked Immunosorbent Assay. PLoS ONE 2012, 7, 51089. [Google Scholar] [CrossRef]
- Cox, S.; Abu-Ghannam, N.; Gupta, S. An assessment of the antioxidant and antimicrobial activity of six species of edible Irish seaweeds. Int. Food Res. J. 2010, 17, 205–220. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Nah, J.W.; Jeon, Y.J. Potential anti-inflammatory natural products from marine algae. Environ. Toxicol. Pharmacol. 2016, 48, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Elnabris, K.J.; Elmanama, A.A.; Chihadeh, W.N. Antibacterial activity of four marine seaweeds collected from the coast of Gaza Strip, Palestine. Mesopot. J. Mar. Sci 2013, 28, 81–92. [Google Scholar]
- Endres, L.; Tit, D.M.; Bungau, S.; Cioca, G.; Daim, M.A.; Buhas, C.; Pop, O.; Sava, C. Markers usefulness in the melanic metastatic cellular epitops identification in the sentinel lymph node. Rev. Chim. 2018, 69, 3675–3679. [Google Scholar] [CrossRef]
- Bungau, S.; Abdel-Daim, M.M.; Tit, D.M.; Ghanem, E.; Sato, S.; Maruyama-Inoue, M.; Yamane, S.; Kadonosono, K. Health Benefits of Polyphenols and Carotenoids in Age-Related Eye Diseases. Oxidative Med. Cell. Longev. 2019, 9783429. [Google Scholar] [CrossRef]
- Mobin, S.M.A.; Chowdhury, H.; Alam, F. Commercially important bioproducts from microalgae and their current applications-A review. Energy Procedia 2019, 160, 752–760. [Google Scholar] [CrossRef]
- Chakraborty, K.; Paulraj, R. Sesquiterpenoids with free-radical-scavenging properties from marine macroalga Ulva fasciata Delile. Food Chem. 2010, 122, 31–41. [Google Scholar] [CrossRef]
- Meenakshi, S.; Gnanambigai, D.M.; Mozhi, S.T.; Arumugam, M.; Balasubramanian, T. Total flavanoid and in vitro antioxidant activity of two seaweeds of Rameshwaram Coast. Glob. J. Pharm. 2009, 3, 59–62. [Google Scholar]
- Roy, S. Screening and Partial Characterization of Natural Antioxidants from Seaweeds Collected From, Rameshwaram Southeast Coast of India. J. Mar. Sci. Res. Oceanogr. 2020, 3, 1–12. [Google Scholar] [CrossRef]
- Balaji Raghavendra Rao, H.; Sathivel, A.; Devaki, T. Antihepatotoxic nature of Ulva reticulata (Chlorophyceae) on acetaminophen-induced hepatoxicity in experimental rats. J. Med. Food 2004, 7, 495–497. [Google Scholar] [CrossRef]
- Mamani, J.; Chávez, J.; Apumayta, E.; Gil-Kodaka, P. Antioxidant activity and total phenolic content in Caulerpa filiformis (Chlorophyta) from Sechura Bay and Paracas Bay, Peru. Rev. Peru. Biol. 2020, 27, 61–66. [Google Scholar] [CrossRef] [Green Version]
- Jesumani, V.; Du, H.; Aslam, M.; Pei, P.; Huang, N. Potential use of seaweed bioactive compounds in skincare—A review. Mar. Drugs 2019, 17, 688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, L. Seaweeds as Source of Bioactive Substances and Skin Care Therapy — Cosmeceuticals, Algotherapy, and Thalassotherapy. Cosmetics 2018, 5, 68. [Google Scholar] [CrossRef] [Green Version]
- Park, N.H.; Choi, J.S.; Hwang, S.Y.; Kim, Y.C.; Hong, Y.K.; Cho, K.K.; Choi, I.S. Antimicrobial activities of stearidonic and gamma-linolenic acids from the green seaweed Enteromorpha linza against several oral pathogenic bacteria. Bot. Stud. 2013, 54, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ismail, A.; Ktari, L.; Ben Redjem Romdhane, Y.; Aoun, B.; Sadok, S.; Boudabous, A.; El Bour, M. Antimicrobial Fatty Acids from Green Alga Ulva rigida (Chlorophyta). BioMed Res. Int. 2018, 2018, 3069595. [Google Scholar] [CrossRef] [Green Version]
- Pereira, L.; Critchley, A.T. The COVID 19 novel coronavirus pandemic 2020: Seaweeds to the rescue? Why does substantial, supporting research about the antiviral properties of seaweed polysaccharides seem to go unrecognized by the pharmaceutical community in these desperate times? J. Appl. Phycol. 2020, 32, 1875–1877. [Google Scholar] [CrossRef]
- Ahmed, S.A.; Abdelrheem, D.A.; El-Mageed, H.R.A.; Mohamed, H.S.; Rahman, A.A.; Elsayed, K.N.M.; Ahmed, S.A. Destabilizing the structural integrity of COVID-19 by caulerpin and its derivatives along with some antiviral drugs: An in silico approaches for a combination therapy. Struct. Chem. 2020. [Google Scholar] [CrossRef]
- Moo-Puc, R.; Robledo, D.; Freile-Pelegrín, Y. In vitro cytotoxic and antiproliferative activities of marine macroalgae from Yucatán, Mexico | Actividad citotóxica y antiproliferativa in vitro de macroalgas marinas de Yucatán, México. Cienc. Mar. 2009, 35, 345–358. [Google Scholar] [CrossRef] [Green Version]
- Paul, S.; Kundu, R. Antiproliferative activity of methanolic extracts from two green algae, Enteromorpha intestinalis and Rizoclonium riparium on HeLa cells. DARU J. Pharm. Sci. 2013, 21, 72. [Google Scholar] [CrossRef] [Green Version]
- Noda, H.; Amano, H.; Arashima, K.; Nisizawa, K. Antitumor activity of marine algae. Hydrobiologia 1990, 204, 577–584. [Google Scholar] [CrossRef]
- Robledo, D.; Moo-Puc, R.; Freile-Pelegrin, Y. Enhanced antitumoral activity of extracts derived from cultured Udotea flabellum (chlorophyta). Evid. -Based Complementary Altern. Med. 2011, 2011, 969275. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.C.; Hou, M.F.; Huang, H.W.; Chang, F.R.; Yeh, C.C.; Tang, J.Y.; Chang, H.W. Marine algal natural products with anti-oxidative, anti-inflammatory, and anti-cancer properties. Cancer Cell Int. 2013, 13, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alves, C.; Silva, J.; Pinteus, S.; Gaspar, H.; Alpoim, M.C.; Botana, L.M.; Pedrosa, R. From marine origin to therapeutics: The antitumor potential of marine algae-derived compounds. Front. Pharmacol. 2018, 9, 777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cinar, E.; Taskin, E.; Tasdemir, D.; Ozkale, E.; Grienke, U.; Firsova, D. Anti-Acetylcholinesterase, Antiprotozoal and Cytotoxic Activities of some turkish marine algae. Fresenius Environ. Bull. 2019, 28, 3991–4000. [Google Scholar]
- Haq, S.H.; Al-Ruwaished, G.; Al-Mutlaq, M.A.; Naji, S.A.; Al-Mogren, M.; Al-Rashed, S.; Ain, Q.T.; Al-Amro, A.A.; Al-Mussallam, A. Antioxidant, Anticancer Activity and Phytochemical Analysis of Green Algae, Chaetomorpha Collected from the Arabian Gulf. Sci. Rep. 2019, 9, 18906. [Google Scholar] [CrossRef] [Green Version]
- Acharya, D.; Satapathy, S.; Somu, P.; Parida, U.K.; Mishra, G. Apoptotic Effect and Anticancer Activity of Biosynthesized Silver Nanoparticles from Marine Algae Chaetomorpha linum Extract Against Human Colon Cancer Cell HCT-116. Biol. Trace Elem. Res. 2020. [Google Scholar] [CrossRef]
- Koyande, A.K.; Chew, K.W.; Rambabu, K.; Tao, Y.; Chu, D.T.; Show, P.L. Microalgae: A potential alternative to health supplementation for humans. Food Sci. Hum. Wellness 2019, 8, 16–24. [Google Scholar] [CrossRef]
- Aditya, T.; Gartia, B.; Mercy, E.G. The Role of Algae in Pharmaceutical Development. Spec. Issue Rev. Pharm. Nanotechnol. Res. Rev. J. Pharm. Nanotechnol. 2016, 4, 82–89. [Google Scholar]
- Crismaru, I.; Pantea Stoian, A.; Bratu, O.G.; Gaman, M.A.; Stanescu, A.M.A.; Bacalbasa, N.; Diaconu, C.C. Low-density lipoprotein cholesterol lowering treatment: The current approach. Lipids Health Dis. 2020, 19, 85. [Google Scholar] [CrossRef]
- Abdel-Daim, M.M.; El-Tawil, O.S.; Bungau, S.G.; Atanasov, A.G. Applications of Antioxidants in Metabolic Disorders and Degenerative Diseases: Mechanistic Approach. Oxidative Med. Cell. Longev. 2019, 2019, 4179676. [Google Scholar] [CrossRef] [Green Version]











| Compound | Species | Bioactivity | Ref. |
|---|---|---|---|
| Racemosin A (1) | Caulerpa racemosa | Neuroprotective | [26] |
| Racemosin B (2) | Caulerpa racemosa | Neuroprotective | [26] |
| Racemosin C (3) | Caulerpa racemosa | Significant PTP1B inhibitor | [27] |
| Caulerpin (4) Caulerpic acid (5) | Caulerpa racemosa and Caulerpa genus | Strong PTP1B inhibitor | [28] |
| Caulersin (6) | Caulerpa serrulata | PTP1B inhibitor | [30] |
| Caulerchlorin (7) | Caulerpa racemose | Weak antifungal | [31] |
| Caulerprenylols A (8) | Caulerpa racemosa | Antifungal | [26] |
| Caulerprenylols B (9) | Caulerpa racemosa | Antifungal | [26] |
| Pyrrolopipera-zine-2,5-dione (10) | Ulva prolifera | Antialgal | [40] |
| 7-Hydroxycymo-pochromanone (PBQI) (11) | Cymopolia barbata | Chemotherapeutic | [41] |
| 7-Hydroxycymo-polone (PBQ2) (12) | Cymopolia barbata | Chemotherapeutic, Anticancer colon cell | [41] |
| 3′-methoxy-7-hydroxycymopolone (13) | Cymopolia barbata | Antimutagenic against S typhimurium | [41] |
| 3-hydroxycymopolone (14) | Cymopolia barbata | Antimutagenic against S typhimurium | [41] |
| 3,7-hydroxycymopolone (15) | Cymopolia barbata | Antimutagenic against S typhimurium | [41] |
| 7-dihydroxycymo-pochromenol (16) | Cymopolia barbata | Antimutagenic against S typhimurium | [41] |
| Derivatives of cymopochromenol (17) | Cymopolia barbata | Antimutagenic- S typhimurium | [41] |
| Compound | Species | Bioactivity | Ref. |
|---|---|---|---|
| Caulerpal A (18) | Caulerpa taxifolia | hPTP1B inhibitor | [21] |
| Caulerpal B (19) | Caulerpa taxifolia | hPTP1B inhibitor | [21] |
| Acetylene Sesquiterpenoid Esters (20–28) | Caulerpa prolifera | Antibacterial | [52] |
| Acetylene Sesquiterpenoid Esters (29–34) | Caulerpa prolifera | Antibacterial | [52] |
| Guai-2-en-10α-ol (35) | Ulva fasciata | Antibacterial | [53] |
| guai-2-en-10α-methanol (36) | Ulva fasciata | Antibacterial | [53] |
| Guai-2-en-10α-methyl methanoate (37) | Ulva fasciata | Antibacterial | [53] |
| Compound | Species | Bioactivity | Ref. |
|---|---|---|---|
| labda-14-ene-8-ol (38) | Ulva fasciata | Antibacterial | [54] |
| labda-14-ene-3α,8α-diol (39) | Ulva fasciata | Antibacterial | [54] |
| labda-14-ene-8α,9α-diol (40) | Ulva fasciata | Antibacterial | [54] |
| labda-14-ene-8α-hydroxy-3-one (41) | Ulva fasciata | Antibacterial | [54] |
| ent-labda-13(16),14-diene-2-one (42) | Ulva fasciata | Antibacterial | [54] |
| ent-labda-13(16),14-diene-3α-ol (43) | Ulva fasciata | Antibacterial | [54] |
| ent-labda-13(16),14-diene-3α-ol (44) | Ulva fasciata | Antibacterial | [54] |
| racemobutenolids A, B (45ab) | Caulerpa racemosa | - | [56] |
| 4,5-dehydrodiodictyonema A (46) | Caulerpa racemosa | PTP1B inhibitor | [56] |
| an α-tocopheroid,α-tocoxylenoxy (47) | Caulerpa racemosa | PTP1B inhibitor | [56] |
| Compound | Species | Bioactivity | Ref. |
|---|---|---|---|
| Lanosta-8-en-3,29-diol-23-oxo-3,29-disodium sulfate (48) | Tydemania expeditionis | cytotoxic tumor cell | [57] |
| Capisterones A (49) | Penicillus capitatus | potent antifungal | [58] |
| Capisterones B (50) | Penicillus capitatus | potent antifungal | [58] |
| Squalene (51) | C. Racemose | - | [59] |
| Dwarkenoic acid (52) | Codium dwarkense | alpha-glucosidase inhibitor | [60] |
| Loliolide (53) | Ulva prolifera | - | [61] |
| Lsololiolide (54) | Ulva prolifera | - | [61] |
| Compound | Species | Bioactivity | Ref. |
|---|---|---|---|
| Cholest-5-en-3-ol (55) | Ulva prolifera | Antialgal | [63] |
| (8E,12Z,15Z)-10-hydroxy-8,12,15-octadecatrien-4,6-diynoic acid (56) | Caulerpa racemosa | - | [64] |
| Hexadeca-4,7,10,13-tetraenoic acid (HDTA) (57) | Ulva fasciata | Algicidal | [63] |
| Octadeca-6,9,12,15-tetraenoic acid (ODTA) (58) | Ulva fasciata | Algicidal | [59] |
| α-linolenic acid (59) | Ulva fasciata | Algicidal | [59] |
| β-sitosterol (60) | Caulerpa racemosa | - | [59] |
| (1, iyengadione) (61) | Codium iyengarii | - | [65] |
| iyengaroside-A (62) and B (63) | Codium iyengarii | Antibacterial | [65] |
| Clerosterol galactoside (64) | Codium iyengarii | - | [65] |
| 3-hydroxy-octadeca-4(E),6(Z),15(Z)-trienoic acid (65) | Tydemania expeditionis | Antitumor | [57] |
| 3-hydroxyhexadeca-4(E),6(Z)-dienoic acid (66) | Tydemania expeditionis | Antitumor | [57] |
| (24R)-5,28-stigmastadiene-3β,24-diol-7-one (67) | Ulva australis | Aldose reductase inhibitor | [66] |
| (24S)-5,28-stigmastadiene-3β,24-diol-7-one (68) | Ulva australis | Aldose reductase inhibitor | [66] |
| 24R and 24S-vinylcholesta-3β,5α,6β,24-tetraol (69) | Ulva australis | Aldose reductase inhibitor | [66] |
| keto-type fatty acid (70) | Ulva lactuca | ARE activators | [67] |
| Shorter chain C16 acid (71) | Ulva lactuca | ARE activators | [67] |
| Amide derivative (72) | Ulva lactuca | ARE activators | [67] |
| (23E)-3b-hydroxy-stigmasta-5,23 dien-28-one (73) | Caulerpa racemosa | PTP1B inhibitor | [56] |
| Compound | Species | Bioactivity | Ref. |
|---|---|---|---|
| 1-O-octadecanoic acid-3-O-β-d-galactopyranosyl glycerol (74) | Ulva prolifera | Antialgal | [61] |
| 1-O-palmitoyl-3-O-β-d-galactopyranosyl glycerol (75) | Ulva prolifera | Antialgal | [61] |
| 1-O-palmitoyl-2-Ooleoyl-3-O-β-d-galactopyranosyl glycerol (76) | Ulva prolifera | Antialgal | [61] |
| Monopalmitate (77) | Ulva prolifera | Antialgal | [61] |
| 9-hexadecenoic acid, 2,3-dihydroxypropyl ester (78) | Ulva prolifera | Antialgal | [61] |
| 1-eicosapentaenoyl-2-linolenoyl-3-galacto-sylglycerol (79) | C. racemosa | Anti-inflammatory | [59] |
| Capsofulvesins (80A–82C) | Capsosiphon fulvescens | Acetylcholinesterase (ache) inhibitor | [68] |
| Galactosylglycerolipid (GGL) (83) | Ulva pertusa | - | [69,70] |
| Sulfoquinovosyl diacylglycerol (SQDG) (84) | Caulerpa racemosa | Antiviral against HSV-2 | [71] |
| Avrainvilloside (85) | Avrainvillea nigricans | Inactive cytotoxic | [73] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shah, S.A.A.; Hassan, S.S.u.; Bungau, S.; Si, Y.; Xu, H.; Rahman, M.H.; Behl, T.; Gitea, D.; Pavel, F.-M.; Corb Aron, R.A.; et al. Chemically Diverse and Biologically Active Secondary Metabolites from Marine Phylum chlorophyta. Mar. Drugs 2020, 18, 493. https://doi.org/10.3390/md18100493
Shah SAA, Hassan SSu, Bungau S, Si Y, Xu H, Rahman MH, Behl T, Gitea D, Pavel F-M, Corb Aron RA, et al. Chemically Diverse and Biologically Active Secondary Metabolites from Marine Phylum chlorophyta. Marine Drugs. 2020; 18(10):493. https://doi.org/10.3390/md18100493
Chicago/Turabian StyleShah, Sayed Asmat Ali, Syed Shams ul Hassan, Simona Bungau, Yongsheng Si, Haiwei Xu, Md. Habibur Rahman, Tapan Behl, Daniela Gitea, Flavia-Maria Pavel, Raluca Anca Corb Aron, and et al. 2020. "Chemically Diverse and Biologically Active Secondary Metabolites from Marine Phylum chlorophyta" Marine Drugs 18, no. 10: 493. https://doi.org/10.3390/md18100493
APA StyleShah, S. A. A., Hassan, S. S. u., Bungau, S., Si, Y., Xu, H., Rahman, M. H., Behl, T., Gitea, D., Pavel, F. -M., Corb Aron, R. A., Pasca, B., & Nemeth, S. (2020). Chemically Diverse and Biologically Active Secondary Metabolites from Marine Phylum chlorophyta. Marine Drugs, 18(10), 493. https://doi.org/10.3390/md18100493