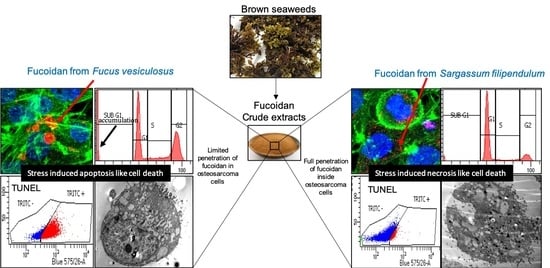
Fucoidan Inhibition of Osteosarcoma Cells is Species and Molecular Weight Dependent
Abstract
:1. Introduction
2. Results
2.1. Natural Sugars, Sulphation and Acid Contents in Fucoidan
2.2. Effect of Fucoidan on MG63 Cell Attachment and Morphology
2.3. Effect of Fucoidan on MG63 Cell Growth and Mitochondrial Activity
2.4. Ultrastructure Examination of MG63 Cells Treated with Fucoidan
2.5. Effect of Fucoidan on Cell Cycle
2.6. Annexin V/PI Staining
2.7. Assessment of DNA Fragmentation in MG63 Cells after Fucoidan Treatment
2.8. Effect of Fucoidan on Mitochondrial Health
2.9. Localization of Fucoidan in MG63 Cells
3. Discussion
3.1. Fucoidan Causes G1 Phase Cell Cycle Arrest in MG63 Cells
3.2. F. vesiculosus Fucoidan Causes Stress-Induced Apoptosis-Like Cell Death in MG63 Cells
3.3. S. filipendula Fucoidan Causes Necrosis in MG63 Cells
3.4. Fucoidan Affects Cell Cytoskeleton Formation and Adhesion
3.5. HMW Fraction is Responsible for Cytotoxicity of Crude Fucoidan from S. filipendula
4. Materials and Methods
4.1. Extraction and Purification of Fucoidan
4.2. Measurement of Neutral Sugars in Fucoidan
4.3. Measurement of Acid Sugars in Fucoidan
4.4. Measurement of Sulphated Sugars Content
4.5. Culturing of MG63 Osteosarcoma Cells
4.6. Cell Metabolic Activity Assay
4.7. Actin Cytoskeleton and Focal Adhesion Staining
4.8. DNA Content Assay
4.9. Giemsa Staining
4.10. Cell Cycle Analysis
4.11. Annexin V/PI Staining
4.12. Transmission Electron Microscopy (TEM)
4.13. JC-1 Staining
4.14. Immunostaining for Cytochrome C (Cyt C) and Fucoidan
4.15. Terminal Deoxynucleotidyl Transferase dUTP Nick End Labelling (TUNEL) Assay
4.16. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kylin, H. Zur Biochemie der Meeresalgen. Hoppe-Seyler s J. Physiol. Chem. 1913, 83, 171–197. [Google Scholar] [CrossRef]
- Li, B.; Lu, F.; Wei, X.; Zhao, R. Fucoidan: Structure and bioactivity. Molecules 2008, 13, 1671–1695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ermakova, S.; Sokolova, R.; Kim, S.; Um, B.-H.; Isakov, V.; Zvyagintseva, T. Fucoidans from Brown Seaweeds Sargassum hornery, Eclonia cava, Costaria costata: Structural Characteristics and Anti-cancer Activity. Appl. Biochem. Biotechnol. 2011, 164, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, H.R.; Biller, P.; Ross, A.B.; Adams, J.M.M. The seasonal variation of fucoidan within three species of brown macroalgae. Algal Res. 2017, 22, 79–86. [Google Scholar] [CrossRef] [Green Version]
- Hahn, T.; Lang, S.; Ulber, R.; Muffler, K. Novel procedures for the extraction of fucoidan from brown algae. Process. Biochem. 2012, 47, 1691–1698. [Google Scholar] [CrossRef]
- Oliveira, C.; Ferreira, A.S.; Novoa-Carballal, R.; Nunes, C.; Pashkuleva, I.; Neves, N.M.; Coimbra, M.A.; Reis, R.L.; Martins, A.; Silva, T.H. The Key Role of Sulfation and Branching on Fucoidan Antitumor Activity. Macromol. Biosci. 2017, 17, 1600340. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Teruya, K.; Eto, H.; Shirahata, S. Induction of Apoptosis by Low-Molecular-Weight Fucoidan through Calcium- and Caspase-Dependent Mitochondrial Pathways in MDA-MB-231 Breast Cancer Cells. Biosci. Biotechnol. Biochem. 2013, 77, 235–242. [Google Scholar] [CrossRef]
- Teruya, T.; Konishi, T.; Uechi, S.; Tamaki, H.; Tako, M. Anti-proliferative activity of oversulfated fucoidan from commercially cultured Cladosiphon okamuranus TOKIDA in U937 cells. Int. J. Biol. Macromol. 2007, 41, 221–226. [Google Scholar] [CrossRef]
- Costa, L.S.; Telles, C.B.S.; Oliveira, R.M.; Nobre, L.T.D.B.; Dantas-Santos, N.; Camara, R.B.G.; Costa, M.S.S.P.; Almeida-Lima, J.; Melo-Silveira, R.F.; Albuquerque, I.R.L.; et al. Heterofucan from Sargassum filipendula induces apoptosis in HeLa cells. Mar. Drugs 2011, 9, 603–614. [Google Scholar] [CrossRef]
- Zhao, Y.; Zheng, Y.; Wang, J.; Ma, S.; Yu, Y.; White, W.L.; Yang, S.; Yang, F.; Lu, J. Fucoidan Extracted from Undaria pinnatifida : Source for Nutraceuticals/Functional Foods. Mar. Drugs 2018, 16, 321. [Google Scholar] [CrossRef] [Green Version]
- Alekseyenko, T.V.; Zhanayeva, S.Y.; Venediktova, A.A.; Zvyagintseva, T.N.; Kuznetsova, T.A.; Besednova, N.N.; Korolenko, T.A. Antitumor and antimetastatic activity of fucoidan, a sulfated polysaccharide isolated from the Okhotsk sea Fucus evanescens brown alga. Bull. Exp. Biol. Med. 2007, 143, 730–732. [Google Scholar] [CrossRef] [PubMed]
- Palanisamy, S.; Vinosha, M.; Marudhupandi, T.; Rajasekar, P.; Prabhu, N.M. Isolation of fucoidan from Sargassum polycystum brown algae: Structural characterization, in vitro antioxidant and anti-cancer activity. Int. J. Biol. Macromol. 2017, 102, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Nagamine, T.; Hayakawa, K.; Kusakabe, T.; Takada, H.; Nakazato, K.; Hisanaga, E.; Iha, M. Inhibitory effect of fucoidan on huh7 hepatoma cells through downregulation of CXCL12. Nutr. Cancer 2009, 61, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Gambato, G.; Baroni, É.G.; Garcia, C.S.C.; Frassini, R.; Frozza, C.O.S.; Moura, S.; Pereira, C.M.P.; Fujii, M.T.; Colepicolo, P.; Lambert, A.P.F.; et al. Brown Algae Himantothallus grandifolius (Desmarestiales, Phaeophyceae) Suppresses Proliferation and Promotes Apoptosis-Mediated Cell Death in Tumor Cells. Adv. Biol. Chem. 2014, 4, 98–108. [Google Scholar] [CrossRef] [Green Version]
- Álvarez-Viñas, M.; Flórez-Fernández, N.; González-Muñoz, M.J.; Domínguez, H. Influence of molecular weight on the properties of Sargassum muticum fucoidan. Algal Res. 2019, 38, 101393. [Google Scholar] [CrossRef]
- Wijesinghe, W.A.J.P.; Jeon, Y.J. Biological activities and potential industrial applications of fucose rich sulfated polysaccharides and fucoidans isolated from brown seaweeds: A review. Carbohydr. Polym. 2012, 88, 13–20. [Google Scholar] [CrossRef]
- Senthilkumar, K.; Ramajayam, G.; Venkatesan, J.; Kim, S.K.; Ahn, B.C. Chapter 14: Biomedical Applications of Fucoidan, Seaweed Polysaccharides. In Seaweed Polysaccharides: Isolation, Biological and Biomedical Applications; Elsevier: Amsterdam, Netherland, 2017; pp. 269–281. ISBN 9780128098172. [Google Scholar]
- Atashrazm, F.; Lowenthal, R.M.; Woods, G.M.; Holloway, A.F.; Dickinson, J.L. Fucoidan and cancer: A multifunctional molecule with anti-tumor potential. Mar. Drugs 2015, 13, 2327–2346. [Google Scholar] [CrossRef] [Green Version]
- Khan, T.; Date, A.; Chawda, H.; Patel, K. Polysaccharides as potential anti-cancer agents—A review of their progress. Carbohydr. Polym. 2019, 210, 412–428. [Google Scholar] [CrossRef]
- Cho, M.L.; Lee, B.Y.; You, S.G. Relationship between oversulfation and conformation of low and high molecular weight fucoidans and evaluation of their in vitro anti-cancer activity. Molecules 2011, 16, 291–297. [Google Scholar] [CrossRef] [Green Version]
- Xue, M.; Ji, X.; Xue, C.; Liang, H.; Ge, Y.; He, X.; Zhang, L.; Bian, K.; Zhang, L. Caspase-dependent and caspase-independent induction of apoptosis in breast cancer by fucoidan via the PI3K/AKT/GSK3β pathway in vivo and in vitro. Biomed. Pharmacother. 2017, 94, 898–908. [Google Scholar] [CrossRef]
- Banafa, A.M.; Roshan, S.; Liu, Y.Y.; Chen, H.J.; Chen, M.J.; Yang, G.X.; He, G.Y. Fucoidan induces G1 phase arrest and apoptosis through caspases-dependent pathway and ROS induction in human breast cancer MCF-7 cells. J. Huazhong Univ. Sci. Technol. Med. Sci. 2013, 33, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.Y.; Lin, T.Y.; Hwang, P.A.; Tseng, L.M.; Chen, R.H.; Tsao, S.M.; Hsu, J. Fucoidan induces changes in the epithelial to mesenchymal transition and decreases metastasis by enhancing ubiquitin-dependent tgfβ receptor degradation in breast cancer. Carcinogenesis 2012, 34, 874–884. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki-Miyamoto, Y.; Yamasaki, M.; Tachibana, H.; Yamada, K. Fucoidan Induces Apoptosis through Activation of Caspase-8 on Human Breast Cancer MCF-7 Cells. J. Agric. Food Chem. 2009, 57, 8677–8682. [Google Scholar] [CrossRef] [PubMed]
- Hyun, J.-H.; Kim, S.-C.; Kang, J.-I.; Kim, M.-K.; Boo, H.-J.; Kwon, J.-M.; Koh, Y.-S.; Hyun, J.-W.; Park, D.-B.; Yoo, E.-S.; et al. Apoptosis inducing activity of fucoidan in HCT-15 colon carcinoma cells. Biol. Pharm. Bull. 2009, 32, 1760–1764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, E.J.; Park, S.Y.; Lee, J.Y.; Park, J.H.Y. Fucoidan present in brown algae induces apoptosis of human colon cancer cells. BMC Gastroenterol. 2010, 10, 96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikeguchi, M.; Yamamoto, M.; Arai, Y.; Maeta, Y.; Ashida, K.; Katano, K.; Miki, Y.; Kimura, T. Fucoidan reduces the toxicities of chemotherapy for patients with unresectable advanced or recurrent colorectal cancer. Oncol. Lett. 2011, 2, 319–322. [Google Scholar] [CrossRef] [PubMed]
- Somasundaram, S.N.; Shanmugam, S.; Subramanian, B.; Jaganathan, R. Cytotoxic effect of fucoidan extracted from Sargassum cinereum on colon cancer cell line HCT-15. Int. J. Biol. Macromol. 2016, 91, 1215–1223. [Google Scholar] [CrossRef]
- Cho, Y.; Yoon, J.-H.; Yoo, J.; Lee, M.; Lee, D.H.; Cho, E.J.; Lee, J.-H.; Yu, S.J.; Kim, Y.J.; Kim, C.Y. Fucoidan protects hepatocytes from apoptosis and inhibits invasion of hepatocellular carcinoma by up-regulating p42/44 MAPK-dependent NDRG-1/CAP43. Acta Pharm. Sin. B 2015, 5, 544–553. [Google Scholar] [CrossRef] [Green Version]
- Huang, T.H.; Chiu, Y.H.; Chan, Y.L.; Chiu, Y.H.; Wang, H.; Huang, K.C.; Li, T.L.; Hsu, K.H.; Wu, C.J. Prophylactic administration of fucoidan represses cancer metastasis by inhibiting vascular endothelial growth factor (VEGF) and matrix metalloproteinases (MMPs) in Lewis tumor-bearing mice. Mar. Drugs 2015, 13, 1882–1900. [Google Scholar] [CrossRef]
- Wang, F.; Schmidt, H.; Pavleska, D.; Wermann, T.; Seekamp, A.; Fuchs, S. Crude fucoidan extracts impair angiogenesis in models relevant for bone regeneration and osteosarcoma via reduction of VEGF and SDF-1. Mar. Drugs 2017, 15, 186. [Google Scholar] [CrossRef] [Green Version]
- Kimura, R.; Rokkaku, T.; Takeda, S.; Senba, M.; Mori, N. Cytotoxic effects of fucoidan nanoparticles against osteosarcoma. Mar. Drugs 2013, 11, 4267–4278. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Jeon, T.J. Fucoidan Induces Cell Aggregation and Apoptosis in Osteosarcoma MG-63 Cells. Anim. Cells Syst. Seoul 2016, 20, 186–192. [Google Scholar] [CrossRef] [Green Version]
- Luetke, A.; Meyers, P.A.; Lewis, I.; Juergens, H. Osteosarcoma treatment—Where do we stand? A state of the art review. Cancer Treat. Rev. 2014, 40, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Broadhead, M.L.; Clark, J.; Myers, D.E.; Dass, C.R.; Choong, P.F. The molecular pathogenesis of osteosarcoma: A review. Sarcoma 2011, 2011. [Google Scholar] [CrossRef] [PubMed]
- Park, H.Y.; Choi, I.W.; Kim, G.Y.; Kim, B.W.; Kim, W.J.; Choi, Y.H. Fucoidan induces G1 arrest of the cell cycle in EJ human bladder cancer cells through down-regulation of pRB phosphorylation. Brazilian J. Pharmacogn. 2015, 25, 246–251. [Google Scholar] [CrossRef] [Green Version]
- Aisa, Y.; Miyakawa, Y.; Nakazato, T.; Shibata, H.; Saito, K.; Ikeda, Y.; Kizaki, M. Fucoidan induces apoptosis of human HS-Sultan cells accompanied by activation of caspase-3 and down-regulation of ERK pathways. Am. J. Hematol. 2005, 78, 7–14. [Google Scholar] [CrossRef]
- Boo, H.J.; Hong, J.Y.; Kim, S.C.; Kang, J.I.; Kim, M.K.; Kim, E.J.; Hyun, J.W.; Koh, Y.S.; Yoo, E.S.; Kwon, J.M.; et al. The anti-cancer effect of fucoidan in PC-3 prostate cancer cells. Mar. Drugs 2013, 11, 2982–2999. [Google Scholar] [CrossRef] [Green Version]
- Ale, M.T.; Maruyama, H.; Tamauchi, H.; Mikkelsen, J.D.; Meyer, A.S. Fucoidan from Sargassum sp. and Fucus vesiculosus reduces cell viability of lung carcinoma and melanoma cells in vitro and activates natural killer cells in mice in vivo. Int. J. Biol. Macromol. 2011, 49, 331–336. [Google Scholar] [CrossRef]
- Yamasaki, Y.; Yamasaki, M.; Tachibana, H.; Yamada, K. Important Role of β1-Integrin in Fucoidan-Induced Apoptosis via Caspase-8 Activation. Biosci. Biotechnol. Biochem. 2012, 76, 1163–1168. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.M.; Bignon, J.; Haroun-Bouhedja, F.; Bittoun, P.; Vassy, J.; Fermandjian, S.; Wdzieczak-Bakala, J.; Boisson-Vidal, C. Inhibitory effect of fucoidan on the adhesion of adenocarcinoma cells to fibronectin. Anti-cancer Res. 2005, 25, 2129–2133. [Google Scholar]
- Lee, H.; Kim, J.S.; Kim, E. Fucoidan from Seaweed Fucus vesiculosus Inhibits Migration and Invasion of Human Lung Cancer Cell via PI3K-Akt-mTOR Pathways. PLoS ONE 2012, 7, e50624. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.-Y.; Lin, T.-Y.; Wu, Y.-C.; Tsao, S.-M.; Hwang, P.-A.; Shih, Y.-W.; Hsu, J. Fucoidan inhibition of lung cancer in vivo and in vitro: Role of the Smurf2-dependent ubiquitin proteasome pathway in TGFβ receptor degradation. Oncotarget 2014, 5, 7870–7885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, S.Y.; Yan, M.D.; Wu, A.T.H.; Yuan, K.S.P.; Liu, S.H. Brown Seaweed Fucoidan Inhibits Cancer Progression by Dual Regulation of mir-29c/ADAM12 and miR-17-5p/PTEN Axes in Human Breast Cancer Cells. J. Cancer 2016, 7, 2408–2419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramu, V.; Gill, M.R.; Jarman, P.J.; Turton, D.; Thomas, J.A.; Das, A.; Smythe, C. A cytostatic ruthenium(II)-platinum(II) bis(terpyridyl) anti-cancer complex that blocks entry into S phase by up-regulating p27KIP1. Chem. A Eur. J. 2015, 21, 9185–9197. [Google Scholar] [CrossRef]
- Han, Y.S.; Lee, J.H.; Lee, S.H. Antitumor effects of fucoidan on human colon cancer cells via activation of Akt signaling. Biomol. Ther. 2015, 23, 225–232. [Google Scholar] [CrossRef] [Green Version]
- Cho, T.M.; Kim, W.J.; Moon, S.K. AKT signaling is involved in fucoidan-induced inhibition of growth and migration of human bladder cancer cells. Food Chem. Toxicol. 2014, 64, 344–352. [Google Scholar] [CrossRef]
- Atashrazm, F.; Lowenthal, R.M.; Woods, G.M.; Holloway, A.F.; Karpiniec, S.S.; Dickinson, J.L. Fucoidan Suppresses the Growth of Human Acute Promyelocytic Leukemia Cells In Vitro and In Vivo. J. Cell. Physiol. 2016, 231, 688–697. [Google Scholar] [CrossRef]
- Wang, P.; Liu, Z.; Liu, X.; Teng, H.; Zhang, C.; Hou, L.; Zou, X. Anti-metastasis effect of fucoidan from Undaria pinnatifida sporophylls in mouse hepatocarcinoma Hca-F cells. PLoS ONE 2014, 9, e106071. [Google Scholar] [CrossRef] [Green Version]
- Kastan, M.B.; Bartek, J. Cell-cycle checkpoints and cancer. Nature 2004, 432, 316–323. [Google Scholar] [CrossRef]
- Schöthal, A.H. Checkpoint Controls and Cancer; Schöthal, A.H., Ed.; Humana Press: Los Angeles, CA, USA, 2004; Volume 2, ISBN 1-59259-788-2. [Google Scholar]
- Zamaraeva, M.V.; Sabirov, R.Z.; Maeno, E.; Ando-Akatsuka, Y.; Bessonova, S.V.; Okada, Y. Cells die with increased cytosolic ATP during apoptosis: A bioluminescence study with intracellular luciferase. Cell Death Differ. 2005, 12, 1390–1397. [Google Scholar] [CrossRef]
- Blondin, A.; Green, E. The mechanism of mitochondrial swelling. VIII. Permeability of mitochondria to alkali metal acetates. J. Bioenerg. 1970, 1, 479–492. [Google Scholar] [CrossRef]
- Blondin, G.A.; Green, D.E. The mechanism of mitochondrial swelling. Proc. Natl. Acad. Sci. 1967, 58, 612–619. [Google Scholar] [CrossRef] [Green Version]
- Wakabayashi, T. Structural changes of mitochondria related to apoptosis: Swelling and megamitochondria formation. Acta Biochim. Pol. 1999, 46, 223–237. [Google Scholar] [CrossRef] [Green Version]
- Van Cruchten, S.; Broeck, W. Van Den Morphological and biochemical aspects of apoptosis, oncosis and necrosis. Anat. Histol. Embryol. 2002, 31, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Karch, J.; Molkentin, J.D. Regulated Necrotic Cell Death: The Passive Aggressive Side of Bax and Bak. Circ. Res. 2015, 116, 1800–1809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higuchi, Y. Chromosomal DNA fragmentation in apoptosis and necrosis induced by oxidative stress. Biochem. Pharmacol. 2003, 66, 1527–1535. [Google Scholar] [CrossRef]
- Yang, L.; Wang, P.; Wang, H.; Li, Q.; Hongming, T.; Liu, Z.; Yang, W.; Hou, L.; Zou, X. Fucoidan Derived from Undaria pinnatifida Induces Apoptosis in Human Hepatocellular Carcinoma SMMC-7721 Cells via the ROS-Mediated Mitochondrial Pathway. Mar. Drugs 2013, 11, 1961–1976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanjeewa, K.K.A.; Lee, J.S.; Kim, W.S.; Jeon, Y.J. The potential of brown-algae polysaccharides for the development of anti-cancer agents: An update on anti-cancer effects reported for fucoidan and laminaran. Carbohydr. Polym. 2017, 177, 451–459. [Google Scholar] [CrossRef]
- Lee, H.E.; Choi, E.S.; Shin, J.A.; Lee, S.O.; Park, K.S.; Cho, N.P.; Cho, S.D. Fucoidan induces caspase-dependent apoptosis in MC3 human mucoepidermoid carcinoma cells. Exp. Ther. Med. 2014, 7, 228–232. [Google Scholar] [CrossRef]
- Choo, G.-S.; Lee, H.-N.; Shin, S.-A.; Kim, H.-J.; Jung, J.-Y. Anti-cancer Effect of Fucoidan on DU-145 Prostate Cancer Cells through Inhibition of PI3K/Akt and MAPK Pathway Expression. Mar. Drugs 2016, 14, 126. [Google Scholar] [CrossRef] [Green Version]
- Cho, Y.; Cho, E.J.; Lee, J.-H.; Yu, S.J.; Kim, Y.J.; Kim, C.Y.; Yoon, J.-H. Fucoidan-induced ID-1 suppression inhibits the in vitro and in vivo invasion of hepatocellular carcinoma cells. Biomed. Pharmacother. 2016, 83, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-Y.; Huang, T.-C.; Lin, L.-C.; Shieh, T.-M.; Wu, C.-H.; Wang, K.-L.; Hong, Y.-H.; Hsia, S.-M. Fucoidan inhibits the proliferation of leiomyoma cells and decreases extracellular matrix-associated protein expression. Cell. Physiol. Biochem. 2018, 49, 1970–1986. [Google Scholar] [CrossRef] [PubMed]
- Han, M.H.; Lee, D.S.; Jeong, J.W.; Hong, S.H.; Choi, I.W.; Cha, H.J.; Kim, S.; Kim, H.S.; Park, C.; Kim, G.Y.; et al. Fucoidan Induces ROS-Dependent Apoptosis in 5637 Human Bladder Cancer Cells by Downregulating Telomerase Activity via Inactivation of the PI3K/Akt Signaling Pathway. Drug Dev. Res. 2017, 78, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Teruya, K.; Eto, H.; Shirahata, S. Fucoidan extract induces apoptosis in MCF-7 cells via a mechanism involving the ros-dependent JNK activation and mitochondria-mediated pathways. PLoS ONE 2011, 6, e27441. [Google Scholar] [CrossRef]
- Liu, B.; Xu, N.; Man, Y.; Shen, H.; Avital, I.; Stojadinovic, A.; Liao, D.J. Apoptosis in Living Animals Is Assisted by Scavenger Cells and Thus May Not Mainly Go through the Cytochrome C-Caspase Pathway. J. Cancer 2013, 4, 716–723. [Google Scholar] [CrossRef] [Green Version]
- Dou, X.; Chen, L.; Lei, M.; Zellmer, L.; Jia, Q.; Ling, P.; He, Y.; Yang, W.; Liao, D.J. Evaluating the remote control of programmed cell death, with or without a compensatory cell proliferation. Int. J. Biol. Sci. 2018, 14, 1800–1812. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Yang, W.; Guan, Z.; Yu, W.; Fan, B.; Xu, N.; Liao, D.J. There are only four basic modes of cell death, although there are many ad-hoc variants adapted to different situations. Cell Biosci. 2018, 8, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Lou, X.; Jin, L.; Zhou, R.; Liu, S.; Xu, N.; Liao, D.J. Necrosis, and then stress induced necrosis-like cell death, but not apoptosis, should be the preferred cell death mode for chemotherapy: Clearance of a few misconceptions. Oncoscience 2015, 1, 407. [Google Scholar] [CrossRef] [Green Version]
- Sano, R.; Reed, J.C. ER stress-induced cell death mechanisms. Biochim. Biophys. Acta-Mol. Cell Res. 2013, 1833, 3460–3470. [Google Scholar] [CrossRef] [Green Version]
- Hsu, H.-Y.; Lin, T.-Y.; Lu, M.-K.; Leng, P.-J.; Tsao, S.-M.; Wu, Y.-C. Fucoidan induces Toll-like receptor 4-regulated reactive oxygen species and promotes endoplasmic reticulum stress-mediated apoptosis in lung cancer. Sci. Rep. 2017, 7, 44990. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Zhao, Y.; Zhang, Y.; Zhang, D. Fucoidan induces cancer cell apoptosis by modulating the endoplasmic reticulum stress cascades. PLoS ONE 2014, 9, e108157. [Google Scholar] [CrossRef]
- Park, H.S.; Kim, G.Y.; Nam, T.J.; Deuk Kim, N.; Hyun Choi, Y. Antiproliferative activity of fucoidan was associated with the induction of apoptosis and autophagy in AGS human gastric cancer cells. J. Food Sci. 2011, 76, T77–T83. [Google Scholar] [CrossRef]
- Balvan, J.; Krizova, A.; Gumulec, J.; Raudenska, M.; Sladek, Z.; Sedlackova, M.; Babula, P.; Sztalmachova, M.; Kizek, R.; Chmelik, R.; et al. Multimodal holographic microscopy: Distinction between apoptosis and oncosis. PLoS ONE 2015, 10, e0121674. [Google Scholar] [CrossRef] [Green Version]
- Cho, Y.S.; Jung, W.K.; Kim, J.A.; Choi, I.W.; Kim, S.K. Beneficial effects of fucoidan on osteoblastic MG-63 cell differentiation. Food Chem. 2009, 116, 990–994. [Google Scholar] [CrossRef]
- Nikoletopoulou, V.; Markaki, M.; Palikaras, K.; Tavernarakis, N. Crosstalk between apoptosis, necrosis and autophagy. Biochim. Biophys. Acta-Mol. Cell Res. 2013, 1833, 3448–3459. [Google Scholar] [CrossRef] [Green Version]
- Watcharasit, P.; Bijur, G.N.; Song, L.; Zhu, J.; Chen, X.; Jope, R.S. Glycogen Synthase Kinase-3β (GSK3β) Binds to and Promotes the Actions of p53. J. Biol. Chem. 2003, 278, 48872–48879. [Google Scholar] [CrossRef] [Green Version]
- Hoeflich, K.P.; Luo, J.; Rubie, E.A.; Tsao, M.-S.; Jin, O.; Woodgett, J.R. Requirement for glycogen synthase kinase-3β in cell survival and NF-κB activation. Nature 2000, 406, 86–90. [Google Scholar] [CrossRef]
- Steinbrecher, K.A.; Wilson, W.; Patricia, C.; Transcription, N.-B.; Iii, W.W.; Cogswell, P.C.; Baldwin, A.S. Glycogen Synthase Kinase 3β Functions To Specify Gene-Specific, Transcription. Mol. Cell. Biol. 2005, 25, 8444–8455. [Google Scholar] [CrossRef] [Green Version]
- Jacobs, K.M.; Bhave, S.R.; Ferraro, D.J.; Jaboin, J.J.; Hallahan, D.E.; Thotala, D. GSK-3β: A bifunctional role in cell death pathways. Int. J. Cell Biol. 2012. [Google Scholar] [CrossRef] [Green Version]
- Hsu, H.Y.; Chiu, S.L.; Wen, M.H.; Chen, K.Y.; Hua, K.F. Ligands of Macrophage Scavenger Receptor Induce Cytokine Expression via Differential Modulation of Protein Kinase Signaling Pathways. J. Biol. Chem. 2001, 276, 28719–28730. [Google Scholar] [CrossRef] [Green Version]
- Brodeur, M.R.; Brissette, L.; Falstrault, L.; Luangrath, V.; Moreau, R. Scavenger receptor of class B expressed by osteoblastic cells are implicated in the uptake of cholesteryl ester and estradiol from LDL and HDL3. J. Bone Miner. Res. 2008, 23, 326–337. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Yang, M.; Yuen, P.M.P.; Chik, K.W.; Li, C.K.; Shing, M.M.K.; Lam, H.K.B.; Fok, T.F. Thrombospondin-1 induces apoptosis in primary leukemia and cell lines mediated by CD36 and Caspase-3. Int. J. Mol. Med. 2003, 12, 995–1001. [Google Scholar] [CrossRef] [PubMed]
- Beltran, B.; Mathur, A.; Duchen, M.R.; Erusalimsky, J.D.; Moncada, S. The effect of nitric oxide on cell respiration: A key to understanding its role in cell survival or death. Proc. Natl. Acad. Sci. USA 2000, 97, 14602–14607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chevolot, L.; Foucault, A.; Chaubet, F.; Kervarec, N.; Sinquin, C.; Fisher, A.M.; Boisson-Vidal, C. Further data on the structure of brown seaweed fucans: Relationships with anticoagulant activity. Carbohydr. Res. 1999, 319, 154–165. [Google Scholar] [CrossRef]
- Synytsya, A.; Kim, W.J.; Kim, S.M.; Pohl, R.; Synytsya, A.; Kvasnička, F.; Čopíková, J.; Il Park, Y. Structure and antitumour activity of fucoidan isolated from sporophyll of Korean brown seaweed Undaria pinnatifida. Carbohydr. Polym. 2010, 81, 41–48. [Google Scholar] [CrossRef]
- Farndale, R.W.; Buttle, D.J.; Barrett, A.J. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. BBA-Gen. Subj. 1986, 883, 173–177. [Google Scholar] [CrossRef]
- Li, J.; Kisara, K.; Danielsson, S.; Lindström, M.E.; Gellerstedt, G. An improved methodology for the quantification of uronic acid units in xylans and other polysaccharides. Carbohydr. Res. 2007, 342, 1442–1449. [Google Scholar] [CrossRef]
- Whitley, C.B.; Ridnour, M.D.; Draper, K.A.; Dutton, C.M.; Neglia, J.P. Diagnostic test for mucopolysaccharidosis. I. Direct method for quantifying excessive urinary glycosaminoglycan excretion. Clin. Chem. 1989, 35, 374–379. [Google Scholar] [CrossRef]
- Perelman, A.; Wachtel, C.; Cohen, M.; Haupt, S.; Shapiro, H.; Tzur, A. JC-1: Alternative excitation wavelengths facilitate mitochondrial membrane potential cytometry. Cell Death Dis. 2012, 3, e430. [Google Scholar] [CrossRef] [Green Version]
- Torode, T.A.; Marcus, S.E.; Jam, M.; Tonon, T.; Blackburn, R.S.; Hervé, C.; Knox, J.P. Monoclonal antibodies directed to fucoidan preparations from brown algae. PLoS ONE 2015, 10, e0118366. [Google Scholar] [CrossRef] [Green Version]









| Fucoidan-Type | Neutral Sugars (%) | Uronic Acids (%) | Sulfation (%) | |
|---|---|---|---|---|
| Glucose | Fucose | |||
| F. vesiculosus Crude | 7.88 ± 0.04 | 56.57 ± 0.01 | 8.28 ± 0.01 | 33.92 ± 0.09 |
| S. filipendula Crude | 11.18 ± 0.01 | 79.48 ± 0.02 | 11.83 ± 0.01 | 16.52 ± 0.02 |
| S. filipendula LMW | 10.95 ± 0.01 | 76.69 ± 0.01 | 10.00 ± 0.04 | 15.05 ± 0.01 |
| S. filipendula MMW | 11.87 ± 0.01 | 82.31 ± 0.01 | 12.66 ± 0.01 | 25.99 ± 0.01 |
| S. filipendula HMW | 10.95 ± 0.02 | 70.28 ± 0.01 | 12.26 ± 0.01 | 20.13 ± 0.03 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gupta, D.; Silva, M.; Radziun, K.; Martinez, D.C.; Hill, C.J.; Marshall, J.; Hearnden, V.; Puertas-Mejia, M.A.; Reilly, G.C. Fucoidan Inhibition of Osteosarcoma Cells is Species and Molecular Weight Dependent. Mar. Drugs 2020, 18, 104. https://doi.org/10.3390/md18020104
Gupta D, Silva M, Radziun K, Martinez DC, Hill CJ, Marshall J, Hearnden V, Puertas-Mejia MA, Reilly GC. Fucoidan Inhibition of Osteosarcoma Cells is Species and Molecular Weight Dependent. Marine Drugs. 2020; 18(2):104. https://doi.org/10.3390/md18020104
Chicago/Turabian StyleGupta, Dhanak, Melissa Silva, Karolina Radziun, Diana C. Martinez, Christopher J. Hill, Julie Marshall, Vanessa Hearnden, Miguel A. Puertas-Mejia, and Gwendolen C. Reilly. 2020. "Fucoidan Inhibition of Osteosarcoma Cells is Species and Molecular Weight Dependent" Marine Drugs 18, no. 2: 104. https://doi.org/10.3390/md18020104
APA StyleGupta, D., Silva, M., Radziun, K., Martinez, D. C., Hill, C. J., Marshall, J., Hearnden, V., Puertas-Mejia, M. A., & Reilly, G. C. (2020). Fucoidan Inhibition of Osteosarcoma Cells is Species and Molecular Weight Dependent. Marine Drugs, 18(2), 104. https://doi.org/10.3390/md18020104