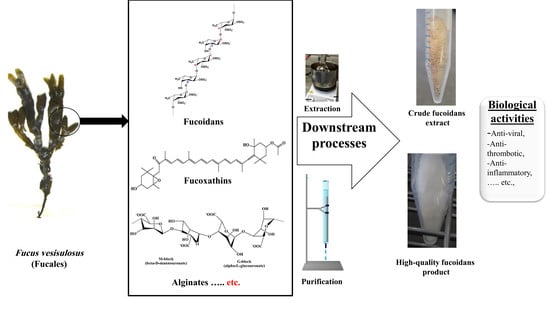
Fucoidans: Downstream Processes and Recent Applications
Abstract
:1. Introduction
2. Global Market and Cultivation of Brown Algae
3. Downstream Processes
3.1. Pre-Treatment
3.2. Extraction
3.3. Separation Physical Methods
3.4. Purification
4. Recent Uncommon Applications
5. Enzymatic Modification of Native Fucoidans
6. Conclusion and Future Prospective
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Munoz-Bonilla, A.; Echeverria, C.; Sonseca, A.; Arrieta, M.P.; Fernandez-Garcia, M. Bio-based polymers with antimicrobial properties towards sustainable development. Materials 2019, 12, 641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amos, R.A.; Mohnen, D. Critical review of plant cell wall matrix polysaccharide glycosyltransferase activities verified by heterologous protein expression. Front. Plant Sci. 2019, 10, 915. [Google Scholar] [CrossRef] [Green Version]
- Lampugnani, E.R.; Flores-Sandoval, E.; Tan, Q.W.; Mutwil, M.; Bowman, J.L.; Persson, S. Cellulose synthesis—central components and their evolutionary relationships. Trends Plant Sci. 2019, 24, 402–412. [Google Scholar] [CrossRef] [PubMed]
- Helle, S.; Bray, F.; Verbeke, J.; Devassine, S.; Courseaux, A.; Facon, M.; Tokarski, C.; Rolando, C.; Szydlowski, N. Proteome analysis of potato starch reveals the presence of new starch metabolic proteins as well as multiple protease inhibitors. Front. Plant Sci. 2018, 9, 746. [Google Scholar] [CrossRef] [PubMed]
- Ball, S.; Colleoni, C.; Cenci, U.; Raj, J.N.; Tirtiaux, C. The evolution of glycogen and starch metabolism in eukaryotes gives molecular clues to understand the establishment of plastid endosymbiosis. J. Exp. Bot. 2011, 62, 1775–1801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edmond Ghanem, M.; Han, R.-M.; Classen, B.; Quetin-Leclerq, J.; Mahy, G.; Ruan, C.-J.; Qin, P.; Pérez-Alfocea, F.; Lutts, S. Mucilage and Polysaccharides in the Halophyte plant species Kosteletzkya virginica: Localization and composition in relation to salt stress. J. Plant Physiol. 2010, 167, 382–392. [Google Scholar] [CrossRef]
- Shukla, P.S.; Mantin, E.G.; Adil, M.; Bajpai, S.; Critchley, A.T.; Prithiviraj, B. Ascophyllum nodosum-based biostimulants: sustainable applications in agriculture for the stimulation of plant growth, stress tolerance, and disease management. Front. Plant Sci. 2019, 10, 655. [Google Scholar] [CrossRef] [Green Version]
- Hamed, I.; Özogul, F.; Özogul, Y.; Regenstein, J.M. Marine bioactive compounds and their health benefits: A review. Compr. Rev. Food Sci. Food Saf. 2015, 14, 446–465. [Google Scholar] [CrossRef]
- Lee, Y.-E.; Kim, H.; Seo, C.; Park, T.; Lee, K.B.; Yoo, S.Y.; Hong, S.C.; Kim, J.T.; Lee, J. Marine polysaccharides: therapeutic efficacy and biomedical applications. Arch. Pharmacal Res. 2017, 40, 1006–1020. [Google Scholar] [CrossRef]
- Ruocco, N.; Costantini, S.; Guariniello, S.; Costantini, M. Polysaccharides from the marine environment with pharmacological, cosmeceutical and nutraceutical potential. Molecules 2016, 21, 551. [Google Scholar] [CrossRef]
- Laurienzo, P. Marine Polysaccharides in Pharmaceutical Applications: An Overview. Mar. Drugs 2010, 8, 2435–2465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meenakshi, S.; Umayaparvathi, S.; Saravanan, R.; Manivasagam, T.; Balasubramanian, T. Hepatoprotective effect of fucoidan isolated from the seaweed turbinaria decurrens in ethanol intoxicated rats. Int. J. Biol. Macromol. 2014, 67, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Cunha, L.; Grenha, A. Sulfated seaweed polysaccharides as multifunctional materials in drug delivery applications. Mar. Drugs 2016, 14, 42. [Google Scholar] [CrossRef] [PubMed]
- Hudak, J.E.; Bertozzi, C.R. Glycotherapy: New advances inspire a reemergence of glycans in medicine. Chem. Biol. 2014, 21, 16–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novotny, M.V.; Alley, W.R., Jr. Recent trends in analytical and structural glycobiology. Curr. Opin. Chem. Biol. 2013, 17, 832–840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caldwell, G.S.; Pagett, H.E. Marine glycobiology: Current status and future perspectives. Mar. Biotechnol. 2010, 12, 241–252. [Google Scholar] [CrossRef]
- Pomin, V.H. Marine medicinal Gglycomics. Front. Cell. Infect. Microbiol. 2014, 4, 5. [Google Scholar] [CrossRef] [Green Version]
- De Jesus Raposo, M.F.; de Morais, A.M.B.; de Morais, R.M.S.C. Marine polysaccharides from algae with potential biomedical applications. Mar. Drugs 2015, 13, 2967–3028. [Google Scholar] [CrossRef]
- Gobet, A.; Barbeyron, T.; Matard-Mann, M.; Magdelenat, G.; Vallenet, D.; Duchaud, E.; Michel, G. Evolutionary evidence of algal polysaccharide degradation acquisition by Pseudoalteromonas carrageenovora 9T to adapt to macroalgal niches. Front. Microbiol. 2018, 9, 2740. [Google Scholar] [CrossRef] [Green Version]
- Maneein, S.; Milledge, J.J.; Nielsen, B.V.; Harvey, P.J. A Review of seaweed pre-treatment methods for enhanced biofuel production by anaerobic digestion or fermentation. Fermentation 2018, 4, 100. [Google Scholar] [CrossRef] [Green Version]
- Afonso, N.C.; Catarino, M.D.; Silva, A.M.S.; Cardoso, S.M. Brown macroalgae as valuable food ingredients. Antioxidants 2019, 8, 365. [Google Scholar] [CrossRef] [Green Version]
- Catarino, M.D.; Silva, A.M.S.; Cardoso, S.M. Phycochemical constituents and biological activities of Fucus spp. Mar. Drugs 2018, 16, 249. [Google Scholar] [CrossRef] [Green Version]
- Deniaud-Bouët, E.; Hardouin, K.; Potin, P.; Kloareg, B.; Hervé, C. A review about brown algal cell walls and fucose-containing sulfated polysaccharides: Cell wall context, biomedical properties and key research challenges. Carbohydr. Polym. 2017, 175, 395–408. [Google Scholar] [CrossRef] [PubMed]
- Deniaud-Bouët, E.; Kervarec, N.; Michel, G.; Tonon, T.; Kloareg, B.; Hervé, C. Chemical and enzymatic fractionation of cell walls from Fucales: Insights into the structure of the extracellular matrix of brown algae. Ann. Bot. 2014, 114, 1203–1216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Generalić Mekinić, I.; Skroza, D.; Šimat, V.; Hamed, I.; Čagalj, M.; Popović Perković, Z. Phenolic content of brown algae (Pheophyceae) species: Extraction, identification, and quantification. Biomolecules 2019, 9, 244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, Y.; Xue, C.; Tang, Q.; Li, D.; Wu, X.; Wang, J. Isolation and characterization of a sea cucumber fucoidan-utilizing marine bacterium. Lett. Appl. Microbiol. 2010, 50, 301–307. [Google Scholar] [CrossRef]
- Zayed, A.; Dienemann, C.; Giese, C.; Krämer, R.; Ulber, R. An immobilized perylene diimide derivative for fucoidan purification from a crude brown algae extract. Process Biochem. 2018, 65, 233–238. [Google Scholar] [CrossRef]
- Citkowska, A.; Szekalska, M.; Winnicka, K. Possibilities of fucoidan utilization in the development of pharmaceutical dosage forms. Mar. Drugs 2019, 17, 458. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Li, J.; Zhi, Z.; Hu, Y.; Ge, J.; Ye, X.; Tian, D.; Linhardt, R.J.; Chen, S. 4-O-sulfation in sea cucumber fucodians contribute to reversing dyslipidiaemia caused by HFD. Int. J. Biol. Macromol. 2017, 99, 96–104. [Google Scholar] [CrossRef] [Green Version]
- Fitton, J.H.; Stringer, D.S.; Park, A.Y.; Karpiniec, S.N. Therapies from fucoidan: new developments. Mar. Drugs 2019, 17, 571. [Google Scholar] [CrossRef] [Green Version]
- Van Weelden, G.; Bobinski, M.; Okla, K.; van Weelden, W.J.; Romano, A.; Pijnenborg, J.M.A. Fucoidan structure and activity in relation to anti-cancer mechanisms. Mar. Drugs 2019, 17, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Xing, M.; Cao, Q.; Ji, A.; Liang, H.; Song, S. Biological activities of fucoidan and the factors mediating its therapeutic effects: A review of recent studies. Mar. Drugs 2019, 17, 183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luthuli, S.; Wu, S.; Cheng, Y.; Zheng, X.; Wu, M.; Tong, H. Therapeutic effects of fucoidan: A review on recent studies. Mar. Drugs 2019, 17, 487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chollet, L.; Saboural, P.; Chauvierre, C.; Villemin, J.N.; Letourneur, D.; Chaubet, F. Fucoidans in nanomedicine. Mar. Drugs 2016, 14, 145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holtkamp, A.D.; Kelly, S.; Ulber, R.; Lang, S. Fucoidans and fucoidanases-focus on techniques for molecular structure elucidation and modification of marine polysaccharides. Appl. Microbiol. Biotechnol. 2009, 82, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ustyuzhanina, N.E.; Bilan, M.I.; Ushakova, N.A.; Usov, A.I.; Kiselevskiy, M.V.; Nifantiev, N.E. Fucoidans: Pro- or antiangiogenic agents? Glycobiology 2014, 24, 1265–1274. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Zheng, Y.; Wang, J.; Ma, S.; Yu, Y.; White, W.L.; Yang, S.; Yang, F.; Lu, J. Fucoidan extracted from Undaria pinnatifida: Source for nutraceuticals/functional foods. Mar. Drugs 2018, 16, 321. [Google Scholar] [CrossRef] [Green Version]
- Arumugam, P.; Arunkumar, K.; Sivakumar, L.; Murugan, M.; Murugan, K. Anticancer effect of fucoidan on cell proliferation, cell cycle progression, genetic damage and apoptotic cell death in HepG2 cancer cells. Toxicol. Rep. 2019, 6, 556–563. [Google Scholar]
- Atashrazm, F.; Lowenthal, R.M.; Woods, G.M.; Holloway, A.F.; Dickinson, J.L. Fucoidan and cancer: A multifunctional molecule with anti-tumor potential. Mar. Drugs 2015, 13, 2327–2346. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.-M.; Liu, P.-Y.; Chen, Y.-A.; Tseng, H.-Y.; Shen, P.-C.; Hwang, P.-A.; Hsu, H.-L. Oligo-fucoidan prevents IL-6 and CCL2 production and cooperates with p53 to suppress ATM signaling and tumor progression. Sci. Rep. 2017, 7, 11864. [Google Scholar] [CrossRef] [Green Version]
- Zayed, A.; Muffler, K.; Hahn, T.; Rupp, S.; Finkelmeier, D.; Burger-Kentischer, A.; Ulber, R. Physicochemical and biological characterization of fucoidan from Fucus vesiculosus purified by dye affinity chromatography. Mar. Drugs 2016, 14, 79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, X.; Dong, S.; Wang, J.; Li, F.; Chen, A.; Li, B. A comparative study of antithrombotic and antiplatelet activities of different fucoidans from Laminaria japonica. Thromb. Res. 2012, 129, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wu, J.; Zhang, X.; Hao, C.; Zhao, X.; Jiao, G.; Shan, X.; Tai, W.; Yu, G. Inhibition of influenza A virus infection by fucoidan targeting viral neuraminidase and cellular EGFR pathway. Sci. Rep. 2017, 7, 40760. [Google Scholar] [CrossRef] [PubMed]
- Prokofjeva, M.M.; Imbs, T.I.; Shevchenko, N.M.; Spirin, P.V.; Horn, S.; Fehse, B.; Zvyagintseva, T.N.; Prassolov, V.S. Fucoidans as potential inhibitors of HIV-1. Mar. Drugs 2013, 11, 3000–3014. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Kawaguchi, M.; Kitamura, K.; Narumiya, S.; Kawamura, M.; Tengan, I.; Nishimoto, S.; Hanamure, Y.; Majima, Y.; Tsubura, S.; et al. An exploratory study on the anti-inflammatory effects of fucoidan in relation to quality of life in advanced cancer patients. Integr. Cancer Ther. 2018, 17, 282–291. [Google Scholar] [CrossRef] [Green Version]
- Park, H.Y.; Han, M.H.; Park, C.; Jin, C.-Y.; Kim, G.-Y.; Choi, I.-W.; Kim, N.D.; Nam, T.-J.; Kwon, T.K.; Choi, Y.H. Anti-inflammatory effects of fucoidan through inhibition of NF-κB, MAPK and Akt activation in lipopolysaccharide-induced BV2 microglia cells. Food Chem. Toxicol. 2011, 49, 1745–1752. [Google Scholar] [CrossRef]
- Li, B.; Lu, F.; Wei, X.; Zhao, R. Fucoidan: Structure and bioactivity. Molecules 2008, 13, 1671–1695. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Shi, K.K.; Chen, S.; Wang, J.; Hassouna, A.; White, L.N.; Merien, F.; Xie, M.; Kong, Q.; Li, J.; et al. Fucoidan extracted from the New Zealand Undaria pinnatifida-Physicochemical comparison against five other fucoidans: Unique low molecular weight fraction bioactivity in breast cancer cell lines. Mar. Drugs 2018, 16, 461. [Google Scholar] [CrossRef] [Green Version]
- Yoo, H.J.; You, D.-J.; Lee, K.-W. Characterization and immunomodulatory effects of high molecular weight fucoidan fraction from the sporophyll of Undaria pinnatifida in cyclophosphamide-induced immunosuppressed mice. Mar. Drugs 2019, 17, 447. [Google Scholar] [CrossRef] [Green Version]
- Zayed, A.; Hahn, T.; Finkelmeier, D.; Burger-Kentischer, A.; Rupp, S.; Krämer, R.; Ulber, R. Phenomenological investigation of the cytotoxic activity of fucoidan isolated from Fucus vesiculosus. Process Biochem. 2019, 81, 182–187. [Google Scholar] [CrossRef]
- Imbs, T.I.; Skriptsova, A.V.; Zvyagintseva, T.N. Antioxidant activity of fucose-containing sulfated polysaccharides obtained from Fucus evanescens by different extraction methods. J. Appl. Phycol. 2015, 27, 545–553. [Google Scholar] [CrossRef]
- Hifney, A.F.; Fawzy, M.A.; Abdel-Gawad, K.M.; Gomaa, M. Industrial optimization of fucoidan extraction from Sargassum sp. and its potential antioxidant and emulsifying activities. Food Hydrocoll. 2016, 54, 77–88. [Google Scholar] [CrossRef]
- Zayed, A.; Ulber, R. Fucoidan Production: Approval Key Challenges and Opportunities. Carbohydr. Polym. 2019, 211, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Fitton, J.H.; Stringer, D.N.; Karpiniec, S.S. Therapies from fucoidan: An update. Mar. Drugs 2015, 13, 5920–5946. [Google Scholar] [CrossRef] [Green Version]
- Mak, W.; Wang, S.K.; Liu, T.; Hamid, N.; Li, Y.; Lu, J.; White, W.L. Anti-proliferation potential and content of fucoidan extracted from sporophyll of New Zealand Undaria pinnatifida. Front. Nutr. 2014, 1, 9. [Google Scholar] [CrossRef] [Green Version]
- Chauvierre, C.; Aid-Launais, R.; Aerts, J.; Chaubet, F.; Maire, M.; Chollet, L.; Rolland, L.; Bonafe, R.; Rossi, S.; Bussi, S.; et al. Pharmaceutical development and safety evaluation of a GMP-grade fucoidan for molecular diagnosis of cardiovascular diseases. Mar. Drugs 2019, 17, 699. [Google Scholar] [CrossRef] [Green Version]
- Torres, M.D.; Flórez-Fernández, N.; Simón-Vázquez, R.; Giménez-Abián, J.F.; Díaz, J.F.; González-Fernández, Á.; Domínguez, H. Fucoidans: The importance of processing on their anti-tumoral properties. Algal Res. 2020, 45, 101748. [Google Scholar] [CrossRef]
- Borazjani, N.J.; Tabarsa, M.; You, S.; Rezaei, M. Improved immunomodulatory and antioxidant properties of unrefined fucoidans from Sargassum angustifolium by hydrolysis. J. Food Sci. Technol. 2017, 54, 4016–4025. [Google Scholar] [CrossRef]
- Koyanagi, S.; Tanigawa, N.; Nakagawa, H.; Soeda, S.; Shimeno, H. Oversulfation of fucoidan enhances its anti-angiogenic and antitumor activities. Biochem. Pharmacol. 2003, 65, 173–179. [Google Scholar] [CrossRef]
- Ale, M.T.; Mikkelsen, J.D.; Meyer, A.S. Important determinants for fucoidan bioactivity: A critical review of structure-function relations and extraction methods for fucose-containing sulfated polysaccharides from brown seaweeds. Mar. Drugs 2011, 9, 2106–2130. [Google Scholar] [CrossRef] [Green Version]
- Silchenko, A.S.; Rasin, A.B.; Kusaykin, M.I.; Malyarenko, O.S.; Shevchenko, N.M.; Zueva, A.O.; Kalinovsky, A.I.; Zvyagintseva, T.N.; Ermakova, S.P. Modification of native fucoidan from fucus evanescens by recombinant fucoidanase from marine bacteria Formosa algae. Carbohydr. Polym. 2018, 193, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Jiao, G.; Yu, G.; Zhang, J.; Ewart, H.S. Chemical structures and bioactivities of sulfated polysaccharides from marine algae. Mar. Drugs 2011, 9, 196–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suprunchuk, V.E. Low-molecular-weight fucoidan: Chemical modification, synthesis of its oligomeric fragments and mimetics. Carbohydr. Res. 2019, 485, 107806. [Google Scholar] [CrossRef] [PubMed]
- Kardeby, C.; Fälker, K.; Haining, E.J.; Criel, M.; Lindkvist, M.; Barroso, R.; Påhlsson, P.; Ljungberg, L.U.; Tengdelius, M.; Rainger, G.E.; et al. Synthetic glycopolymers and natural fucoidans cause human platelet aggregation via PEAR1 and GPIbα. Blood Adv. 2019, 3, 275–287. [Google Scholar] [CrossRef] [Green Version]
- Kasai, A.; Arafuka, S.; Koshiba, N.; Takahashi, D.; Toshima, K. Systematic synthesis of low-molecular weight fucoidan derivatives and their effect on cancer cells. Org. Biomol. Chem. 2015, 13, 10556–10568. [Google Scholar] [CrossRef]
- Rhein-Knudsen, N.; Ale, M.T.; Meyer, A.S. Seaweed hydrocolloid production: An update on enzyme assisted extraction and modification technologies. Mar. Drugs 2015, 13, 3340–3359. [Google Scholar] [CrossRef]
- Buschmann, A.H.; Camus, C.; Infante, J.; Neori, A.; Israel, Á.; Hernández-González, M.C.; Pereda, S.V.; Gomez-Pinchetti, J.L.; Golberg, A.; Tadmor-Shalev, N.; et al. Seaweed production: Overview of the global state of exploitation, farming and emerging research activity. Eur. J. Phycol. 2017, 52, 391–406. [Google Scholar] [CrossRef]
- Duarte, C.M.; Wu, J.; Xiao, X.; Bruhn, A.; Krause-Jensen, D. Can seaweed farming play a role in climate change mitigation and adaptation? Front. Mar. Sci. 2017, 4, 100. [Google Scholar]
- Zayed, A.; Kovacheva, M.; Muffler, K.; Breiner, H.-W.; Stoeck, T.; Ulber, R. Induction and genetic identification of a callus-like growth developed in the brown alga Fucus vesiculosus. Eng. Life Sci. 2019, 19, 363–369. [Google Scholar] [CrossRef] [Green Version]
- Cho, G.Y.; Rousseau, F.; de Reviers, B.; Boo, S.M. Phylogenetic relationships within the fucales (Phaeophyceae) assessed by the photosystem I coding psaa sequences. Phycologia 2006, 45, 512–519. [Google Scholar] [CrossRef]
- Wahl, M.; Molis, M.; Hobday, A.J.; Dudgeon, S.; Neumann, R.; Steinberg, P.; Campbell, A.H.; Marzinelli, E.; Connell, S. The responses of brown macroalgae to environmental change from local to global scales: Direct versus ecologically mediated effects. Perspect. Phycol. 2015, 2, 11–29. [Google Scholar] [CrossRef] [Green Version]
- Crous, P.W.; Wingfield, M.J.; Burgess, T.I.; Hardy, G.S.J.; Gené, J.; Guarro, J.; Baseia, I.G.; García, D.; Gusmão, L.F.P.; Souza-Motta, C.M.; et al. Fungal planet description sheets: 716–784. Persoonia 2018, 40, 240–393. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.R.; Reddy, C.R.K.; Jha, B. Callus induction and thallus regeneration from callus of phycocolloid yielding seaweeds from the Indian coast. J. Appl. Phycol. 2007, 19, 15–25. [Google Scholar] [CrossRef]
- Muhamad, S.N.S.; Ling, A.P.-K.; Wong, C.-L. Effect of plant growth regulators on direct regeneration and callus induction from Sargassum polycystum C. Agardh. J. Appl. Phycol. 2018, 30, 3299–3310. [Google Scholar] [CrossRef]
- Avila-Peltroche, J.; Won, B.Y.; Cho, T.O. Protoplast isolation and regeneration from Hecatonema terminale (Ectocarpales, Phaeophyceae) using a simple mixture of commercial enzymes. J. Appl. Phycol. 2019, 31, 1873–1881. [Google Scholar] [CrossRef]
- Luiten, E.E.; Akkerman, I.; Koulman, A.; Kamermans, P.; Reith, H.; Barbosa, M.J.; Sipkema, D.; Wijffels, R.H. Realizing the promises of marine biotechnology. Biomol. Eng. 2003, 20, 429–439. [Google Scholar] [CrossRef]
- Baweja, P.; Sahoo, D.; García-Jiménez, P.; Robaina, R.R. Review: Seaweed tissue culture as applied to biotechnology: Problems, achievements and prospects. Phycol. Res. 2009, 57, 45–58. [Google Scholar] [CrossRef]
- Huang, Y.M.; Rorrer, G.L. Cultivation of microplantlets derived from the marine red alga Agardhiella subulata in a stirred tank photobioreactor. Biotechnol. Prog. 2003, 19, 418–427. [Google Scholar] [CrossRef]
- Hahn, T.; Lang, S.; Ulber, R.; Muffler, K. Novel procedures for the extraction of fucoidan from brown algae. Process Biochem. 2012, 47, 1691–1698. [Google Scholar] [CrossRef]
- Thinh, P.D.; Menshova, R.V.; Ermakova, S.P.; Anastyuk, S.D.; Ly, B.M.; Zvyagintseva, T.N. Structural characteristics and anticancer activity of fucoidan from the brown alga Sargassum mcclurei. Mar. Drugs 2013, 11, 1456–1476. [Google Scholar] [CrossRef] [Green Version]
- De Reviers, B. Fucans and alginates without phenolic compounds. J. Appl. Phycol. 1989, 1, 75–76. [Google Scholar] [CrossRef]
- Yang, W.N.; Chen, P.W.; Huang, C.Y. Compositional characteristics and in vitro evaluations of antioxidant and neuroprotective properties of crude extracts of fucoidan prepared from compressional puffing-pretreated Sargassum crassifolium. Mar. Drugs 2017, 15, 183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, R.; Zhang, X.; Tang, Y.; Mao, J. Composition, isolation, purification and biological activities of Sargassum fusiforme polysaccharides: A review. Carbohydr. Polym. 2020, 228, 115381. [Google Scholar] [CrossRef] [PubMed]
- Spicer, S.E.; Adams, J.M.M.; Thomas, D.S.; Gallagher, J.A.; Winters, A.L. Novel rapid method for the characterisation of polymeric sugars from macroalgae. J. Appl. Phycol. 2017, 29, 1507–1513. [Google Scholar] [CrossRef] [Green Version]
- Zou, P.; Lu, X.; Zhao, H.; Yuan, Y.; Meng, L.; Zhang, C.; Li, Y. Polysaccharides derived from the brown algae Lessonia nigrescens enhance salt stress tolerance to wheat seedlings by enhancing the antioxidant system and modulating intracellular ion concentration. Front. Plant Sci. 2019, 10, 48. [Google Scholar] [CrossRef] [Green Version]
- Peng, J.; Yuan, J.-P.; Wu, C.-F.; Wang, J.-H. Fucoxanthin, a marine carotenoid present in brown seaweeds and diatoms: Metabolism and bioactivities relevant to human health. Mar. Drugs 2011, 9, 1806–1828. [Google Scholar] [CrossRef]
- Da Costa, E.; Domingues, P.; Melo, T.; Coelho, E.; Pereira, R.; Calado, R.; Abreu, M.H.; Domingues, M.R. Lipidomic signatures reveal seasonal shifts on the relative abundance of high-valued lipids from the brown algae Fucus vesiculosus. Mar. Drugs 2019, 17, 335. [Google Scholar] [CrossRef] [Green Version]
- Terasaki, M.; Hirose, A.; Narayan, B.; Baba, Y.; Kawagoe, C.; Yasui, H.; Saga, N.; Hosokawa, M.; Miyashita, K. Evaluation of recoverable functional lipid components of several brown seaweeds (Phaeophyta) from Japan with special reference to fucoxanthin and fucosterol contents. J. Phycol. 2009, 45, 974–980. [Google Scholar] [CrossRef]
- Zhu, F. Interactions between cell wall polysaccharides and polyphenols. Crit. Rev. Food Sci. Nutr. 2018, 58, 1808–1831. [Google Scholar] [CrossRef]
- Li, Y.; Fu, X.; Duan, D.; Liu, X.; Xu, J.; Gao, X. Extraction and Identification of Phlorotannins from the Brown Alga, Sargassum fusiforme (Harvey) Setchell. Mar. Drugs 2017, 15, 49. [Google Scholar] [CrossRef] [Green Version]
- Bertoni, G. A key step in phlorotannin biosynthesis revealed. Plant Cell 2013, 25, 2770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Catarino, M.D.; Silva, A.M.S.; Cardoso, S.M. Fucaceae: A source of bioactive phlorotannins. Int. J. Mol. Sci. 2017, 18, 1327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, N.V.; Kim, S.K. Potential pharmacological applications of polyphenolic derivatives from marine brown algae. Environ. Toxicol. Pharmacol. 2011, 32, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Agregán, R.; Munekata, P.E.S.; Franco, D.; Carballo, J.; Barba, F.J.; Lorenzo, J.M. Antioxidant potential of extracts obtained from macro- (Ascophyllum nodosum, Fucus vesiculosus and Bifurcaria bifurcata) and micro-Algae (Chlorella vulgaris and Spirulina platensis) assisted by ultrasound. Medicines 2018, 5, 33. [Google Scholar]
- Zhang, M.Y.; Guo, J.; Hu, X.M.; Zhao, S.Q.; Li, S.L.; Wang, J. An in vivo anti-tumor effect of eckol from marine brown algae by improving the immune response. Food Funct. 2019, 10, 4361–4371. [Google Scholar] [CrossRef]
- Gall, E.A.; Lelchat, F.; Hupel, M.; Jégou, C.; Stiger-Pouvreau, V. Extraction and purification of phlorotannins from brown algae. In Natural Products from Marine Algae: Methods and Protocols; Stengel, D.B., Connan, S., Eds.; Springer: New York, NY, USA, 2015; pp. 131–143. [Google Scholar]
- Brzonova, I.; Kozliak, E.I.; Andrianova, A.A.; LaVallie, A.; Kubátová, A.; Ji, Y. Production of lignin based insoluble polymers (anionic hydrogels) by C. versicolor. Sci. Rep. 2017, 7, 17507. [Google Scholar] [CrossRef]
- Hahn, T.; Zayed, A.; Kovacheva, M.; Stadtmüller, R.; Lang, S.; Muffler, K.; Ulber, R. Dye affinity chromatography for fast and simple purification of fucoidan from marine brown algae. Eng. Life Sci. 2016, 16, 78–87. [Google Scholar] [CrossRef] [Green Version]
- Chades, T.; Scully, S.M.; Ingvadottir, E.M.; Orlygsson, J. Fermentation of mannitol extracts from brown macro algae by Thermophilic clostridia. Front. Microbiol. 2018, 9, 1931. [Google Scholar] [CrossRef]
- Balboa, E.M.; Rivas, S.; Moure, A.; Dominguez, H.; Parajo, J.C. Simultaneous extraction and depolymerization of fucoidan from Sargassum muticum in aqueous media. Mar. Drugs 2013, 11, 4612–4627. [Google Scholar] [CrossRef] [Green Version]
- Descamps, V.; Colin, S.; Lahaye, M.; Jam, M.; Richard, C.; Potin, P.; Barbeyron, T.; Yvin, J.-C.; Kloareg, B. Isolation and culture of a marine bacterium degrading the sulfated fucans from marine brown algae. Mar. Biotechnol. 2006, 8, 27–39. [Google Scholar] [CrossRef] [Green Version]
- Abdella, A.A.; Ulber, R.; Zayed, A. Chitosan-toluidine blue beads for purification of fucoidans. Carbohydr. Polym. 2020, 231, 115686. [Google Scholar] [CrossRef] [PubMed]
- Kadam, S.U.; Tiwari, B.K.; O’Donnell, C.P. Application of novel extraction technologies for bioactives from marine algae. J. Agric. Food Chem. 2013, 61, 4667–4675. [Google Scholar] [CrossRef]
- Pozharitskaya, O.N.; Shikov, A.N.; Faustova, N.M.; Obluchinskaya, E.D.; Kosman, V.M.; Vuorela, H.; Makarov, V.G. Pharmacokinetic and tissue distribution of fucoidan from Fucus vesiculosus after oral administration to rats. Mar. Drugs 2018, 16, 132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hadj Ammar, H.; Lajili, S.; Ben Said, R.; Le Cerf, D.; Bouraoui, A.; Majdoub, H. Physico-chemical characterization and pharmacological evaluation of sulfated polysaccharides from three species of Mediterranean brown algae of the genus Cystoseira. DARU J. Pharm. Sci. 2015, 23, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Xu, M.; Hu, C.; Liu, A.; Chen, J.; Gu, C.; Zhang, X.; You, C.; Tong, H.; Wu, M.; et al. Sargassum fusiforme fucoidan SP2 extends the lifespan of Drosophila melanogaster by upregulating the Nrf2-mediated antioxidant signaling pathway. Oxidative Med. Cell. Longev. 2019, 2019, 8918914. [Google Scholar] [CrossRef] [Green Version]
- Saepudin, E.; Sinurat, E.; Suryabrata, I.A. Depigmentation and characterization of fucoidan from brown seaweed Sargassum binderi Sonder. IOP Conference Series: Mater. Sci. Eng. 2018, 299, 012027. [Google Scholar] [CrossRef]
- Patel, A.; Mikes, F.; Matsakas, L. An overview of current pretreatment methods used to improve lipid extraction from Oleaginous micro-organisms. Molecules 2018, 23, 1562. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.-Y.; Kuo, C.-H.; Chen, P.-W. Compressional-puffing pretreatment enhances neuroprotective effects of fucoidans from the brown seaweed Sargassum hemiphyllum on 6-hydroxydopamine-induced apoptosis in SH-SY5Y cells. Molecules 2017, 23, 78. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.-Y.; Wu, S.-J.; Yang, W.-N.; Kuan, A.-W.; Chen, C.-Y. Antioxidant activities of crude extracts of fucoidan extracted from Sargassum glaucescens by a compressional-puffing-hydrothermal extraction process. Food Chem. 2016, 197, 1121–1129. [Google Scholar] [CrossRef]
- Kordjazi, M.; Shabanpour, B.; Zabihi, E.; Faramarzi, M.A.; Feizi, F.; Ahmadi Gavlighi, H.; Feghhi, M.A.; Hosseini, S.A. Sulfated polysaccharides purified from two species of Padina improve collagen and epidermis formation in the rat. Int. J. Mol. Cell. Med. 2013, 2, 156–163. [Google Scholar]
- Cho, M.L.; Lee, B.-Y.; You, S.G. Relationship between oversulfation and conformation of low and high molecular weight fucoidans and evaluation of their in vitro anticancer activity. Molecules 2010, 16, 291–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, R.M.; Câmara, R.B.G.; Monte, J.F.S.; Viana, R.L.S.; Melo, K.R.T.; Queiroz, M.F.; Filgueira, L.G.A.; Oyama, L.M.; Rocha, H.A.O. Commercial fucoidans from Fucus vesiculosus can be grouped into antiadipogenic and adipogenic agents. Mar. Drugs 2018, 16, 193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kusaykin, M.I.; Silchenko, A.S.; Zakharenko, A.M.; Zvyagintseva, T.N. Fucoidanases. Glycobiology 2015, 26, 3–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ale, M.T.; Meyer, A.S. Fucoidans from brown seaweeds: An update on structures, extraction techniques and use of enzymes as tools for structural elucidation. RSC Adv. 2013, 3, 8131–8141. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.-Y.; Kuo, C.-H.; Lee, C.-H. Antibacterial and antioxidant capacities and attenuation of lipid accumulation in 3T3-L1 adipocytes by low-molecular-weight fucoidans prepared from compressional-puffing-pretreated Sargassum crassifolium. Mar. Drugs 2018, 16, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imbs, T.I.; Shevchenko, N.M.; Sukhoverkhov, S.V.; Semenova, T.L.; Skriptsova, A.V.; Zvyagintseva, T.N. Seasonal variations of the composition and structural characteristics of polysaccharides from the brown alga Costaria costata. Chem. Nat. Compd. 2009, 45, 786–791. [Google Scholar] [CrossRef]
- Fidelis, G.P.; Silva, C.H.F.; Nobre, L.; Medeiros, V.P.; Rocha, H.A.O.; Costa, L.S. Antioxidant fucoidans obtained from tropical seaweed protect pre-osteoblastic cells from hydrogen peroxide-induced damage. Mar. Drugs 2019, 17, 506. [Google Scholar] [CrossRef] [Green Version]
- Rohwer, K.; Neupane, S.; Bittkau, K.S.; Galarza Perez, M.; Dorschmann, P.; Roider, J.; Alban, S.; Klettner, A. Effects of Crude Fucus distichus Subspecies evanescens Fucoidan Extract on Retinal Pigment Epithelium Cells-Implications for Use in Age-Related Macular Degeneration. Mar. Drugs 2019, 17, 538. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Jasso, R.M.; Mussatto, S.I.; Pastrana, L.; Aguilar, C.N.; Teixeira, J.A. Microwave-assisted extraction of sulfated polysaccharides (fucoidan) from brown seaweed. Carbohydr. Polym. 2011, 86, 1137–1144. [Google Scholar] [CrossRef] [Green Version]
- Mussatto, S.I. Microwave-assisted extraction of fucoidan from marine algae. In Natural Products from Marine Algae: Methods and Protocols; Stengel, D.B., Connan, S., Eds.; Springer: New York, NY, USA, 2015; pp. 151–157. [Google Scholar]
- Alboofetileh, M.; Rezaei, M.; Tabarsa, M.; You, S. Ultrasound-assisted extraction of sulfated polysaccharide from Nizamuddinia zanardinii: Process optimization, structural characterization, and biological properties. J. Food Process Eng. 2019, 42, e12979. [Google Scholar] [CrossRef]
- Garcia-Vaquero, M.; O’Doherty, J.V.; Tiwari, B.K.; Sweeney, T.; Rajauria, G. Enhancing the extraction of polysaccharides and antioxidants from macroalgae using sequential hydrothermal-assisted extraction followed by ultrasound and thermal technologies. Mar. Drugs 2019, 17, 457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alboofetileh, M.; Rezaei, M.; Tabarsa, M.; You, S.; Mariatti, F.; Cravotto, G. Subcritical water extraction as an efficient technique to isolate biologically-active fucoidans from Nizamuddinia zanardinii. Int. J. Biol. Macromol. 2019, 128, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Yuan, Q.; Zhang, Y.; Li, J.; Zhu, X.; Zhao, L.; Wen, J.; Liu, J.; Zhao, L.; Zhao, J. Enzyme-assisted extraction optimization, characterization and antioxidant activity of polysaccharides from sea cucumber Phyllophorus proteus. Molecules 2018, 23, 590. [Google Scholar] [CrossRef] [Green Version]
- Wijesinghe, W.A.; Jeon, Y.J. Enzyme-assistant extraction (EAE) of bioactive components: A useful approach for recovery of industrially important metabolites from seaweeds: A review. Fitoterapia 2012, 83, 6–12. [Google Scholar] [CrossRef]
- Ahn, G.; Lee, W.; Kim, K.-N.; Lee, J.-H.; Heo, S.-J.; Kang, N.; Lee, S.-H.; Ahn, C.-B.; Jeon, Y.-J. A sulfated polysaccharide of Ecklonia cava inhibits the growth of colon cancer cells by inducing apoptosis. EXCLI J. 2015, 14, 294–306. [Google Scholar]
- Badrinathan, S.; Shiju, T.M.; Sharon Christa, A.S.; Arya, R.; Pragasam, V. Purification and structural characterization of sulfated polysaccharide from Sargassum myriocystum and its efficacy in scavenging free radicals. Indian J. Pharm. Sci. 2012, 74, 549–555. [Google Scholar] [PubMed] [Green Version]
- Liu, Y.; Huang, G. Extraction and derivatisation of active polysaccharides. J. Enzym. Inhib. Med. Chem. 2019, 34, 1690–1696. [Google Scholar] [CrossRef]
- Zhao, D.; Xu, J.; Xu, X. Bioactivity of fucoidan extracted from Laminaria japonica using a novel procedure with high yield. Food Chem. 2018, 245, 911–918. [Google Scholar] [CrossRef]
- Xing, R.; Liu, S.; Yu, H.; Chen, X.; Qin, Y.; Li, K.; Li, P. Extraction and separation of fucoidan from Laminaria japonica with chitosan as extractant. BioMed Res. Int. 2013, 2013, 193689. [Google Scholar] [CrossRef] [Green Version]
- Ertani, A.; Francioso, O.; Tinti, A.; Schiavon, M.; Pizzeghello, D.; Nardi, S. Evaluation of seaweed extracts from Laminaria and Ascophyllum nodosum spp. as biostimulants in Zea mays L. using a combination of chemical, biochemical and morphological approaches. Front. Plant Sci. 2018, 9, 428. [Google Scholar] [CrossRef]
- Lee, J.M.; Oh, S.Y.; Johnston, T.V.; Ku, S.; Ji, G.E. Biocatalysis of fucodian in Undaria pinnatifida sporophyll using Bifidobacterium longum RD47 for production of prebiotic fucosylated oligosaccharide. Mar. Drugs 2019, 17, 117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Somasundaram, S.N.; Shanmugam, S.; Subramanian, B.; Jaganathan, R. Cytotoxic effect of fucoidan extracted from Sargassum cinereum on colon cancer cell line HCT-15. Int. J. Biol. Macromol. 2016, 91. [Google Scholar] [CrossRef] [PubMed]
- Ustyuzhanina, N.E.; Ushakova, N.A.; Zyuzina, K.A.; Bilan, M.I.; Elizarova, A.L.; Somonova, O.V.; Madzhuga, A.V.; Krylov, V.B.; Preobrazhenskaya, M.E.; Usov, A.I.; et al. Influence of fucoidans on hemostatic system. Mar. Drugs 2013, 11, 2444–2458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saboural, P.; Chaubet, F.; Rouzet, F.; Al-Shoukr, F.; Azzouna, R.B.; Bouchemal, N.; Picton, L.; Louedec, L.; Maire, M.; Rolland, L.; et al. Purification of a low molecular weight fucoidan for SPECT molecular imaging of myocardial infarction. Mar. Drugs 2014, 12, 4851–4867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Vaquero, M.; Rajauria, G.; O’Doherty, J.V.; Sweeney, T. Polysaccharides from macroalgae: Recent advances, innovative technologies and challenges in extraction and purification. Food Res. Int. 2017, 99, 1011–1020. [Google Scholar] [CrossRef] [Green Version]
- Hahn, T.; Schulz, M.; Stadtmüller, R.; Zayed, A.; Muffler, K.; Lang, S.; Ulber, R. Cationic dye for the specific determination of sulfated polysaccharides. Anal. Lett. 2016, 49, 1948–1962. [Google Scholar] [CrossRef]
- Lee, J.M.; Shin, Z.U.; Mavlonov, G.T.; Abdurakhmonov, I.Y.; Yi, T.-H. Solid-phase colorimetric method for the quantification of fucoidan. Appl. Biochem. Biotechnol. 2012, 168, 1019–1024. [Google Scholar] [CrossRef] [PubMed]
- Palanisamy, S.; Vinosha, M.; Manikandakrishnan, M.; Anjali, R.; Rajasekar, P.; Marudhupandi, T.; Manikandan, R.; Vaseeharan, B.; Prabhu, N.M. Investigation of antioxidant and anticancer potential of fucoidan from Sargassum polycystum. Int. J. Biol. Macromol. 2018, 116, 151–161. [Google Scholar] [CrossRef]
- Cong, Q.; Chen, H.; Liao, W.; Xiao, F.; Wang, P.; Qin, Y.; Dong, Q.; Ding, K. Structural characterization and effect on anti-angiogenic activity of a fucoidan from Sargassum fusiforme. Carbohydr. Polym. 2016, 136, 899–907. [Google Scholar] [CrossRef]
- Li, G.; Row, K.H. Magnetic molecularly imprinted polymers for recognition and enrichment of polysaccharides from seaweed. J. Sep. Sci. 2017, 40, 4765–4772. [Google Scholar] [CrossRef]
- Guthrie, L.; Wolfson, S.; Kelly, L. The human gut chemical landscape predicts microbe-mediated biotransformation of foods and drugs. eLife 2019, 8, e42866. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Q.; Zhang, Z.; Song, H.; Li, P. Potential antioxidant and anticoagulant capacity of low molecular weight fucoidan fractions extracted from Laminaria japonica. Int. J. Biol. Macromol. 2010, 46, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.B.; Hayashi, K.; Hashimoto, M.; Nakano, T.; Hayashi, T. Novel antiviral fucoidan from sporophyll of Undaria pinnatifida (Mekabu). Chem. Pharm. Bull. 2004, 52, 1091–1094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elizondo-Gonzalez, R.; Cruz-Suarez, L.E.; Ricque-Marie, D.; Mendoza-Gamboa, E.; Rodriguez-Padilla, C.; Trejo-Avila, L.M. In vitro characterization of the antiviral activity of fucoidan from Cladosiphon okamuranus against Newcastle disease virus. Virol. J. 2012, 9, 307. [Google Scholar] [CrossRef] [Green Version]
- Jeong, J.-W.; Hwang, S.J.; Han, M.H.; Lee, D.-S.; Yoo, J.S.; Choi, I.-W.; Cha, H.-J.; Kim, S.; Kim, H.-S.; Kim, G.-Y.; et al. Fucoidan inhibits lipopolysaccharide-induced inflammatory responses in RAW 264.7 macrophages and zebrafish larvae. Mol. Cell. Toxicol. 2017, 13, 405–417. [Google Scholar] [CrossRef]
- Klettner, A. Fucoidan as a potential therapeutic for major blinding diseases - a hypothesis. Mar. Drugs 2016, 14, 31. [Google Scholar] [CrossRef] [Green Version]
- Barbosa, A.I.; Coutinho, A.J.; Costa Lima, S.A.; Reis, S. Marine polysaccharides in pharmaceutical applications: Fucoidan and chitosan as key players in the drug delivery match field. Mar. Drugs 2019, 17, 654. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Kankala, R.K.; Fan, J.; Long, R.; Liu, Y.; Wang, S. Poly-L-ornithine/fucoidan-coated calcium carbonate microparticles by layer-by-layer self-assembly technique for cancer theranostics. J. Mater. Sci. Mater. Med. 2018, 29, 68. [Google Scholar] [CrossRef]
- Wang, X.; Shan, X.; Dun, Y.; Cai, C.; Hao, J.; Li, G.; Cui, K.; Yu, G. Anti-metabolic syndrome effects of fucoidan from Fucus vesiculosus via reactive oxygen species-mediated regulation of JNK, Akt, and AMPK signaling. Molecules 2019, 24, 3319. [Google Scholar] [CrossRef] [Green Version]
- Sharma, G.; Kar, S.; Basu Ball, W.; Ghosh, K.; Das, P.K. The curative effect of fucoidan on visceral leishmaniasis is mediated by activation of MAP kinases through specific protein kinase C isoforms. Cell. Mol. Immunol. 2014, 11, 263–274. [Google Scholar] [CrossRef] [Green Version]
- Varikuti, S.; Jha, B.K.; Volpedo, G.; Ryan, N.M.; Halsey, G.; Hamza, O.M.; McGwire, B.S.; Satoskar, A.R. Host-directed drug therapies for neglected tropical diseases caused by protozoan parasites. Front. Microbiol. 2018, 9, 2655. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.O.; Zhang, W.; Du, J.Y.; Wong, K.W.; Oda, T.; Yu, Q. Fucoidan can function as an adjuvant in vivo to enhance dendritic cell maturation and function and promote antigen-specific T cell immune responses. PLoS ONE 2014, 9, e99396. [Google Scholar] [CrossRef] [PubMed]
- Tabarsa, M.; Dabaghian, E.H.; You, S.; Yelithao, K.; Cao, R.; Rezaei, M.; Alboofetileh, M.; Bita, S. The activation of NF-κB and MAPKs signaling pathways of RAW264.7 murine macrophages and natural killer cells by fucoidan from Nizamuddinia zanardinii. Int. J. Biol. Macromol. 2020, 148, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Liu, Z.; Liu, X.; Teng, H.; Zhang, C.; Hou, L.; Zou, X. Anti-metastasis effect of fucoidan from Undaria pinnatifida sporophylls in mouse hepatocarcinoma Hca-F cells. PLoS ONE 2014, 9, e106071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Zhao, W.; Wang, L.; Chen, Y.; Zhang, H.; Wang, T.; Yang, X.; Xing, F.; Yan, J.; Fang, X. Protective effects of fucoidan against hydrogen peroxide-induced oxidative damage in porcine intestinal epithelial cells. Animals 2019, 9, 1108. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.H.; Lim, J.D.; Sohn, E.H.; Choi, Y.S.; Han, E.T. Growth-inhibitory effect of a fucoidan from brown seaweed Undaria pinnatifida on Plasmodium parasites. Parasitol. Res. 2009, 104, 245–250. [Google Scholar] [CrossRef]
- Jia, Y.; Sun, Y.; Weng, L.; Li, Y.; Zhang, Q.; Zhou, H.; Yang, B. Low molecular weight fucoidan protects renal tubular cells from injury induced by albumin overload. Sci. Rep. 2016, 6, 31759. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.-H.; Sue, Y.-M.; Cheng, C.-Y.; Chen, Y.-C.; Liu, C.-T.; Hsu, Y.-H.; Hwang, P.-A.; Huang, N.-J.; Chen, T.-H. Oligo-fucoidan prevents renal tubulointerstitial fibrosis by inhibiting the CD44 signal pathway. Sci. Rep. 2017, 7, 40183. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Daim, M.M.; Abushouk, A.I.; Bahbah, E.I.; Bungău, S.G.; Alyousif, M.S.; Aleya, L.; Alkahtani, S. Fucoidan protects against subacute diazinon-induced oxidative damage in cardiac, hepatic, and renal tissues. Environ. Sci. Pollut. Res. 2020. Online ahead of print. [Google Scholar] [CrossRef]
- Chen, M.-C.; Hsu, W.-L.; Hwang, P.-A.; Chou, T.-C. Low molecular weight fucoidan inhibits tumor angiogenesis through downregulation of HIF-1/VEGF signaling under hypoxia. Mar. Drugs 2015, 13, 4436–4451. [Google Scholar] [CrossRef]
- Marinval, N.; Saboural, P.; Haddad, O.; Maire, M.; Bassand, K.; Geinguenaud, F.; Djaker, N.; Ben Akrout, K.; Lamy de la Chapelle, M.; Robert, R.; et al. Identification of a pro-angiogenic potential and cellular uptake mechanism of a LMW highly sulfated fraction of fucoidan from Ascophyllum nodosum. Mar. Drugs 2016, 14, 185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, Y.; Sibusiso, L.; Hou, L.; Jiang, H.; Chen, P.; Zhang, X.; Wu, M.; Tong, H. Sargassum fusiforme fucoidan modifies the gut microbiota during alleviation of streptozotocin-induced hyperglycemia in mice. Int. J. Biol. Macromol. 2019, 131, 1162–1170. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Juenet, M.; Aid-Launais, R.; Maire, M.; Ollivier, V.; Letourneur, D.; Chauvierre, C. Development of polymer microcapsules functionalized with fucoidan to target p-selectin overexpressed in cardiovascular diseases. Adv. Healthc. Mater. 2017, 6, 1601200. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-I.; Oh, W.-S.; Song, P.H.; Yun, S.; Kwon, Y.-S.; Lee, Y.J.; Ku, S.-K.; Song, C.-H.; Oh, T.-H. Anti-photoaging effects of low molecular-weight fucoidan on ultraviolet B-irradiated mice. Mar. Drugs 2018, 16, 286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.H.; Lee, J.-E.; Kim, K.H.; Kang, N.J. Beneficial effects of marine algae-derived carbohydrates for skin health. Mar. Drugs 2018, 16, 459. [Google Scholar] [CrossRef] [Green Version]
- Fitton, J.H.; Dell’Acqua, G.; Gardiner, V.-A.; Karpiniec, S.S.; Stringer, D.N.; Davis, E. Topical benefits of two fucoidan-rich extracts from marine macroalgae. Cosmetics 2015, 2, 66–81. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.S.; Li, H.; Balcos, M.C.; Yun, H.-Y.; Baek, K.J.; Kwon, N.S.; Choi, H.-R.; Park, K.-C.; Kim, D.-S. Fucoidan promotes the reconstruction of skin equivalents. Korean J. Physiol. Pharmacol. 2014, 18, 327–331. [Google Scholar] [CrossRef]
- Venkatesan, J.; Anil, S.; Kim, S.-K.; Shim, M.S. Seaweed polysaccharide-based nanoparticles: Preparation and applications for drug delivery. Polymers 2016, 8, 30. [Google Scholar] [CrossRef] [Green Version]
- Pinheiro, A.C.; Bourbon, A.I.; Cerqueira, M.A.; Maricato, É.; Nunes, C.; Coimbra, M.A.; Vicente, A.A. Chitosan/fucoidan multilayer nanocapsules as a vehicle for controlled release of bioactive compounds. Carbohydr. Polym. 2015, 115, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Choi, D.G.; Venkatesan, J.; Shim, M.S. Selective anticancer therapy using pro-oxidant drug-loaded chitosan-fucoidan nanoparticles. Int. J. Mol. Sci. 2019, 20, 3220. [Google Scholar] [CrossRef] [Green Version]
- Tsai, M.-h.; Chuang, C.-c.; Chen, C.-c.; Yen, H.-j.; Cheng, K.-m.; Chen, X.-a.; Shyu, H.-f.; Lee, C.-y.; Young, J.-j.; Kau, J.-h. Nanoparticles assembled from fucoidan and trimethylchitosan as anthrax vaccine adjuvant: In vitro and in vivo efficacy in comparison to CpG. Carbohydr. Polym. 2020, 236, 116041. [Google Scholar] [CrossRef]
- Venkatesan, J.; Singh, S.K.; Anil, S.; Kim, S.-K.; Shim, M.S. Preparation, characterization and biological applications of biosynthesized silver nanoparticles with chitosan-fucoidan coating. Molecules 2018, 23, 1429. [Google Scholar] [CrossRef] [Green Version]
- Hwang, P.-A.; Yan, M.-D.; Lin, H.-T.V.; Li, K.-L.; Lin, Y.-C. Toxicological evaluation of low molecular weight fucoidan in vitro and in vivo. Mar. Drugs 2016, 14, 121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Z.; Zhu, B.; Ai, C.; Lu, J.; Wu, S.; Liu, Y.; Wang, L.; Yang, J.; Song, S.; Liu, X. Development and application of a HPLC-MS/MS method for quantitation of fucosylated chondroitin sulfate and fucoidan in sea cucumbers. Carbohydr. Res. 2018, 466, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Xue, C.; Chang, Y.; Xu, X.; Ge, L.; Liu, G.; Wang, Y. Structure elucidation of fucoidan composed of a novel tetrafucose repeating unit from sea cucumber Thelenota ananas. Food Chem. 2014, 146, 113–119. [Google Scholar] [CrossRef]
- Zhao, X.; Guo, F.; Hu, J.; Zhang, L.; Xue, C.; Zhang, Z.; Li, B. Antithrombotic activity of oral administered low molecular weight fucoidan from Laminaria Japonica. Thromb. Res. 2016, 144, 46–52. [Google Scholar] [CrossRef]
- Tsai, H.L.; Tai, C.J.; Huang, C.W.; Chang, F.R.; Wang, J.Y. Efficacy of low-molecular-weight fucoidan as a supplemental therapy in metastatic colorectal cancer patients: A double-blind randomized controlled trial. Mar. Drugs 2017, 15, 122. [Google Scholar] [CrossRef] [Green Version]
- Jonsson, M.; Allahgholi, L.; Sardari, R.R.R.; Hreggviethsson, G.O.; Nordberg Karlsson, E. Extraction and modification of macroalgal polysaccharides for current and next-generation applications. Molecules 2020, 25, 930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cumashi, A.; Ushakova, N.A.; Preobrazhenskaya, M.E.; D’Incecco, A.; Piccoli, A.; Totani, L.; Tinari, N.; Morozevich, G.E.; Berman, A.E.; Bilan, M.I.; et al. A comparative study of the anti-inflammatory, anticoagulant, antiangiogenic, and antiadhesive activities of nine different fucoidans from brown seaweeds. Glycobiology 2007, 17, 541–552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silchenko, A.S.; Ustyuzhanina, N.E.; Kusaykin, M.I.; Krylov, V.B.; Shashkov, A.S.; Dmitrenok, A.S.; Usoltseva, R.V.; Zueva, A.O.; Nifantiev, N.E.; Zvyagintseva, T.N. Expression and biochemical characterization and substrate specificity of the fucoidanase from Formosa algae. Glycobiology 2017, 27, 254–263. [Google Scholar]
- Silchenko, A.S.; Rasin, A.B.; Kusaykin, M.I.; Kalinovsky, A.I.; Miansong, Z.; Changheng, L.; Malyarenko, O.; Zueva, A.O.; Zvyagintseva, T.N.; Ermakova, S.P. Structure, enzymatic transformation, anticancer activity of fucoidan and sulphated fucooligosaccharides from Sargassum horneri. Carbohydr. Polym. 2017, 175, 654–660. [Google Scholar] [CrossRef] [PubMed]
- Colin, S.; Deniaud, E.; Jam, M.; Descamps, V.; Chevolot, Y.; Kervarec, N.; Yvin, J.C.; Barbeyron, T.; Michel, G.; Kloareg, B. Cloning and biochemical characterization of the fucanase FcnA: Definition of a novel glycoside hydrolase family specific for sulfated fucans. Glycobiology 2006, 16, 1021–1032. [Google Scholar] [CrossRef] [PubMed]
- Nagao, T.; Arai, Y.; Yamaoka, M.; Komatsu, F.; Yagi, H.; Suzuki, H.; Ohshiro, T. Identification and characterization of the fucoidanase gene from Luteolibacter algae H18. J. Biosci. Bioeng. 2018, 126, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Bakunina, I.; Nedashkovskaia, O.I.; Alekseeva, S.A.; Ivanova, E.P.; Romanenko, L.A.; Gorshkova, N.M.; Isakov, V.V.; Zviagintseva, T.N.; Mikhailov, V.V. Degradation of fucoidan by the marine proteobacterium Pseudoalteromonas citrea. Mikrobiologiia 2002, 71, 49–55. [Google Scholar] [PubMed]
- Wu, Q.; Zhang, M.; Wu, K.; Liu, B.; Cai, J.; Pan, R. Purification and characteristics of fucoidanase obtained from Dendryphiella arenaria TM94. J. Appl. Phycol. 2011, 23, 197–203. [Google Scholar] [CrossRef]
- Sakai, T.; Kimura, H.; Kojima, K.; Shimanaka, K.; Ikai, K.; Kato, I. Marine bacterial sulfated fucoglucuronomannan (SFGM) lyase digests brown algal SFGM into trisaccharides. Mar. Biotechnol. 2003, 5, 70–78. [Google Scholar] [CrossRef]
- Sakai, T.; Ishizuka, K.; Shimanaka, K.; Ikai, K.; Kato, I. Structures of oligosaccharides derived from Cladosiphon okamuranus fucoidan by digestion with marine bacterial enzymes. Mar. Biotechnol. 2003, 5, 536–544. [Google Scholar]
- Ohshiro, T.; Ohmoto, Y.; Ono, Y.; Ohkita, R.; Miki, Y.; Kawamoto, H.; Izumi, Y. Isolation and characterization of a novel fucoidan-degrading microorganism. Biosci. Biotechnol. Biochem. 2010, 74, 1729–1732. [Google Scholar] [CrossRef] [Green Version]
- Bilan, M.I.; Kusaykin, M.I.; Grachev, A.A.; Tsvetkova, E.A.; Zvyagintseva, T.N.; Nifantiev, N.E.; Usov, A.I. Effect of enzyme preparation from the marine mollusk Littorina kurila on fucoidan from the brown alga Fucus distichus. Biochemistry 2005, 70, 1321–1326. [Google Scholar] [CrossRef]
- Kim, W.J.; Kim, S.M.; Lee, Y.H.; Kim, H.G.; Kim, H.K.; Moon, S.H.; Suh, H.H.; Jang, K.H.; Park, Y.I. Isolation and characterization of marine bacterial strain degrading fucoidan from korean Undaria pinnatifida Sporophylls. J. Microbiol. Biotechnol. 2008, 18, 616–623. [Google Scholar]
- Kim, W.J.; Park, J.W.; Park, J.K.; Choi, D.J.; Park, Y.I. Purification and characterization of a fucoidanase (FNase S) from a marine bacterium Sphingomonas paucimobilis PF-1. Mar. Drugs 2015, 13, 4398–4417. [Google Scholar] [CrossRef] [Green Version]
- Kitamura, K.; Matsuo, M.; Tsuneo, Y. Enzymic degradation of fucoidan by fucoidanase from the hepatopancreas of Patinopecten yessoensis. Biosci. Biotechnol. Biochem. 1992, 56, 490–494. [Google Scholar] [CrossRef]
- Daniel, R.; Berteau, O.; Chevolot, L.; Varenne, A.; Gareil, P.; Goasdoue, N. Regioselective desulfation of sulfated l-fucopyranoside by a new sulfoesterase from the marine mollusk Pecten maximus. Eur. J. Biochem. 2001, 268, 5617–5626. [Google Scholar] [CrossRef]
- Berteau, O.; McCort, I.; Goasdoué, N.; Tissot, B.; Daniel, R. Characterization of a new alpha-L-fucosidase isolated from the marine mollusk Pecten maximus that catalyzes the hydrolysis of alpha-L-fucose from algal fucoidan (Ascophyllum nodosum). Glycobiology 2002, 12, 273–282. [Google Scholar] [CrossRef] [Green Version]
- Dong, S.; Chang, Y.; Shen, J.; Xue, C.; Chen, F. Purification, expression and characterization of a novel α-l-fucosidase from a marine bacteria Wenyingzhuangia fucanilytica. Protein Expr. Purif. 2017, 129, 9–17. [Google Scholar] [CrossRef]
- Tanaka, R.; Mizutani, Y.; Shibata, T.; Miyake, H.; Iehata, S.; Mori, T.; Kuroda, K.; Ueda, M. Genome sequence of Formosa haliotis strain MA1, a brown alga-degrading bacterium isolated from the gut of Abalone Haliotis gigantea. Genome Announc. 2016, 4, e01312-16. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.; Chang, Y.; Dong, S.; Xue, C. Wenyingzhuangia fucanilytica sp. nov., a sulfated fucan utilizing bacterium isolated from shallow coastal seawater. Int. J. Syst. Evol. Microbiol. 2016, 66, 3270–3275. [Google Scholar] [CrossRef]
- Li, J.; Cheng, Y.; Wang, D.; Li, J.; Wang, Y.; Han, W.; Li, F. Draft genome sequence of the polysaccharide-degrading marine bacterium Pseudoalteromonas sp. Strain A601. Genome Announc. 2017, 5, e00590-17. [Google Scholar] [CrossRef] [Green Version]
- Cao, H.T.T.; Mikkelsen, M.D.; Lezyk, M.J.; Bui, L.M.; Tran, V.T.T.; Silchenko, A.S.; Kusaykin, M.I.; Pham, T.D.; Truong, B.H.; Holck, J.; et al. Novel enzyme actions for sulphated galactofucan depolymerisation and a new engineering strategy for molecular stabilisation of fucoidan degrading enzymes. Mar. Drugs 2018, 16, 422. [Google Scholar] [CrossRef] [Green Version]
- Tran, P.H.L.; Duan, W.; Tran, T.T.D. Fucoidan-based nanostructures: A focus on its combination with chitosan and the surface functionalization of metallic nanoparticles for drug delivery. Int. J. Pharm. 2019, 575, 118956. [Google Scholar] [CrossRef]
- Jesumani, V.; Du, H.; Pei, P.; Aslam, M.; Huang, N. Comparative study on skin protection activity of polyphenol-rich extract and polysaccharide-rich extract from Sargassum vachellianum. PLoS ONE 2020, 15, e0227308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunt, E.G.; Burgess, J.G. The promise of marine molecules as cosmetic active ingredients. Int. J. Cosmet. Sci. 2018, 40, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, R.M.; Ramalho Ribeiro, A.; Patinha, C.; Silva, A.M.S.; Cardoso, S.M.; Costa, R. Water extraction kinetics of bioactive compounds of Fucus vesiculosus. Molecules 2019, 24, 3408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michel, G.; Tonon, T.; Scornet, D.; Cock, J.M.; Kloareg, B. The cell wall polysaccharide metabolism of the brown alga Ectocarpus siliculosus. Insights into the evolution of extracellular matrix polysaccharides in eukaryotes. New Phytol. 2010, 188, 82–97. [Google Scholar] [CrossRef]
- Chi, S.; Liu, T.; Wang, X.; Wang, R.; Wang, S.; Wang, G.; Shan, G.; Liu, C. Functional genomics analysis reveals the biosynthesis pathways of important cellular components (alginate and fucoidan) of Saccharina. Curr. Genet. 2018, 64, 259–273. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.; Zhang, P.; Lu, C.; Li, S.; Chen, Z.; Wang, X.; Duan, D. Transcriptome sequencing of Saccharina Japonica Sporophytes during whole developmental periods reveals regulatory networks underlying alginate and mannitol biosynthesis. BMC Genom. 2019, 20, 975. [Google Scholar] [CrossRef] [Green Version]



| Application | Biogenic Source | Quality Grade/Purification Method | Structural Features | Involved Mechanism | Ref. |
|---|---|---|---|---|---|
| Therapeutic | |||||
| Anti-viral (IAV) | Kjellmaniella crassifolia (Laminariales) | * | Inhibition of the viral neuraminidase (NA) Interference with the cellular EGFR pathway | [43] | |
| Anti-metabolic syndrome | Fucus vesiculosus (Fucales) | Dialysis of crude alginate-free fucoidans | Alternating α(1→3)/α(1→4)linked fucose, Mw > 7.0 × 103 g/mol | Regulation of jnk, akt, and ampk signaling Alleviation of insulin resistance Regulation of lipid metabolism | [151] |
| Anti-leishmaniasis | Commercial product purchased from Sigma-Aldrich® | Polymer of α-(1→3) linked fucose | Activation of the mitogen-activated protein kinase (MAPK)/NF-κB pathway against Leishmania donovani-infected macrophages | [152] | |
| Enhancement of dendritic cells maturation, production of pro-inflammatory cytokines, and down-regulation of anti-inflammatory cytokines | [153,154] | ||||
| Immunostimulant | Nizamuddinia zanardinii (Fucales) | A fraction of DEAE Sepharose Fast Flow column | Highly branched polymer Mw: 953.6 × 103 g/mol | Stimulation of RAW264.7 murine macrophage and NK cells | [155] |
| Anti-metastasis | Undaria pinnatifida (Laminariales) | DEAE-cellulose, and Sephadex G-100 column chromatography (purity>90%) | Mw: of 10.4356 × 104 g/mol | - Suppression of Hca-F cell growth, adhesion, invasion, and metastasis capabilities, - Inactivation of the NF-κB pathway | [156] |
| Gastrointestinal tract protective | Purity ≥ 95% (Commercial product purchased from Sigma-Aldrich®) | Protection against H2O2-induced damage via activation of the NRF2 signaling pathway | [157] | ||
| Anti-malaria | - Partial purification by cetylpyridinum chloride Fractionation by DEAE-Sephadex A-25 column | Sugar monomers, and uronic acid, M.wt: approx. 15 × 103 g/mol | In-vitro and in-vivo inhibition of erythrocytes invasion by P. falciparum merozoites | [158] | |
| Renal protective | Laminaria japonica (Laminariales) | LMWF (Mw: 7 x 103 g/mol) | Inhibition of overexpression of pro-inflammatory and pro-fibrotic factors, oxidative stress and apoptosis | [159,160] | |
| Cardio-, hepatic- and renal protective | Commercial product purchased from Absunutrix Lyfetrition® | Reduction of oxidative stress, pro-inflammatory effects and injuries to the cardiac, hepatic, and renal tissues | [161] | ||
| Inhibition of tumor angiogenesis | Sargassum hemiphyllum (Fucales) | Hydrolyzed crude extract | LMWF; 760 g/mol | Suppression of HIF-1/VEGF-regulated signaling pathway | [162] |
| Pro-angiogenic | Ascophyllum nodosum (Fucales) | Fractionated with dialysis commercial crude fucoidan (ASPHY) | LMWF (<4.9 x 103 g/mol) | Increase of the vascular network formation regulated via Erk1/2 and PI3K/AKT cell signaling pathways | [163] |
| Alleviation of diabetic complications | S. Fusiforme (Fucales) | Crude extract | Mw: 205.89x103 g/mol, high sulfate content | - Suppression of oxidative stress - Alteration of the gut microbiota - Attenuation of the pathological changes in heart and liver | [164] |
| Diagnostic | |||||
| Imaging of cardiovascular diseases | Ascophyllum nodosum (Fucales) | An oxidative-reductive degraded crude extract (purchased from Algues and Mer, Ascophyscient®) | GMP-grade LMWF (7.1x103 g/mol) | Synthesis of technetium-99m-fucoidan radiotracer for detection of P-selectin | [56] |
| Commercial product from Algues and Mer | Synthesis of polycyanoacrylate-fucoidan microcapsules (Fuco-MCs) for detection of P-selectin | [165] | |||
| Cosmeceutical | |||||
| Anti-Photoaging | Ecklonia cava (Laminariales) | Enzymatic degradation of a commercial HMWF | LMWF (Mw: 8 × 103 g/mol) | Anti-oxidant, anti-apoptotic, and MMP-9-inhibiting effects | [166] |
| Skin brightening and age spot reduction | F. vesiculosus (Fucales) | Crude extracts purchased from Marinova® Pty Ltd. | 58.6% fucoidans, 33.7% polyphenol | Increase of Sirtuin 1 (SIRT1) expression in vitro | [167,168] |
| Skin immunity, soothing and protection | U. pinnatifida (Laminariales) | 89.6% fucoidans, <2% polyphenol | |||
| Reconstruction of skin | F. vesiculosus (Fucales) | Commercial product from Sigma-Aldrich® (not determined the degree of purity) | Increase of proliferating cell nuclear antigen (PCNA) p63 and α6-integrin expression | [169] | |
| Pharmaceutical technology | |||||
| As vehicle for drug delivery | F. vesiculosus (Fucales) | Commercial product purchased from Sigma-Aldrich® | Mw: 57.26 ×103 g/mol | - Chitosan-fucoidans-based nanoparticles for delivery of anti-cancers (e.g., curcumin-loaded NPs) - Nanoencapsulation of poly L-lysine | [170,171] |
| Piperlongumine (PL)-loaded chitosan-fucoidan nanoparticles (PL-CS-F NPs) | [172] | ||||
| Synthesis of fucoidan/trimethylchitosan nanoparticles (FUC-TMC-NPs) as adjuvant in anthrax vaccine adsorbed | [173] | ||||
| Green synthesis of silver nanoparticles | Synthesis of chitosan-fucoidan complex-coated AgNPs | [174] | |||
| Biogenic Source of Fucoidans | Fucoidanase Source | Mode of Action | Ref. |
|---|---|---|---|
| F. evanescens | Formosa algae KMM 3553 | Endo α-1→4 | [61,182] |
| Pseudoalteromonas citrea strains KMM 3296, KMM 3297, KMM 3298 | Endo α-1→3 | [186] | |
| F. vesiculosus | Dendryphiella. arenaria TM94 | Endo n.d. * | [187] |
| Kjellmaniella crassifolia | Fucobacter marina SA-0082 | Endo β-1→4 | [188] |
| Cladosiphon okamuranus | Fucophilus fucoidanolyticus SI-1234 | Endo α-1→3 | [189] |
| Flavobacterium sp. F-31 | Endo n.d. | [190] | |
| F. distichus | Littorina kurila | Endo α-1→3 | [191] |
| Pelvetia canaliculata | Mariniflexile fucanivorans SW5T | Endo α-1→4 | [184] |
| Undara pinnatifida | Sphingomonas paucimobilis PF-1 | Endo n.d. | [192,193] |
| Saccharina cichorioides | Pseudoalteromonas citrea strains KMM 3296, KMM 3297, KMM 3298 | Endo α-1→3 | [186] |
| Nemacystus decipiens | Mizuhopecten yessoensis | Endo n.d. | [194] |
| Ascophyllum nodosum | Pecten maximus | Exo n.d. | [195,196] |
| Thelenota ananas (Wild sea cucumber) | Wenyingzhuangia Fucanilytica | Endo n.d. | [197] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zayed, A.; Ulber, R. Fucoidans: Downstream Processes and Recent Applications. Mar. Drugs 2020, 18, 170. https://doi.org/10.3390/md18030170
Zayed A, Ulber R. Fucoidans: Downstream Processes and Recent Applications. Marine Drugs. 2020; 18(3):170. https://doi.org/10.3390/md18030170
Chicago/Turabian StyleZayed, Ahmed, and Roland Ulber. 2020. "Fucoidans: Downstream Processes and Recent Applications" Marine Drugs 18, no. 3: 170. https://doi.org/10.3390/md18030170
APA StyleZayed, A., & Ulber, R. (2020). Fucoidans: Downstream Processes and Recent Applications. Marine Drugs, 18(3), 170. https://doi.org/10.3390/md18030170