Clavukoellians G–K, New Nardosinane and Aristolane Sesquiterpenoids with Angiogenesis Promoting Activity from the Marine Soft Coral Lemnalia sp.
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. General Experimental Procedures
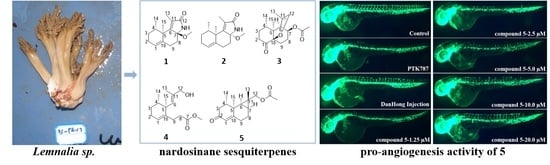
4.2. Soft Coral Material
4.3. Extraction and Isolation
4.4. Computational Section
4.5. Promoting Angiogenesis Assay
4.5.1. Zebrafish Maintenance
4.5.2. PTK787-Induced Vessel Loss Model of Zebrafish
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wu, Q.H.; Sun, J.D.; Chen, J.W.; Zhang, H.W.; Guo, Y.W.; Wang, H. Terpenoids from marine soft coral of the genus Lemnalia: Chemistry and biological activities. Mar. Drugs 2018, 16, 320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bishara, A.; Yeffet, D.; Sisso, M.; Shmul, G.; Schleyer, M.; Benayahu, Y.; Rudi, A.; Kashman, Y. Nardosinanols A-I and lemnafricanol, sesquiterpenes from several soft corals, Lemnalia sp., Paralemnalia clavata, Lemnalia Africana, and Rhytisma fulvum fulvum. J. Nat. Prod. 2008, 71, 375–380. [Google Scholar] [CrossRef] [PubMed]
- El-Gamal, A.A.H.; Wang, S.K.; Dai, C.F.; Duh, C.Y. New nardosinanes and 19-oxygenated ergosterols from the soft coral Nephthea armata collected in Taiwan. J. Nat. Prod. 2004, 67, 1455–1458. [Google Scholar] [CrossRef] [PubMed]
- Kapojos, M.M.; Mangindaan, R.E.P.; Nakazawa, T.; Oda, T.; Ukai, K.; Namikoshi, M. Three new nardosinane type sesquiterpenes from an Indonesian soft coral Nephthea sp. Chem. Pharm. Bull. 2008, 56, 332–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Y.; Li, P.J.; Hung, W.Y.; Su, J.H.; Wen, Z.H.; Hsu, C.H.; Dai, C.F.; Chiang, M.Y.; Sheu, J.H. Nardosinane sesquiterpenoids from the Formosan soft coral Lemnalia flava. J. Nat. Prod. 2011, 74, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Su, J.H.; Lu, Y.; Hung, W.Y.; Huang, C.Y.; Chiang, M.Y.; Sung, P.J.; Kuo, Y.H.; Sheu, J.H. Sesquiterpenoids from the Formosan soft coral Lemnalia flava. Chem. Pharm. Bull. 2011, 59, 698–702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Hu, Z.Q.; Luo, X.C.; Liu, J.; Li, G.Q.; Cao, S.G.; Liu, Q.S. Clavukoellians A–F, highly rearranged nardosinane sesquiterpenoids with antiangiogenic activity from Clavularia koellikeri. J. Nat. Prod. 2019, 82, 1331–1337. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.H.; Ye, F.; Li, X.L.; Liang, L.F.; Sun, J.D.; Sun, H.; Guo, Y.W.; Wang, H. Uncommon polyoxygenated sesquiterpenoids from South China Sea soft coral Lemnalia flava. J. Org. Chem. 2019, 84, 3083–3092. [Google Scholar] [CrossRef] [PubMed]
- Jurek, J.; Scheuer, P.J. Sesquiterpenoids and norsesquiterpenoids from the soft coral Lemnalia Africana. J. Nat. Prod. 1993, 56, 508–513. [Google Scholar] [CrossRef] [PubMed]
- He, J.W.; Liang, H.X.; Gao, H.; Kuang, R.Q.; Chen, G.D.; Hu, D.; Wang, C.X.; Liu, X.Z.; Li, Y.; Yao, X.S. Talaflavuterpenoid A, a new nardosinane-type sesquiterpene from Talaromyces flavus. J. Asian. Nat. Prod. Res. 2014, 16, 1029–1034. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.C.; Zhong, S.Y.; Li, X.Y.; Wang, Y.H.; Xun, M.M.; Bai, Y.L.; Zhu, K.K. Total synthesis, structural revision and biological evaluation of γ-elemene-type sesquiterpenes. Org. Biomol. Chem. 2018, 16, 7843–7850. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Han, L.; Zheng, X.; Wang, X.; Zhang, P.; Wu, J.; Liu, R.; Fu, Y.; Sun, J.; Kang, X.; et al. Tanshinol borneol ester, a novel synthetic small molecule angiogenesis stimulator inspired by botanical formulations for angina pectoris. Br. J. Pharmacol. 2019, 176, 3143–3160. [Google Scholar] [CrossRef] [PubMed]
- Cross, L.M.; Cook, M.A.; Lin, S.; Chen, J.N.; Rubinstein, A.L. Rapid analysis of angiogenesis drugs in a live fluorescent zebrafish assay. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 911–912. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Dang, Y.Y.; Oi Lam Che, G.; Kwan, Y.W.; Chan, S.W.; Leung, G.P.; Lee, S.M.; Hoi, M.P. VEGFR tyrosine kinase inhibitor Ⅱ (VRI) induced vascular insufficiency in zebrafish as a model for studying vascular toxicity and vascular preservation. Toxicol. Appl. Pharmacol. 2014, 280, 408–420. [Google Scholar] [CrossRef] [PubMed]
- Gaumann, A.K.; Drexler, H.C.; Lang, S.A.; Stoeltzing, O.; Diermeier-Daucher, S.; Buchdunger, E.; Wood, J.; Bold, G.; Breier, G. The inhibition of tyrosine kinase receptor signaling in leiomyosarcoma cells using the small molecule kinase inhibitor PTK787/ZK222584 (Vatalanib). Int. J. Oncol. 2014, 45, 2267–2277. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| No. | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| NH | 5.94, brs | 5.91, brs | |||
| 1 | 5.40, s | 5.47, d (4.5) | 5.38, m | 5.78, s | |
| 2 | 2.20, m | 1.96, m | 2.32, d (5.6) | 2.06, m | |
| 2.04, m | |||||
| 3 | 1.61, m | 1.96, m | 2.19, m | 1.44, m | 2.28, m |
| 1.54, m | 1.46, m | 2.24, m | |||
| 4 | 1.93, m | 2.36, m | 2.46, m | 1.86, m | 2.28, m |
| 5 | |||||
| 6 | 2.39, d (3.5) | 5.37, s | 0.91, d (9.6) | ||
| 7 | 5.41, m | 1.14, dd (9.8, 3.2) | |||
| 8 | 2.26, m 2.04, m | 2.28, d (12.7) 1.49, m | 1.91, m | 2.43, m | 2.22, m 1.58, m |
| 9 | 2.15, m | 2.73, t (14.2) 2.04, m | 1.72, m | 2.22, t (7.8) | 2.47, m 2.09, m |
| 10 | |||||
| 11 | |||||
| 12 | 6.24, s | 3.96, s | 3.88, d (10.7) 3.63, d (11.0) | ||
| 13 | 1.99, s | 2.04, s | 1.62, s | 1.66, s | 1.05, s |
| 14 | 1.00, d (6.9) | 1.07, d (6.5) | 0.85, d (6.8) | 0.86, d (6.8) | 1.08, d (4.5) |
| 15 | 1.34, s | 1.31, s | 1.25, s | 1.05, s | 1.26, d (0.6) |
| Ac | 2.04, s | 2.06, d (0.6) | |||
| OMe-7 | 3.00, s | 3.07, s | 3.66, s |
| Carbon | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| 1 | 120.2, CH | 122.8, CH | 208.0, C | 120.9, CH | 125.6, CH |
| 2 | 25.0, CH2 | 28.9, CH2 | 49.1, CH2 | 25.8, CH2 | 198.9, C |
| 3 | 26.1, CH2 | 27.0, CH2 | 45.3, CH2 | 26.9, CH2 | 42.6, CH2 |
| 4 | 35.6, CH | 34.6, CH | 33.4, CH | 38.3, CH | 36.5, CH |
| 5 | 43.5, C | 45.9, C | 37.2, C | 43.2, C | 38.3, C |
| 6 | 157.1, C | 155.8, C | 43.9, CH | 134.7, CH | 31.0, CH |
| 7 | 90.5, C | 90.7, C | 72.9, CH | 174.5, C | 16.7, CH |
| 8 | 37.4, CH2 | 40.2, CH2 | 23.8, CH2 | 33.6, CH2 | 19.4, CH2 |
| 9 | 28.0, CH2 | 25.6, CH2 | 34.7, CH2 | 27.1, CH2 | 30.4, CH2 |
| 10 | 142.5, C | 142.1, C | 80.2, C | 143.0, C | 172.8, C |
| 11 | 131.5, C | 127.2, C | 104.9, C | 135.7, C | 23.2, C |
| 12 | 173.1, C | 172.4, C | 139.3, CH | 70.3, CH2 | 74.7, CH2 |
| 13 | 11.0, CH3 | 11.0, CH3 | 19.1, CH3 | 14.2, CH3 | 13.3, CH3 |
| 14 | 17.5, CH3 | 20.3, CH3 | 14.9, CH3 | 17.4, CH3 | 15.2, CH3 |
| 15 | 19.0, CH3 | 16.8, CH3 | 14.7, CH3 | 22.1, CH3 | 21.7, CH3 |
| Ac | 170.7, C | 171.4, C | |||
| 21.5, CH3 | 21.2, CH3 | ||||
| OMe-7 | 50.3, CH3 | 50.3, CH3 | 51.7, CH3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Tang, X.; Liu, H.; Luo, X.; Sung, P.J.; Li, P.; Li, G. Clavukoellians G–K, New Nardosinane and Aristolane Sesquiterpenoids with Angiogenesis Promoting Activity from the Marine Soft Coral Lemnalia sp. Mar. Drugs 2020, 18, 171. https://doi.org/10.3390/md18030171
Wang Q, Tang X, Liu H, Luo X, Sung PJ, Li P, Li G. Clavukoellians G–K, New Nardosinane and Aristolane Sesquiterpenoids with Angiogenesis Promoting Activity from the Marine Soft Coral Lemnalia sp. Marine Drugs. 2020; 18(3):171. https://doi.org/10.3390/md18030171
Chicago/Turabian StyleWang, Qi, Xuli Tang, Hui Liu, Xiangchao Luo, Ping Jyun Sung, Pinglin Li, and Guoqiang Li. 2020. "Clavukoellians G–K, New Nardosinane and Aristolane Sesquiterpenoids with Angiogenesis Promoting Activity from the Marine Soft Coral Lemnalia sp." Marine Drugs 18, no. 3: 171. https://doi.org/10.3390/md18030171
APA StyleWang, Q., Tang, X., Liu, H., Luo, X., Sung, P. J., Li, P., & Li, G. (2020). Clavukoellians G–K, New Nardosinane and Aristolane Sesquiterpenoids with Angiogenesis Promoting Activity from the Marine Soft Coral Lemnalia sp. Marine Drugs, 18(3), 171. https://doi.org/10.3390/md18030171