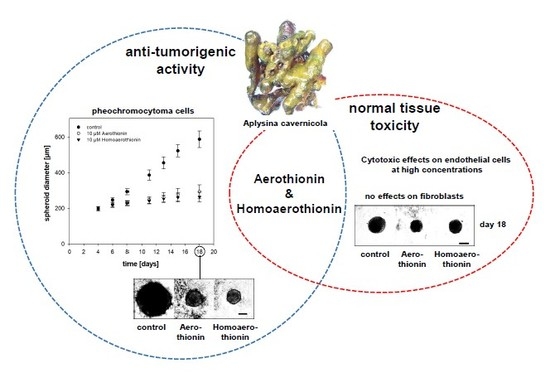
Anti-Tumor Activity vs. Normal Cell Toxicity: Therapeutic Potential of the Bromotyrosines Aerothionin and Homoaerothionin In Vitro
Abstract
:1. Introduction
2. Results
2.1. Anti-Tumor Activity of Aerothionin and Homoaerothionin In Vitro
2.1.1. Aerothionin and Homoaerothionin Decreased Proliferating Cell Characteristics
2.1.2. Aerothionin and Homoaerothionin Diminished Spheroid Growth
2.2. Effects of Aerothionin and Homoaerothionin on Cells of the Normal Tissue
2.2.1. Effects of Aerothionin and Homoaerothionin on Endothelial Cells
2.2.2. Aerothionin and Homoaerothionin Stimulated the Viability of Normal Fibroblasts
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Viability Assay
4.3. Proliferation Assay
4.4. Generation and Cultivation of Tumor Cell Spheroids
4.5. Generation and Cultivation of Fibroblast Spheroids
4.6. Spheroid Treatment and Growth Measurement
4.7. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Altmann, K.-H. Drugs from the oceans: Marine natural products as leads for drug discovery. CHIMIA 2017, 71, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Nieto, S.; González-Iriarte, M.; Carmona, R.; Muñoz-Chápuli, R.; Medina, M.A.; Quesada, A.R. Antiangiogenic activity of aeroplysinin-1, a brominated compound isolated from a marine sponge. FASEB J. 2002, 16, 261–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thoms, C.; Wolff, M.; Padmakumar, K.; Ebel, R.; Proksch, P. Chemical defense of mediterranean sponges aplysina cavernicola and aplysina aerophoba. Z. Naturforsch C J. Biosci. 2004, 59, 113–122. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Marine-sourced anti-cancer and cancer pain control agents in clinical and late preclinical development. Mar. Drugs 2014, 12, 255–278. [Google Scholar] [CrossRef] [Green Version]
- Dyshlovoy, S.A.; Honecker, F. Marine compounds and cancer: 2017 updates. Mar. Drugs 2018, 16, 41. [Google Scholar] [CrossRef] [Green Version]
- Schwartsmann, G.; da Rocha, A.B.; Berlinck, R.G.; Jimeno, J. Marine organisms as a source of new anticancer agents. Lancet Oncol. 2001, 2, 221–225. [Google Scholar] [CrossRef]
- Mehbub, M.F.; Lei, J.; Franco, C.; Zhang, W. Marine sponge derived natural products between 2001 and 2010: Trends and opportunities for discovery of bioactives. Mar. Drugs 2014, 12, 4539–4577. [Google Scholar] [CrossRef] [Green Version]
- Peng, J.; Li, J.; Hamann, M.T. The marine bromotyrosine derivatives. In The alkaloids: Chemistry and Biology; Elsevier: Amsterdam, The Netherlands, 2005; Volume 61, pp. 59–262. [Google Scholar]
- Kovalchuk, V.; Voronkina, A.; Binnewerg, B.; Schubert, M.; Muzychka, L.; Wysokowski, M.; Tsurkan, M.V.; Bechmann, N.; Petrenko, I.; Fursov, A.; et al. Naturally drug-loaded chitin: Isolation and applications. Mar. Drugs 2019, 17, 574. [Google Scholar] [CrossRef] [Green Version]
- Moody, K.; Thomson, R.; Fattorusso, E.; Minale, L.T.; Sodano, G. Aerothionin and homoaerothionin: Two tetrabromo spirocyclohexadienylisoxazoles from verongia sponges. J. Chem. Soc. Perkin 1972, 1, 18–24. [Google Scholar] [CrossRef]
- Shaala, L.A.; Youssef, D.T.; Badr, J.M.; Sulaiman, M.; Khedr, A. Bioactive secondary metabolites from the red sea marine verongid sponge suberea species. Mar. Drugs 2015, 13, 1621–1631. [Google Scholar] [CrossRef] [Green Version]
- Kalaitzis, J.A.; Leone, P.d.A.; Hooper, J.N.; Quinn, R.J. Ianthesine e, a new bromotyrosine-derived metabolite from the great barrier reef sponge pseudoceratina sp. Nat. Prod. Res. 2008, 22, 1257–1263. [Google Scholar] [CrossRef] [PubMed]
- Mani, L.; Jullian, V.; Mourkazel, B.; Valentin, A.; Dubois, J.; Cresteil, T.; Folcher, E.; Hooper, J.N.; Erpenbeck, D.; Aalbersberg, W.; et al. New antiplasmodial bromotyrosine derivatives from suberea ianthelliformis lendenfeld, 1888. Chem. Biodivers 2012, 9, 1436–1451. [Google Scholar] [CrossRef] [PubMed]
- Encarnación-Dimayuga, R.; Ramirez, M.; Luna-Herrera, J. Aerothionin, a bromotyrosine derivative with antimycobacterial activity from the marine sponge aplysina gerardogreeni (demospongia). Pharm. Biol. 2003, 41, 384–387. [Google Scholar] [CrossRef]
- Gil, A.M.; Gordillo, D.A.; Diaz, I.D.; Palomero, E.G.; De Luque, C.D.A.; Egea, P.U.; Millan, M.D.M.; Padilla, M.M. Marine compounds with calcium channel blocking properties for the treatment of cognitive or neurodegenerative diseases. US Patent 2008/0039513 A1, 17 June 2005. [Google Scholar]
- Ehrlich, H.; Bazhenov, V.V.; Debitus, C.; de Voogd, N.; Galli, R.; Tsurkan, M.V.; Wysokowski, M.; Meissner, H.; Bulut, E.; Kaya, M.; et al. Isolation and identification of chitin from heavy mineralized skeleton of suberea clavata (verongida: Demospongiae: Porifera) marine demosponge. Int. J. Biol. Macromol. 2017, 104, 1706–1712. [Google Scholar] [CrossRef] [PubMed]
- Fromont, J.; Żółtowska-Aksamitowska, S.; Galli, R.; Meissner, H.; Erpenbeck, D.; Vacelet, J.; Diaz, C.; Tsurkan, M.V.; Petrenko, I.; Youssef, D.T.; et al. New family and genus of a dendrilla-like sponge with characters of verongiida. Part ii. Discovery of chitin in the skeleton of ernstilla lacunosa. Zool. Anz. 2019, 280, 21–29. [Google Scholar] [CrossRef]
- Mutsenko, V.V.; Gryshkov, O.; Lauterboeck, L.; Rogulska, O.; Tarusin, D.N.; Bazhenov, V.V.; Schütz, K.; Brüggemeier, S.; Gossla, E.; Akkineni, A.R.; et al. Novel chitin scaffolds derived from marine sponge ianthella basta for tissue engineering approaches based on human mesenchymal stromal cells: Biocompatibility and cryopreservation. Int. J. Biol. Macromol. 2017, 104, 1955–1965. [Google Scholar] [CrossRef]
- Mutsenko, V.V.; Bazhenov, V.V.; Rogulska, O.; Tarusin, D.N.; Schütz, K.; Brüggemeier, S.; Gossla, E.; Akkineni, A.R.; Meißner, H.; Lode, A.; et al. 3d chitinous scaffolds derived from cultivated marine demosponge aplysina aerophoba for tissue engineering approaches based on human mesenchymal stromal cells. Int. J. Biol. Macromol. 2017, 104, 1966–1974. [Google Scholar] [CrossRef]
- Ehrlich, H.; Steck, E.; Ilan, M.; Maldonado, M.; Muricy, G.; Bavestrello, G.; Kljajic, Z.; Carballo, J.; Schiaparelli, S.; Ereskovsky, A.; et al. Three-dimensional chitin-based scaffolds from verongida sponges (demospongiae: Porifera). Part ii: Biomimetic potential and applications. Int. J. Biol. Macromol. 2010, 47, 141–145. [Google Scholar] [CrossRef]
- Shaala, L.A.; Asfour, H.Z.; Youssef, D.T.; Żółtowska-Aksamitowska, S.; Wysokowski, M.; Tsurkan, M.; Galli, R.; Meissner, H.; Petrenko, I.; Tabachnick, K.; et al. New source of 3d chitin scaffolds: The red sea demosponge pseudoceratina arabica (pseudoceratinidae, verongiida). Mar. Drugs 2019, 17, 92. [Google Scholar] [CrossRef] [Green Version]
- Schubert, M.; Binnewerg, B.; Voronkina, A.; Muzychka, L.; Wysokowski, M.; Petrenko, I.; Kovalchuk, V.; Tsurkan, M.; Martinovic, R.; Bechmann, N.; et al. Naturally prefabricated marine biomaterials: Isolation and applications of flat chitinous 3d scaffolds from ianthella labyrinthus (demospongiae: Verongiida). Int. J. Mol. Sci. 2019, 20, 5105. [Google Scholar] [CrossRef] [Green Version]
- Klinger, C.; Żółtowska-Aksamitowska, S.; Wysokowski, M.; Tsurkan, M.V.; Galli, R.; Petrenko, I.; Machałowski, T.; Ereskovsky, A.; Martinović, R.; Muzychka, L.; et al. Express method for isolation of ready-to-use 3d chitin scaffolds from aplysina archeri (aplysineidae: Verongiida) demosponge. Mar. Drugs 2019, 17, 131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wysokowski, M.; Machałowski, T.; Petrenko, I.; Schimpf, C.; Rafaja, D.; Galli, R.; Ziętek, J.; Pantović, S.; Voronkina, A.; Kovalchuk, V.; et al. 3d chitin scaffolds of marine demosponge origin for biomimetic mollusk hemolymph-associated biomineralization ex-vivo. Mar. Drugs 2020, 18, 123. [Google Scholar] [CrossRef] [Green Version]
- Bechmann, N.; Ehrlich, H.; Eisenhofer, G.; Ehrlich, A.; Meschke, S.; Ziegler, C.G.; Bornstein, S.R. Anti-tumorigenic and anti-metastatic activity of the sponge-derived marine drugs aeroplysinin-1 and isofistularin-3 against pheochromocytoma in vitro. Mar. Drugs 2018, 16, 172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eisenhofer, G.; Bornstein, S.R.; Brouwers, F.M.; Cheung, N.-K.V.; Dahia, P.L.; De Krijger, R.R.; Giordano, T.J.; Greene, L.A.; Goldstein, D.S.; Lehnert, H.; et al. Malignant pheochromocytoma: Current status and initiatives for future progress. Endocr. Relat. Cancer 2004, 11, 423–436. [Google Scholar] [CrossRef] [PubMed]
- Bechmann, N.; Poser, I.; Seifert, V.; Greunke, C.; Ullrich, M.; Qin, N.; Walch, A.; Peitzsch, M.; Robledo, M.; Pacak, K.; et al. Impact of extrinsic and intrinsic hypoxia on catecholamine biosynthesis in absence or presence of hif2α in pheochromocytoma cells. Cancers 2019, 11, 594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khedr, S.; Deussen, A.; Kopaliani, I.; Zatschler, B.; Martin, M. Effects of tryptophan-containing peptides on angiotensin-converting enzyme activity and vessel tone ex vivo and in vivo. Eur. J. Nutr. 2018, 57, 907–915. [Google Scholar] [CrossRef]
- Binnewerg, B.; Schubert, M.; Voronkina, A.; Muzychka, L.; Wysokowski, M.; Petrenko, I.; Djurović, M.; Kovalchuk, V.; Tsurkan, M.; Martinovic, R.; et al. Marine biomaterials: Biomimetic and pharmacological potential of cultivated aplysina aerophoba marine demosponge. Mater. Sci. Eng. C 2020, 109, 110566. [Google Scholar] [CrossRef]
- Teeyapant, R.; Woerdenbag, H.; Gross, H.; Proksch, P. Biotransformation of the brominated compounds in the marine sponge verongia aerophoba: Evidence for an induced chemical defense? Planta Med. 1993, 59, A641–A642. [Google Scholar] [CrossRef]
- Teeyapant, R.; Kreis, P.; Wray, V.; Witte, L.; Proksch, P. Brominated secondary compounds from the marine sponge verongia aerophoba and the sponge feeding gastropod tylodina perversa. Z. Naturforsch C J. Biosci. 1993, 48, 640–644. [Google Scholar] [CrossRef]
- Kerbel, R.S. Tumor angiogenesis. N. Engl. J. Med. 2008, 358, 2039–2049. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Poveda, B.; Rodríguez-Nieto, S.; García-Caballero, M.; Medina, M.-Á.; Quesada, A.R. The antiangiogenic compound aeroplysinin-1 induces apoptosis in endothelial cells by activating the mitochondrial pathway. Mar. Drugs 2012, 10, 2033–2046. [Google Scholar] [CrossRef] [PubMed]
- Darby, I.A.; Laverdet, B.; Bonté, F.; Desmoulière, A. Fibroblasts and myofibroblasts in wound healing. Clin. Cosmet Investig. Dermatol. 2014, 7, 301. [Google Scholar] [PubMed] [Green Version]
- Werner, S.; Krieg, T.; Smola, H. Keratinocyte–fibroblast interactions in wound healing. J. Investig. Dermatol. 2007, 127, 998–1008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, Y.; Xie, Z.; Kim, G.B.; Dong, C.; Yang, J. Design strategies and applications of circulating cell-mediated drug delivery systems. ACS Biomater. Sci. Eng. 2015, 1, 201–217. [Google Scholar] [CrossRef] [Green Version]
- Gabizon, A.A. Selective tumor localization and improved therapeutic index of anthracyclines encapsulated in long-circulating liposomes. Cancer Res. 1992, 52, 891–896. [Google Scholar]
- Bechmann, N.; Kniess, T.; Pietzsch, J. Nitric oxide-releasing selective estrogen receptor modulators: A bifunctional approach to improve the therapeutic index. J. Med. Chem. 2019, 62, 6525–6539. [Google Scholar] [CrossRef]
- Nölting, S.; Grossman, A.; Pacak, K. Metastatic phaeochromocytoma: Spinning towards more promising treatment options. Exp. Clin. Endocrinol. Diabetes 2019, 127, 117–128. [Google Scholar] [CrossRef] [Green Version]
- Fankhauser, M.; Bechmann, N.; Lauseker, M.; Goncalves, J.; Favier, J.; Klink, B.; William, D.; Gieldon, L.; Maurer, J.; Spöttl, G.; et al. Synergistic highly potent targeted drug combinations in different pheochromocytoma models including human tumor cultures. Endocrinology 2019, 160, 2600–2617. [Google Scholar] [CrossRef] [Green Version]
- Powers, J.F.; Evinger, M.J.; Tsokas, P.; Bedri, S.; Alroy, J.; Shahsavari, M.; Tischler, A.S. Pheochromocytoma cell lines from heterozygous neurofibromatosis knockout mice. Cell Tissue Res. 2000, 302, 309–320. [Google Scholar] [CrossRef]
- Martiniova, L.; Lai, E.W.; Elkahloun, A.G.; Abu-Asab, M.; Wickremasinghe, A.; Solis, D.C.; Perera, S.M.; Huynh, T.-T.; Lubensky, I.A.; Tischler, A.S.; et al. Characterization of an animal model of aggressive metastatic pheochromocytoma linked to a specific gene signature. Clin. Exp. Metastasis 2009, 26, 239–250. [Google Scholar] [CrossRef] [Green Version]
- Tischler, A.S.; Greene, L.A.; Kwan, P.W.; Slayton, V.W. Ultrastructural effects of nerve growth factor on pc 12 pheochromocytoma cells in spinner culture. Cell Tissue Res. 1983, 228, 641–648. [Google Scholar] [CrossRef] [PubMed]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drechsel, A.; Helm, J.; Ehrlich, H.; Pantovic, S.; Bornstein, S.R.; Bechmann, N. Anti-Tumor Activity vs. Normal Cell Toxicity: Therapeutic Potential of the Bromotyrosines Aerothionin and Homoaerothionin In Vitro. Mar. Drugs 2020, 18, 236. https://doi.org/10.3390/md18050236
Drechsel A, Helm J, Ehrlich H, Pantovic S, Bornstein SR, Bechmann N. Anti-Tumor Activity vs. Normal Cell Toxicity: Therapeutic Potential of the Bromotyrosines Aerothionin and Homoaerothionin In Vitro. Marine Drugs. 2020; 18(5):236. https://doi.org/10.3390/md18050236
Chicago/Turabian StyleDrechsel, Antje, Jana Helm, Hermann Ehrlich, Snezana Pantovic, Stefan R. Bornstein, and Nicole Bechmann. 2020. "Anti-Tumor Activity vs. Normal Cell Toxicity: Therapeutic Potential of the Bromotyrosines Aerothionin and Homoaerothionin In Vitro" Marine Drugs 18, no. 5: 236. https://doi.org/10.3390/md18050236
APA StyleDrechsel, A., Helm, J., Ehrlich, H., Pantovic, S., Bornstein, S. R., & Bechmann, N. (2020). Anti-Tumor Activity vs. Normal Cell Toxicity: Therapeutic Potential of the Bromotyrosines Aerothionin and Homoaerothionin In Vitro. Marine Drugs, 18(5), 236. https://doi.org/10.3390/md18050236