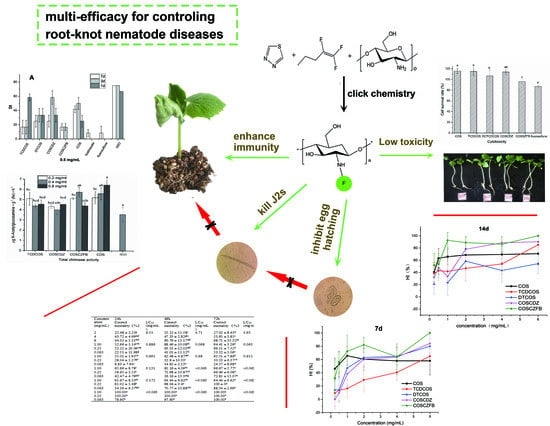
Chitosan Oligosaccharide Fluorinated Derivative Control Root-Knot Nematode (Meloidogyne incognita) Disease Based on the Multi-Efficacy Strategy
Abstract
:1. Introduction
2. Results
2.1. Characterization of FTIR, NMR, Elemental Analysis, and TG/DTG
2.1.1. FTIR
2.1.2. 1H NMR and 13C NMR
2.1.3. Elemental Analysis
2.1.4. TG/DTG
2.2. Egg Hatch Inhibitory Activity
2.3. Nematicidal Activity against J2s
2.4. Actual Control RKN Disease Ability
2.5. Effect on Photosynthetic Pigment Content
2.6. Effect on Immune Enzyme
2.7. Cytotoxicity
2.8. Phytotoxicity
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Characterization Methods
4.3. Synthesis of COS Thiadiazole Trifluorobutene Derivative
4.4. Egg Hatching Assay In Vitro
4.5. Nematicidal Assay In Vitro
4.6. Greenhouse Test Tube Assay In Vivo
4.7. Plant-Regulation Assays
4.8. Chitinase and β-1,3-glucanase Assay
4.9. Cell Toxicity
4.10. Phytotoxicity
Author Contributions
Funding
Conflicts of Interest
References
- Siddique, S.; Grundler, F.M. Parasitic nematodes manipulate plant development to establish feeding sites. Curr. Opin. Microbiol. 2018, 46, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, H.M.M.; Ahmad, E.M.; Martinez-Medina, A.; Aly, M.A.M. Effective approaches to study the plant-root knot nematode interaction. Plant Physiol. Biochem. 2019, 141, 332–342. [Google Scholar] [CrossRef] [PubMed]
- Bresso, E.; Fernandez, D.; Amora, D.X.; Noel, P.; Petitot, A.S.; de Sa, M.L.; Albuquerque, E.V.S.; Danchin, E.G.J.; Maigret, B.; Martins, N.F. A Chemosensory GPCR as a Potential Target to Control the Root-Knot Nematode Meloidogyne incognita Parasitism in Plants. Molecules 2019, 24, 3798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lagos, S.; Perruchon, C.; Katsoula, A.; Karpouzas, D.G. Isolation and characterization of soil bacteria able to rapidly degrade the organophosphorus nematicide fosthiazate. Lett. Appl. Microbiol. 2019, 68, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Wang, Q.; Guo, M.; Fang, W.; Wang, X.; Wang, Q.; Yan, D.; Ouyang, C.; Li, Y.; Cao, A. The synergistic advantage of combining chloropicrin or dazomet with fosthiazate nematicide to control root-knot nematode in cucumber production. J. Integr. Agric. 2019, 18, 2093–2106. [Google Scholar] [CrossRef]
- Huang, W.K.; Wu, Q.S.; Peng, H.; Kong, L.A.; Liu, S.M.; Yin, H.Q.; Cui, R.Q.; Zhan, L.P.; Cui, J.K.; Peng, D.L. Mutations in Acetylcholinesterase2 (ace2) increase the insensitivity of acetylcholinesterase to fosthiazate in the root-knot nematode Meloidogyne incognita. Sci. Rep. 2016, 6, 38102. [Google Scholar] [CrossRef] [Green Version]
- Fu, Y.; He, H.; Liu, R.; Zhu, L.; Xia, Y.; Qiu, J. Preparation and performance of a BTDA-modified polyurea microcapsule for encapsulating avermectin. Colloids Surf. Biointerfaces 2019, 183, 110400. [Google Scholar] [CrossRef]
- Lu, H.; Xu, S.; Zhang, W.; Xu, C.; Li, B.; Zhang, D.; Mu, W.; Liu, F. Nematicidal Activity of trans-2-Hexenal against Southern Root-Knot Nematode (Meloidogyne incognita) on Tomato Plants. J. Agric. Food Chem. 2017, 65, 544–550. [Google Scholar] [CrossRef]
- Ntalli, N.G.; Caboni, P. Botanical nematicides: A review. J. Agric. Food Chem. 2012, 60, 9929–9940. [Google Scholar] [CrossRef]
- Liu, T.; Wu, H.; Jiang, H.; Zhang, L.; Zhang, Y.; Mao, L. Thiophenes from echinops grijsii as a preliminary approach to control disease complex of root-knot nematodes and soil-borne fungi: Isolation, activities, and structure-nonphototoxic activity relationship analysis. J. Agric. Food Chem. 2019, 67, 6160–6168. [Google Scholar] [CrossRef]
- Oliveira, D.F.; Santos Junior, H.M.; Nunes, A.S.; Campos, V.P.; Pinho, R.S.; Gajo, G.C. Purification and identification of metabolites produced by Bacillus cereus and B. subtilis active against Meloidogyne exigua, and their in silico interaction with a putative phosphoribosyltransferase from M. incognita. An. Acad. Bras. Cienc. 2014, 86, 525–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castaneda-Alvarez, C.; Aballay, E. Rhizobacteria with nematicide aptitude: Enzymes and compounds associated. World J. Microbiol. Biotechnol. 2016, 32, 203. [Google Scholar] [CrossRef] [PubMed]
- El-Ansary; Hamouda, M.S.M.R.A.; Eldemery, S.M.M. Eco-friendl Approaches for Controlling Root-knot Nematode Meloidogyne javanica by Alginate and its Effect on Eggplant DNA Pattern. Egypt. J. Biol. Pest Control 2017, 27, 155–163. [Google Scholar]
- Yuan, X.; Zheng, J.; Jiao, S.; Cheng, G.; Feng, C.; Du, Y.; Liu, H. A review on the preparation of chitosan oligosaccharides and application to human health, animal husbandry and agricultural production. Carbohydr. Polym. 2019, 220, 60–70. [Google Scholar] [CrossRef]
- Radwan, M.A.; Farrag, S.A.A.; Abu-Elamayem, M.M.; Ahmed, N.S. Extraction, characterization, and nematicidal activity of chitin and chitosan derived from shrimp shell wastes. Biol. Fertil. Soils 2011, 48, 463–468. [Google Scholar] [CrossRef]
- Khalil, M.S.; Badawy, M.E.I. Nematicidal activity of a biopolymer chitosan at different molecular weights against root-knot nematode, Meloidogyne incognita. Plant Prot. Sci. 2012, 48, 170–178. [Google Scholar] [CrossRef] [Green Version]
- Bell, N.I.; Watson, R.N.; Sarathchandra, S.U. Suppression of plant parasitic nematodes in pastoral soils amended with chitin. N. Z. Plant Prot. 2000, 53, 44–47. [Google Scholar] [CrossRef]
- Fan, Z.; Qin, Y.; Liu, S.; Xing, R.; Yu, H.; Chen, X.; Li, K.; Li, R.; Wang, X.; Li, P. The bioactivity of new chitin oligosaccharide dithiocarbamate derivatives evaluated against nematode disease (Meloidogyne incognita). Carbohydr. Polym. 2019, 224, 115155. [Google Scholar] [CrossRef]
- Wood, W.W.; Kuhn, D.; Hu, Y.; Tecle, B. Pesticidal and Parasitical di-and Trifluorosubstituted Alkene Compounds. EP1427287B1, 6 June 2007. [Google Scholar]
- DR Udo, K.; DR Wolfram, A.; DR Andreas, T.; DR Norbert, M. New Benzoic acid-(4,4-di:fluoro-but-3-ene-yl)ester Derivs. Patent DE19531276, 27 February 2020. [Google Scholar]
- Yukiyoshi, W.; Koichi, I.; Shinichi, N.; Takuya, G.; Yuichi, O.; Katsuhiko, S. Nematicidal Trifluorobutenes. Patent US20030109563A1, 12 June 2003. [Google Scholar]
- Desaeger, J.A.; Watson, T.T. Evaluation of new chemical and biological nematicides for managing Meloidogyne javanica in tomato production and associated double-crops in Florida. Pest Manag. Sci. 2019, 75, 3363–3370. [Google Scholar] [CrossRef]
- Oka, Y.; Saroya, Y. Effect of fluensulfone and fluopyram on the mobility and infection of second-stage juveniles of Meloidogyne incognita and M. javanica. Pest Manag. Sci. 2019, 75, 2095–2106. [Google Scholar] [CrossRef]
- Morris, K.A.; Langston, D.B.; Davis, R.F.; Noe, J.P.; Timper, P. Efficacy of various application methods of fluensulfone for managing root-knot nematodes in vegetables. J. Nematol. 2016, 48, 65–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, Y.; Xing, R.; Liu, S.; Li, K.; Meng, X.; Li, R.; Cui, J.; Li, B.; Li, P. Novel thiosemicarbazone chitosan derivatives: Preparation, characterization, and antifungal activity. Carbohydr. Polym. 2012, 87, 2664–2670. [Google Scholar] [CrossRef]
- Turnbull, M.D. 2-(3,4,4,-Trifluorobutenylmercapto) Alkoxy or Nitro Benzoxazoyl Compounds. Patent US005273988A, 28 December 1993. [Google Scholar]
- Cullen, T.G.; Willut, J.M.; Disanzo, C.P.; Martinez, A.J. Nematicidal activity of 5-substituted-2-s-(3,4,4-trifluoro-3-butenyl)-1,3,4-thiadiazoles. In Synthesis and Chemistry of Agrochemicals, 3rd ed.; Don, R.B., Joseph, G.F., James, J.S., Eds.; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1992; Volume 504, pp. 361–370. [Google Scholar]
- Hubl, D.; Pieroh, E.; Joppien, H. 5-Substituted 1,3,4-Thiadiazole Derivatives, Their Preparation and Their Use as Pesticides. Patent EP0410551A1, 30 January 1991. [Google Scholar]
- Aranda-Martinez, A.; Lenfant, N.; Escudero, N.; Zavala-Gonzalez, E.A.; Henrissat, B.; Lopez-Llorca, L.V. CAZyme content of Pochonia chlamydosporia reflects that chitin and chitosan modification are involved in nematode parasitism. Environ. Microbiol. 2016, 18, 4200–4215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Degenkolb, T.; Vilcinskas, A. Metabolites from nematophagous fungi and nematicidal natural products from fungi as an alternative for biological control. Part I: Metabolites from nematophagous ascomycetes. Appl. Microbiol. Biotechnol. 2016, 100, 3799–3812. [Google Scholar] [CrossRef]
- Badawy, M.E.I.; Rabea, E.I. A biopolymer chitosan and its derivatives as promising antimicrobial agents against plant pathogens and their applications in crop protection. Int. J. Carbohyd. Chem. 2011, 2011, 460381. [Google Scholar] [CrossRef]
- Davies, K.; Spiegel, Y. Biological Control. of Plant-Parasitic Nematodes: Building Coherence between Microbial Ecology and Molecular Mechanisms, 1st ed.; Springer: Berlin, Germany, 2011; Volume 11, p. 248. [Google Scholar]
- Jia, X.C.; Zeng, H.H.; Wang, W.X.; Zhang, F.Y.; Yin, H. Chitosan oligosaccharide induces resistance to pseudomonas syringae pv. Tomato dc3000 in arabidopsis thaliana by activating both salicylic acid- and jasmonic acid-mediated pathways. Mol. Plant.-Microbe Interact. 2018, 31, 1271–1279. [Google Scholar] [CrossRef] [Green Version]
- Song, S.; Wang, Y.; Xie, J.; Sun, B.; Zhou, N.; Shen, H.; Shen, J. Carboxymethyl chitosan modified carbon nanoparticle for controlled emamectin benzoate delivery: Improved solubility, ph-responsive release, and sustainable pest control. ACS Appl. Mater. Interfaces 2019, 11, 34258–34267. [Google Scholar] [CrossRef]
- Xu, C.; Cao, L.; Zhao, P.; Zhou, Z.; Cao, C.; Zhu, F.; Li, F.; Huang, Q. Synthesis and characterization of stimuli-responsive poly(2-dimethylamino-ethylmethacrylate)-grafted chitosan microcapsule for controlled pyraclostrobin release. Int. J. Mol. Sci. 2018, 19, 854. [Google Scholar] [CrossRef] [Green Version]
- Muxika, A.; Etxabide, A.; Uranga, J.; Guerrero, P.; de la Caba, K. Chitosan as a bioactive polymer: Processing, properties and applications. Int. J. Biol. Macromol. 2017, 105 Pt 2, 1358–1368. [Google Scholar] [CrossRef]
- Singh, R.R.; Chinnasri, B.; De Smet, L.; Haeck, A.; Demeestere, K.; Van Cutsem, P.; Van Aubel, G.; Gheysen, G.; Kyndt, T. Systemic defense activation by COS-OGA in rice against root-knot nematodes depends on stimulation of the phenylpropanoid pathway. Plant Physiol. Biochem. 2019, 142, 202–210. [Google Scholar] [CrossRef] [Green Version]
- Korayem, A.; Youssef, M.; Mohamed, M. Effect of chitin and abamectin on Meloidogyne incognita infesting rapeseed. J. Plant. Prot. Res. 2008, 48, 365–370. [Google Scholar] [CrossRef]
- Escudero, N.; Ferreira, S.R.; Lopez-Moya, F.; Naranjo-Ortiz, M.A.; Marin-Ortiz, A.I.; Thornton, C.R.; Lopez-Llorca, L.V. Chitosan enhances parasitism of Meloidogyne javanica eggs by the nematophagous fungus Pochonia chlamydosporia. Fungal Biol. 2016, 120, 572–585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srivastava, N.; Haseeb, A. Biomanagement of Phytonematodes; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014. [Google Scholar]
- Mcsorley, R. Overview of organic amendments for management of plant-parasitic nematodes, with case studies from florida. J. Nematol. 2011, 43, 69–81. [Google Scholar] [PubMed]
- Algam, S.A.E.; Xie, G.; Li, B.; Yu, S.; Su, T.; Larsen, J. Effects of paenibacillus strains and chitosan on plant growth promotion and control of ralstonia wilt in tomato. J. Plant. Pathol. 2010, 92, 593–600. [Google Scholar]
- Bogner, C.W.; Kamdem, R.S.; Sichtermann, G.; Matthaus, C.; Holscher, D.; Popp, J.; Proksch, P.; Grundler, F.M.; Schouten, A. Bioactive secondary metabolites with multiple activities from a fungal endophyte. Microb. Biotechnol. 2017, 10, 175–188. [Google Scholar] [CrossRef]
- Caboni, P.; Aissani, N.; Demurtas, M.; Ntalli, N.; Onnis, V. Nematicidal activity of acetophenones and chalcones against Meloidogyne incognita and structure-activity considerations. Pest Manag. Sci. 2016, 72, 125–130. [Google Scholar] [CrossRef]
- Wahla, V.; Maheshwari, D.K.; Bajpai, V.K. Nematicidal fluorescent pseudomonads for the in vitro and in vivo suppression of root knot (Meloidogyne incognita) of Capsicum annuum L. Pest. Manag. Sci. 2012, 68, 1148–1155. [Google Scholar] [CrossRef]
- Andres, M.F.; Gonzalez-Coloma, A.; Munoz, R.; De la Pena, F.; Julio, L.F.; Burillo, J. Nematicidal potential of hydrolates from the semi industrial vapor-pressure extraction of Spanish aromatic plants. Environ. Sci. Pollut. Res. Int. 2018, 25, 29834–29840. [Google Scholar] [CrossRef]
- Zong, H.Y.; Liu, S.; Xing, R.E.; Chen, X.L.; Li, P.C. Protective effect of chitosan on photosynthesis and antioxidative defense system in edible rape (Brassica rapa L.) in the presence of cadmium. Ecotoxicol. Environ. Saf. 2017, 138, 271–278. [Google Scholar] [CrossRef]
- Ramkissoon, A.; Ramsubhag, A.; Jayaraman, J. Phytoelicitor activity of three Caribbean seaweed species on suppression of pathogenic infections in tomato plants. J. Appl. Phycol. 2017, 29, 3235–3244. [Google Scholar] [CrossRef]
- Magdaleno, A.; Peralta Gavensky, M.; Fassiano, A.V.; Rios de Molina, M.C.; Santos, M.; March, H.; Moretton, J.; Juarez, A.B. Phytotoxicity and genotoxicity assessment of imazethapyr herbicide using a battery of bioassays. Environ. Sci. Pollut. Res. Int. 2015, 22, 19194–19202. [Google Scholar] [CrossRef] [PubMed]










| Step (x) | Compound | Elemental Containing | DS (%) | Group | DD (%) | |||
|---|---|---|---|---|---|---|---|---|
| C% | N% | S% 1 | C/N | |||||
| COS | 43.79 | 6.75 | 0.03 | 6.49 | 35.24 | acetyl | 64.76 | |
| 1 | TCDCOS | 37.00 | 11.55 | 9.12 | 3.20 | 44.31 | thiourea | 70.08 |
| 2 | DTCOS | 37.15 | 10.74 | 7.66 | 3.46 | 62.03 | dithioformyl | 72.47 |
| COSCDZ | 34.71 | 9.10 | 5.75 | 3.81 | <44.31 | thiadiazole | 72.84 | |
| 3 | COSCZFB | 33.57 | 8.41 | 6.99 | 3.99 | 29.34 | trifluorobutenyl | 78.34 |
| Samples | Concentration (mg/mL) | 24 h | 48 h | 72 h | |||
|---|---|---|---|---|---|---|---|
| Correct Mortality (%) | LC50 (mg/mL) | Correct Mortality (%) | LC50 (mg/mL) | Correct Mortality (%) | LC50 (mg/mL) | ||
| COS | 2 | 22.48 ± 2.25 g | 6.51 | 25.13 ± 11.58 j | 4.71 | 27.02 ± 8.43 d | 3.93 |
| 4 | 43.72 ± 4.99 d,e,f | 47.33 ± 2.82 g,h,i | 53.95 ± 3.85 c | ||||
| 6 | 44.33 ± 3.31 d,e,f | 60.79 ± 13.17 d,e,f | 68.71 ± 35.32 b,c | ||||
| TCDCOS | 1.00 | 52.96 ± 3.14 c,d,e | 0.886 | 88.49 ± 10.08 a,b | 0.098 | 94.41 ± 5.29 a | 0.063 |
| 0.25 | 52.22 ± 20.09 c,d,e | 63.33 ± 12.02 d,e,f | 66.11 ± 7.52 b | ||||
| 0.063 | 22.51 ± 11.98 g | 41.01 ± 11.12 h,i | 53.52 ± 3.06 c | ||||
| DTCOS | 1.00 | 55.01 ± 1.95 c,d | 0.691 | 62.48 ± 4.87 d,e,f | 0.68 | 65.51 ± 7.86 b | 0.611 |
| 0.25 | 28.04 ± 5.57 g,h | 32.8 ± 10.33 i | 50.53 ± 6.37 c,d | ||||
| 0.063 | 8.83 ± 7.94 i | 14.85 ± 2.13 k | 24.57 ± 8.98 d | ||||
| COSCDZ | 1.00 | 65.66 ± 8.78 c | 0.131 | 81.16 ± 4.09 b,c | <0.063 | 96.67 ± 5.77 a | <0.063 |
| 0.25 | 58.65 ± 3.53 c | 72.98 ± 10.87 c,d | 96.49 ± 6.08 a | ||||
| 0.063 | 42.47 ± 4.79 d,e,f | 56.16 ± 13.3 e,f,g | 72.81 ± 15.07 b | ||||
| COSSZFB | 1.00 | 91.67 ± 8.33 a,b | 0.172 | 94.44 ± 9.62 a,b | <0.063 | 94.44 ± 9.62 a | <0.063 |
| 0.25 | 81.02 ± 5.48 b | 98.04 ± 3.4 a | 100 ± 0 a | ||||
| 0.063 | 34.36 ± 6.37 f,g,h | 70.77 ± 10.88 c,d,e | 88.54 ± 2.99 a | ||||
| fluensulfone | 1.00 | 100.00 a | <0.063 | 100.00 a | <0.063 | 100.00 a | <0.063 |
| 0.25 | 100.00 a | 100.00 a | 100.00 a | ||||
| 0.063 | 78.90 b | 97.60 a | 100.00 a | ||||
| Samples | Concentration (mg/mL) | Chl a (mg/g FW) | Chl b (mg/g FW) | Chl a + Chl b (mg/g FW) | Car (mg/g FW) | ||
|---|---|---|---|---|---|---|---|
| TCDCOS | 0.2 | 1.27 ± 0.03 b,c | 0.79 ± 0.04 c,d | 2.07 ± 0.07 c | 0.084 ± 0.003 c | ||
| 0.4 | 1.37 ± 0.20 b,c | 0.85 ± 0.08 b,c,d | 2.22 ± 0.27 b,c | 0.082 ± 0.035 b,c | |||
| 0.8 | 1.35 ± 0.22 b,c | 0.85 ± 0.11 b,c,d | 2.20 ± 0.33 b,c | 0.084 ± 0.012 b,c | |||
| COSCDZ | 0.2 | 1.22 ± 0.05 c | 0.76 ± 0.0 2c,d | 1.99 ± 0.08 c | 0.035 ± 0.009 b,c | ||
| 0.4 | 1.55 ± 0.32 b,c | 0.94 ± 0.16 b,c | 2.48 ± 0.47 b,c | 0.072 ± 0.035 b,c | |||
| 0.8 | 1.54 ± 0.22 b,c | 0.93 ± 0.10 b,c | 2.47 ± 0.31 b,c | 0.109 ± 0.038 b | |||
| COSCDZFB | 0.2 | 1.32 ± 0.35 b,c | 0.82 ± 0.14 b,c,d | 2.14 ± 0.49 b,c | 0.084 ± 0.053 b | ||
| 0.4 | 1.58 ± 0.23 b,c | 0.94 ± 0.09 b,c | 2.52 ± 0.32 b,c | 0.100 ± 0.032 b | |||
| 0.8 | 1.70 ± 0.24 b | 0.99 ± 0.12 a,b | 2.69 ± 0.36 b | 0.127 ± 0.034 b | |||
| COS | 0.2 | 1.82 ± 0.29 c | 1.05 ± 0.11 b,c | 2.87 ± 0.4 b,c | 0.194 ± 0.032 b,c | ||
| 0.4 | 1.55 ± 0.15 b,c | 0.92 ± 0.02 b,c,d | 2.47 ± 0.17 b,c | 0.104 ± 0.024 b,c | |||
| 0.8 | 1.41 ± 0.09 b,c | 0.83 ± 0.03 b,c,d | 2.24 ± 0.11 b,c | 0.092 ± 0.018 b,c | |||
| H2O | 1.39 ± 0.19 b,c | 0.83 ± 0.08 d | 2.22 ± 0.27 c | 0.098 ± 0.033 b,c | |||
| Concentration (mg/mL) | Percentage Germination | Germination Index (GI) | Relative Growth Index (RGI) | |
|---|---|---|---|---|
| COS | 0.5 | 90 ± 10 a | 0.81 ± 0.17 b,c,d,e | 0.84 ± 0.18 b,c,d |
| 1.0 | 90 ± 10 a | 0.8 ± 0.16 d,e | 0.83 ± 0.16 b,c,d | |
| 2.0 | 100 ± 0 a | 0.81 ± 0.27 b,c,d,e | 0.81 ± 0.14 c,d | |
| TCDCOS | 0.5 | 96.7 ± 5.8 a | 0.91 ± 0.14 a,b,c | 0.88 ± 0.14 b,c,d |
| 1.0 | 100 ± 0 a | 0.83 ± 0.24 a,b,c,d | 0.79 ± 0.23 d | |
| 2.0 | 96.7 ± 5.8 a | 0.93 ± 0.13 a,b,c | 0.9 ± 0.13 b,c,d | |
| DTCOS | 0.5 | 100 ± 0 a | 1.09 ± 0.34 a | 1.02 ± 0.31 b,c |
| 1.0 | 96.7 ± 5.8 a | 1.06 ± 0.23 a,b,c,d,e | 1.03 ± 0.22 b,c | |
| 2.0 | 100 ± 0 a | 1.08 ± 0.26 a,b | 1.01 ± 0.24 b,c,d | |
| COSCDZ | 0.25 | 93.3 ± 11.5 a | 1.23 ± 0.19 a,b,c,d,e | 1.23 ± 0.19 a |
| 0.5 | 100 ± 0 a | 1.02 ± 0.24 b,c,d,e | 0.95 ± 0.22 b,c,d | |
| 1 | 100 ± 0 a | 1.13 ± 0.27 a,b,c,d,e | 1.05 ± 0.25 a,b | |
| COSCZFB | 0.25 | 100 ± 0 a | 1.03 ± 0.18 e | 0.93 ± 0.16 b,c,d |
| 0.5 | 96.7 ± 5.8 a | 0.92 ± 0.12 e | 0.89 ± 0.12 b,c,d | |
| 1 | 100 ± 0 a | 1.01 ± 0.17 c,d,e | 0.94 ± 0.16 b,c,d | |
| H2O | 93.3 ± 5.8 a | 1 a,b,c,d,e | 1 b,c,d |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, Z.; Qin, Y.; Liu, S.; Xing, R.; Yu, H.; Li, P. Chitosan Oligosaccharide Fluorinated Derivative Control Root-Knot Nematode (Meloidogyne incognita) Disease Based on the Multi-Efficacy Strategy. Mar. Drugs 2020, 18, 273. https://doi.org/10.3390/md18050273
Fan Z, Qin Y, Liu S, Xing R, Yu H, Li P. Chitosan Oligosaccharide Fluorinated Derivative Control Root-Knot Nematode (Meloidogyne incognita) Disease Based on the Multi-Efficacy Strategy. Marine Drugs. 2020; 18(5):273. https://doi.org/10.3390/md18050273
Chicago/Turabian StyleFan, Zhaoqian, Yukun Qin, Song Liu, Ronge Xing, Huahua Yu, and Pengcheng Li. 2020. "Chitosan Oligosaccharide Fluorinated Derivative Control Root-Knot Nematode (Meloidogyne incognita) Disease Based on the Multi-Efficacy Strategy" Marine Drugs 18, no. 5: 273. https://doi.org/10.3390/md18050273
APA StyleFan, Z., Qin, Y., Liu, S., Xing, R., Yu, H., & Li, P. (2020). Chitosan Oligosaccharide Fluorinated Derivative Control Root-Knot Nematode (Meloidogyne incognita) Disease Based on the Multi-Efficacy Strategy. Marine Drugs, 18(5), 273. https://doi.org/10.3390/md18050273