Algae Metabolites in Cosmeceutical: An Overview of Current Applications and Challenges
Abstract
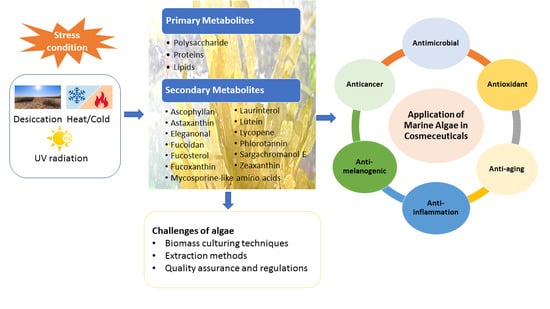
:1. Introduction
1.1. Synthetic Versus Natural Ingredients in Cosmetic Industry
1.2. Current Applications of Algae-Derived Metabolites in Cosmeceutical Industrial
1.3. UV Radiation and Skin-Related Diseases
2. Methods
3. Marine Algae-Derived Compounds in Cosmeceutical Application
3.1. Anti-Aging Activity
3.1.1. Photo-Protectivity and Antioxidant Activities
3.1.2. MMP Inhibition and Prevention of Collagen Degradation
3.1.3. Anti-Inflammatory Activity
3.2. Anti-Melanogenic Activity
3.3. Anticancer Activity
3.4. Antimicrobial Activity
4. Challenges of Algae in Cosmeceuticals
4.1. Biomass Culturing Techniques
4.2. Biometabolites Extraction Methods
4.2.1. Enzyme-Assisted Extraction (EAE)
4.2.2. Microwave-Assisted Extraction (MAE)
4.2.3. Supercritical Fluid Extraction (SFE)
4.2.4. Ultrasound-Assisted Extraction (UAE)
4.3. Quality Assurance and Regulations
5. Conclusions and Future Studies
Author Contributions
Funding
Conflicts of Interest
References
- Kligman, D. Cosmeceuticals. Dermatol. Clin. 2000, 18, 609–615. [Google Scholar] [CrossRef]
- Dureja, H.; Kaushik, D.; Gupta, M.; Kumar, V.; Lather, V. Cosmeceuticals: An emerging concept. Indian J. Pharm. 2005, 37, 155–159. [Google Scholar] [CrossRef] [Green Version]
- Smit, N.; Vicanova, J.; Pavel, S. The Hunt for Natural Skin Whitening Agents. Int. J. Mol. Sci. 2009, 10, 5326–5349. [Google Scholar] [CrossRef] [PubMed]
- Bos, J.D.; Meinardi, M.M. The 500 Dalton rule for the skin penetration of chemical compounds and drugs. Exp. Dermatol. 2000, 9, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Kerdudo, A.; Burger, P.; Merck, F.; Dingas, A.; Rolland, Y.; Michel, T.; Fernandez, X. Development of a natural ingredient–Natural preservative: A case study. Comptes Rendus Chimie 2016, 19, 1077–1089. [Google Scholar] [CrossRef] [Green Version]
- Barrett, J. Chemical Exposures: The Ugly Side of Beauty Products. Environ Health Perspect. 2005, 113, A24. [Google Scholar] [CrossRef] [Green Version]
- Global Market Value for Natural Cosmetics in 2018–2027|Statista. Available online: https://www.statista.com/statistics/673641/global-market-value-for-natural-cosmetics/ (accessed on 18 November 2019).
- Ariede, M.B.; Candido, T.M.; Jacome, A.L.M.; Velasco, M.V.R.; de Carvalho, J.C.M.; Baby, A.R. Cosmetic attributes of algae—A review. Algal Res. 2017, 25, 483–487. [Google Scholar] [CrossRef]
- Wang, H.M.D.; Chen, C.C.; Huynh, P.; Chang, J.S. Exploring the potential of using algae in cosmetics. Bioresour. Technol. 2015, 184, 355–362. [Google Scholar] [CrossRef]
- Fu, W.; Nelson, D.R.; Yi, Z.; Xu, M.; Khraiwesh, B.; Jijakli, K.; Chaiboonchoe, A.; Alzahmi, A.; Al-Khairy, D.; Brynjolfsson, S.; et al. Bioactive compounds from microalgae: Current development and prospects. Stud. Nat. Prod. Chem. 2017, 54, 199–225. [Google Scholar]
- Freile-Pelegrín, Y.; Robledo, D. Bioactive phenolic compounds from algae. In Bioactive Compounds from Marine Foods: Plant and Animal Sources, 1st ed.; Hernández-Ledesma, B., Herrero, M., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2014; pp. 113–129. [Google Scholar]
- Thomas, N.V.; Kim, S.K. Beneficial effects of marine algal compounds in cosmeceuticals. Mar. Drugs 2013, 11, 146–164. [Google Scholar] [CrossRef] [Green Version]
- Fernando, I.S.; Nah, J.W.; Jeon, Y.J. Potential anti-inflammatory natural products from marine algae. Environ. Toxicol. Pharm. 2016, 48, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Yuan, J.-P.; Wu, C.-F.; Wang, J.-H. Fucoxanthin, a marine carotenoid present in brown seaweeds and diatoms: Metabolism and bioactivities relevant to human health. Mar. Drugs 2011, 9, 1806–1828. [Google Scholar] [CrossRef] [PubMed]
- Talero, E.; García-Mauriño, S.; Ávila-Román, J.; Rodríguez-Luna, A.; Alcaide, A.; Motilva, V. Bioactive compounds isolated from microalgae in chronic inflammation and cancer. Mar. Drugs 2015, 13, 6152–6209. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.M.D.; Li, X.C.; Lee, D.J.; Chang, J.S. Potential biomedical applications of marine algae. Bioresour. Technol. 2017, 244, 1407–1415. [Google Scholar] [CrossRef] [PubMed]
- Saidani, K.; Bedjou, F.; Benabdesselam, F.; Touati, N. Antifungal activity of methanolic extracts of four Algerian marine algae species. Afr. J. Biotechnol. 2012, 11, 9496–9500. [Google Scholar] [CrossRef]
- Hwang, E.; Park, S.Y.; Sun, Z.W.; Shin, H.S.; Lee, D.G.; Yi, T.H. The protective effects of fucosterol against skin damage in UVB-irradiated human dermal fibroblasts. Mar. Biotechnol. 2014, 16, 361–370. [Google Scholar] [CrossRef]
- Martins, A.; Vieira, H.; Gaspar, H.; Santos, S. Marketed marine natural products in the pharmaceutical and cosmeceutical industries: Tips for success. Mar. Drugs 2014, 12, 1066–1101. [Google Scholar] [CrossRef] [Green Version]
- Nayaka, S.; Toppo, K.; Verma, S. Adaptation in Algae to Environmental Stress and Ecological Conditions. In Plant Adaptation Strategies in Changing Environment; Springer: Singapore, 2017; pp. 103–115. [Google Scholar] [CrossRef]
- Katz, A.; Waridel, P.; Shevchenko, A.; Pick, U. Salt-induced changes in the plasma membrane proteome of the halotolerant alga Dunaliella salina as revealed by blue native gel electrophoresis and nano-LC-MS/MS analysis. Mol. Cell. Proteom. 2007, 6, 1459–1472. [Google Scholar] [CrossRef] [Green Version]
- Sydney, E.B.; Sydney, A.C.N.; de Carvalho, J.C.; Soccol, C.R. Potential carbon fixation of industrially important microalgae. In Biofuels from Algae; Elsevier: Amsterdam, The Netherlands, 2019; pp. 67–88. [Google Scholar]
- Usher, P.K.; Ross, A.B.; Camargo-Valero, M.A.; Tomlin, A.S.; Gale, W.F. An overview of the potential environmental impacts of large-scale microalgae cultivation. Biofuels 2014, 5, 331–349. [Google Scholar] [CrossRef]
- Álvarez-Gómez, F.; Korbee, N.; Casas-Arrojo, V.; Abdala-Díaz, R.T.; Figueroa, F.L. UV photoprotection, cytotoxicity and immunology capacity of red algae extracts. Molecules 2019, 24, 341. [Google Scholar] [CrossRef] [Green Version]
- Khanra, S.; Mondal, M.; Halder, G.; Tiwari, O.N.; Gayen, K.; Bhowmick, T.K. Downstream processing of microalgae for pigments, protein and carbohydrate in industrial application: A review. Food Bioprod. Process. 2018, 110, 60–84. [Google Scholar] [CrossRef]
- Pagels, F.; Guedes, A.C.; Amaro, H.M.; Kijjoa, A.; Vasconcelos, V. Phycobiliproteins from cyanobacteria: Chemistry and biotechnological applications. Biotechnol. Adv. 2019, 37, 422–443. [Google Scholar] [CrossRef] [PubMed]
- Ambati, R.R.; Gogisetty, D.; Aswathanarayana, R.G.; Ravi, S.; Bikkina, P.N.; Bo, L.; Yuepeng, S. Industrial potential of carotenoid pigments from microalgae: Current trends and future prospects. Crit. Rev. Food Sci. Nutr. 2019, 59, 1880–1902. [Google Scholar] [CrossRef] [PubMed]
- Galasso, C.; Corinaldesi, C.; Sansone, C. Carotenoids from marine organisms: Biological functions and industrial applications. Antioxidant 2017, 6, 96. [Google Scholar] [CrossRef] [Green Version]
- Torres, P.; Santos, J.P.; Chow, F.; dos Santos, D.Y. A comprehensive review of traditional uses, bioactivity potential, and chemical diversity of the genus Gracilaria (Gracilariales, Rhodophyta). Algal Res. 2019, 37, 288–306. [Google Scholar] [CrossRef]
- Jahan, A.; Ahmad, I.Z.; Fatima, N.; Ansari, V.A.; Akhtar, J. Algal bioactive compounds in the cosmeceutical industry: A review. Phycologia 2017, 56, 410–422. [Google Scholar] [CrossRef]
- Siezen, R.J. Microbial sunscreens. Microb. Biotechnol. 2011, 4, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Muñoz, R.; Gonzalez-Fernandez, C. (Eds.) Microalgae-Based Biofuels and Bioproducts: From Feedstock Cultivation to End-Products; Woodhead Publishing: Duxford, UK, 2017. [Google Scholar]
- Berthon, J.Y.; Nachat-Kappes, R.; Bey, M.; Cadoret, J.P.; Renimel, I.; Filaire, E. Marine algae as attractive source to skin care. Free Radic. Res. 2017, 51, 555–567. [Google Scholar] [CrossRef]
- Tabassum, N.; Hamdani, M. Plants used to treat skin diseases. Pharm. Rev. 2014, 8, 52–60. [Google Scholar] [CrossRef] [Green Version]
- Skin Cancer|Skin Cancer Facts|Common Skin Cancer Types. Available online: https://www.cancer.org/cancer/skin-cancer.html (accessed on 27 August 2018).
- Tan, L.T.; Mahendra, C.K.; Yow, Y.Y.; Chan, K.G.; Khan, T.M.; Lee, L.H.; Goh, B.H. Streptomyces sp. MUM273b: A mangrove-derived potential source for antioxidant and UVB radiation protectants. Microbiol. Open 2019, 8, e859. [Google Scholar] [CrossRef] [Green Version]
- D’Orazio, J.; Jarrett, S.; Amaro-Ortiz, A.; Scott, T. UV radiation and the skin. Int. J. Mol. Sci. 2013, 14, 12222–12248. [Google Scholar] [CrossRef] [Green Version]
- Amaro-Ortiz, A.; Yan, B.; D’Orazio, J.A. Ultraviolet radiation, aging and the skin: Prevention of damage by topical cAMP manipulation. Molecules 2014, 19, 6202–6219. [Google Scholar] [CrossRef] [PubMed]
- Azam, M.S.; Choi, J.; Lee, M.S.; Kim, H.R. Hypopigmenting effects of brown algae-derived phytochemicals: A review on molecular mechanisms. Mar. Drugs 2017, 15, 297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, H.; Chen, T.; Nines, R.G.; Shin, H.C.; Stoner, G.D. Photochemoprevention of UVB-induced skin carcinogenesis in SKH-1 mice by brown algae polyphenols. Int. J. Cancer 2006, 119, 2742–2749. [Google Scholar] [CrossRef] [PubMed]
- Mahendra, C.K.; Tan, L.T.H.; Yap, W.H.; Chan, C.K.; Pusparajah, P.; Goh, B.H. An optimized cosmetic screening assay for ultraviolet B (UVB) protective property of natural products. Prog. Drug Discov. Biomed. Sci. 2019, 2. [Google Scholar] [CrossRef]
- Shibata, T.; Fujimoto, K.; Nagayama, K.; Yamaguchi, K.; Nakamura, T. Inhibitory activity of brown algal phlorotannins against hyaluronidase. Int. J. Food Sci. Technol. 2002, 37, 703–709. [Google Scholar] [CrossRef]
- Joe, M.J.; Kim, S.N.; Choi, H.Y.; Shin, W.S.; Park, G.M.; Kang, D.W.; Kim, Y.K. The inhibitory effects of eckol and dieckol from Ecklonia stolonifera on the expression of matrix metalloproteinase-1 in human dermal fibroblasts. Biol. Pharm. Bull. 2006, 29, 1735–1739. [Google Scholar] [CrossRef] [Green Version]
- Yoon, N.Y.; Eom, T.K.; Kim, M.M.; Kim, S.K. Inhibitory effect of phlorotannins isolated from Ecklonia cava on mushroom tyrosinase activity and melanin formation in mouse B16F10 melanoma cells. J. Agric. Food Chem. 2009, 57, 4124–4129. [Google Scholar] [CrossRef]
- Anastyuk, S.; Shervchenko, N.; Ermakova, S.; Vishchuk, O.; Nazarenko, E.; Dmitrenok, P.; Zvyagintseva, T. Anticancer activity in vitro of a fucoidan from the brown algae Fucus evanescens and its low-molecular fragments, structurally characterized by tandem mass-spectrometry. Carbohydr. Polym. 2012, 87, 186–194. [Google Scholar] [CrossRef]
- Wang, Z.-J.; Xu, W.; Liang, J.-W.; Wang, C.-S.; Kang, Y. Effect of fucoidan on B16 murine melanoma cell melanin formation and apoptosis. Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 149–155. [Google Scholar] [CrossRef] [Green Version]
- Fernando, I.S.; Sanjeewa, K.A.; Samarakoon, K.W.; Lee, W.W.; Kim, H.S.; Kang, N.; Ranasinghe, P.; Lee, H.S.; Jeon, Y.J. A fucoidan fraction purified from Chnoospora minima; a potential inhibitor of LPS-induced inflammatory responses. Int. J. Boil. Macromol. 2017, 104, 1185–1193. [Google Scholar] [CrossRef] [PubMed]
- Shimoda, H.; Tanaka, J.; Shan, S.J.; Maoka, T. Anti-pigmentary activity of fucoxanthin and its influence on skin mRNA expression of melanogenic molecules. J. Pharm. Pharm. 2010, 62, 1137–1145. [Google Scholar] [CrossRef]
- Mise, T.; Ueda, M.; Yasumoto, T. Production of fucoxanthin-rich powder from Cladosiphon okamuranus. Adv. J. Food Sci. Technol. 2011, 3, 73–76. [Google Scholar]
- Urikura, I.; Sugawara, T.; Hirata, T. Protective effect of fucoxanthin against UVB-induced skin photoaging in hairless mice. Biosci. Biotechnol. Biochem. 2011, 75, 757–760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakai, S.; Komura, Y.; Nishimura, Y.; Sugawara, T.; Hirata, T. Inhibition of mast cell degranulation by phycoerythrin and its pigment moiety phycoerythrobilin, prepared from Porphyra yezoensis. Food Sci. Technol. Res. 2011, 17, 171–177. [Google Scholar] [CrossRef] [Green Version]
- Suh, S.S.; Hwang, J.; Park, M.; Seo, H.; Kim, H.S.; Lee, J.; Moh, S.; Lee, T.K. Anti-inflammation activities of mycosporine-like amino acids (MAAs) in response to UV radiation suggest potential anti-skin aging activity. Mar. Drugs 2014, 12, 5174–5187. [Google Scholar] [CrossRef] [Green Version]
- Hartmann, A.; Gostner, J.; Fuchs, J.E.; Chaita, E.; Aligiannis, N.; Skaltsounis, L.; Ganzera, M. Inhibition of collagenase by mycosporine-like amino acids from marine sources. Planta Medica 2015, 81, 813–820. [Google Scholar] [CrossRef] [Green Version]
- Abu, R.; Jiang, Z.; Ueno, M.; Isaka, S.; Nakazono, S.; Okimura, T.; Cho, K.; Yamaguchi, K.; Kim, D.; Oda, T. Anti-metastatic effects of the sulfated polysaccharide ascophyllan isolated from Ascophyllum nodosum on B16 melanoma. Biochem. Biophys. Res. Commun. 2015, 458, 727–732. [Google Scholar] [CrossRef]
- Silva, J.; Alves, C.; Freitas, R.; Martins, A.; Pinteus, S.; Ribeiro, J.; Gaspar, H.; Alfonso, A.; Pedrosa, R. Antioxidant and Neuroprotective Potential of the Brown Seaweed Bifurcaria bifurcata in an in vitro Parkinson’s Disease Model. Mar. Drugs 2019, 17, 85. [Google Scholar] [CrossRef] [Green Version]
- Muñoz-Ochoa, M.; Murillo-Álvarez, J.I.; Zermeño-Cervantes, L.A.; Martínez-Díaz, S.; Rodríguez-Riosmena, R. Screening of extracts of algae from Baja California Sur, Mexico as reversers of the antibiotic resistance of some pathogenic bacteria. Eur. Rev. Med. Pharmacol. Sci. 2010, 14, 739–747. [Google Scholar]
- Panayotova, V.; Merzdhanova, A.; Dobreva, D.A.; Zlatanov, M.; Makedonski, L. Lipids of black sea algae: Unveiling their potential for pharmaceutical and cosmetic applications. J. IMAB–Ann. Proc. Sci. Pap. 2017, 23, 1747–1751. [Google Scholar] [CrossRef] [Green Version]
- Messina, C.M.; Renda, G.; Laudicella, V.A.; Trepos, R.; Fauchon, M.; Hellio, C.; Santulli, A. From ecology to biotechnology, study of the defense strategies of algae and halophytes (from Trapani Saltworks, NW Sicily) with a focus on antioxidants and antimicrobial properties. Int. J. Mol. Sci. 2019, 20, 881. [Google Scholar] [CrossRef] [Green Version]
- Airanthi, M.W.A.; Hosokawa, M.; Miyashita, K. Comparative antioxidant activity of edible Japanese brown seaweeds. J. Food Sci. 2011, 76, C104–C111. [Google Scholar] [CrossRef] [PubMed]
- Kosanić, M.; Ranković, B.; Stanojković, T. Brown macroalgae from the Adriatic Sea as a promising source of bioactive nutrients. J. Food Meas. Charact. 2019, 13, 330–338. [Google Scholar] [CrossRef]
- Jung, W.K.; Heo, S.J.; Jeon, Y.J.; Lee, C.M.; Park, Y.M.; Byun, H.G.; Choi, Y.H.; Park, S.G.; Choi, I.W. Inhibitory effects and molecular mechanism of dieckol isolated from marine brown alga on COX-2 and iNOS in microglial cells. J. Agric. Food Chem. 2009, 57, 4439–4446. [Google Scholar] [CrossRef]
- Kang, K.A.; Lee, K.H.; Chae, S.; Koh, Y.S.; Yoo, B.S.; Kim, J.H.; Ham, Y.M.; Baik, J.S.; Lee, N.H.; Hyun, J.W. Triphlorethol-A from Ecklonia cava protects V79-4 lung fibroblast against hydrogen peroxide induced cell damage. Free Radic. Res. 2005, 39, 883–892. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Kwon, M.S.; Choi, J.W.; Shin, T.; No, H.K.; Choi, J.S.; Byun, D.S.; Kim, J.I.; Kim, H.R. Anti-inflammatory activities of an ethanol extract of Ecklonia stolonifera in lipopolysaccharide-stimulated RAW 264.7 murine macrophage cells. J. Agric. Food Chem. 2012, 60, 9120–9129. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, Y.; Takeuchi, Y.; Kakinuma, M.; Amano, H. Inhibitory effects of seaweeds on histamine release from rat basophile leukemia cells (RBL-2H3). Fish. Sci. 2006, 72, 1286–1291. [Google Scholar] [CrossRef]
- Teas, J.; Irhimeh, M.R. Melanoma and brown seaweed: An integrative hypothesis. J. Appl. Phycol. 2017, 29, 941–948. [Google Scholar] [CrossRef] [Green Version]
- Zaragozá, M.C.; López, D.P.; Sáiz, M.; Poquet, M.; Pérez, J.; Puig-Parellada, P.; Marmol, F.; Simonetti, P.; Gardana, C.; Lerat, Y.; et al. Toxicity and antioxidant activity in vitro and in vivo of two Fucus vesiculosus extracts. J. Agric. Food Chem. 2008, 56, 7773–7780. [Google Scholar] [CrossRef]
- Campos, A.M.; Matos, J.; Afonso, C.; Gomes, R.; Bandarra, N.M.; Cardoso, C. Azorean macroalgae (Petalonia binghamiae, Halopteris scoparia and Osmundea pinnatifida) bioprospection: A study of fatty acid profiles and bioactivity. Int. J. Food Sci. Technol. 2018, 54, 880–890. [Google Scholar] [CrossRef]
- Plaza, M.; Santoyo, S.; Jaime, L.; Reina, G.G.B.; Herrero, M.; Señoráns, F.J.; Ibáñez, E. Screening for bioactive compounds from algae. J. Pharm. Biomed. Anal. 2010, 51, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.O.; Kim, H.S.; Han, M.H.; Choi, Y.H.; Park, C.; Kim, B.W.; Hwang, H.J. Effects of Hizikia fusiforme fractions on melanin synthesis in B16F10 melanoma cells. J. Life Sci. 2013, 23, 1495–1500. [Google Scholar] [CrossRef]
- Yan, X.; Chuda, Y.; Suzuki, M.; Nagata, T. Fucoxanthin as the major antioxidant in Hijikia fusiformis, a common edible seaweed. Biosci. Biotechnol. Biochem. 1999, 63, 605–607. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.N.; Yang, H.M.; Kang, S.M.; Kim, D.; Ahn, G.; Jeon, Y.J. Octaphlorethol A isolated from Ishige foliacea inhibits α-MSH-stimulated induced melanogenesis via ERK pathway in B16F10 melanoma cells. Food Chem. Toxicol. 2013, 59, 521–526. [Google Scholar] [CrossRef]
- Kim, K.N.; Yang, H.M.; Kang, S.M.; Ahn, G.N.; Roh, S.W.; Lee, W.; Kim, D.K.; Jeon, Y.J. Whitening effect of octaphlorethol A isolated from Ishige foliacea in an in vivo zebrafish model. J. Microbiol. Biotechnol. 2015, 25, 448–451. [Google Scholar] [CrossRef] [Green Version]
- Heo, S.J.; Hwang, J.Y.; Choi, J.I.; Lee, S.H.; Park, P.J.; Kang, D.H.; Oh, C.; Kim, D.W.; Han, J.S.; Jeon, Y.J.; et al. Protective effect of diphlorethohydroxycarmalol isolated from Ishige okamurae against high glucose-induced-oxidative stress in human umbilical vein endothelial cells. Food Chem. Toxicol. 2010, 48, 1448–1454. [Google Scholar] [CrossRef]
- Del Olmo, A.; Picon, A.; Nuñez, M. High pressure processing for the extension of Laminaria ochroleuca (kombu) shelf-life: A comparative study with seaweed salting and freezing. Innov. Food Sci. Emerg. Technol. 2019, 52, 420–428. [Google Scholar] [CrossRef]
- SpecialChem—The Universal Selection Source: Cosmetics Ingredients. Available online: https://cosmetics.specialchem.com/ (accessed on 5 May 2020).
- Antony, T.; Chakraborty, K. Xenicanes attenuate pro-inflammatory 5-lipoxygenase: Prospective natural anti-inflammatory leads from intertidal brown seaweed Padina tetrastromatica. Med. Chem. Res. 2019, 28, 591–607. [Google Scholar] [CrossRef]
- Mohsin, S.; Kurup, G.M. Mechanism underlying the anti-inflammatory effect of sulphated polysaccharide from Padina tetrastromatica against carrageenan induced paw edema in rats. Biomed. Prev. Nutr. 2011, 1, 294–301. [Google Scholar] [CrossRef]
- Yoon, H.S.; Koh, W.B.; Oh, Y.S.; Kim, I.J. The Anti-Melanogenic Effects of Petalonia binghamiae extarcts in α-melanocyte stimulating hormone-induced B16/F10 murine melanoma cells. J. Korean Soc. Appl. Biol. Chem. 2009, 52, 564–567. [Google Scholar] [CrossRef]
- Sappati, P.K.; Nayak, B.; VanWalsum, G.P.; Mulrey, O.T. Combined effects of seasonal variation and drying methods on the physicochemical properties and antioxidant activity of sugar kelp (Saccharina latissima). J. Appl. Phycol. 2019, 31, 1311–1332. [Google Scholar] [CrossRef]
- Vasconcelos, J.B.; de Vasconcelos, E.R.; Urrea-Victoria, V.; Bezerra, P.S.; Reis, T.N.; Cocentino, A.L.; Navarro, D.M.; Chow, F.; Areces, A.J.; Fujii, M.T. Antioxidant activity of three seaweeds from tropical reefs of Brazil: Potential sources for bioprospecting. J. Appl. Phycol. 2019, 31, 835–846. [Google Scholar] [CrossRef]
- Hwang, P.A.; Chien, S.Y.; Chan, Y.L.; Lu, M.K.; Wu, C.H.; Kong, Z.L.; Wu, C.J. Inhibition of lipopolysaccharide (LPS)-induced inflammatory responses by Sargassum hemiphyllum sulfated polysaccharide extract in RAW 264.7 macrophage cells. J. Agric. Food Chem. 2011, 59, 2062–2068. [Google Scholar] [CrossRef] [PubMed]
- Ale, M.T.; Maruyama, H.; Tamauchi, H.; Mikkelsen, J.D.; Meyer, A.S. Fucose-containing sulfated polysaccharides from brown seaweeds inhibit proliferation of melanoma cells and induce apoptosis by activation of caspase-3 in vitro. Mar. Drugs 2011, 9, 2605–2621. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.A.; Ahn, B.N.; Kong, C.S.; Kim, S.K. The chromene sargachromanol E inhibits ultraviolet A-induced ageing of skin in human dermal fibroblasts. Br. J. Dermatol. 2013, 168, 968–976. [Google Scholar] [CrossRef] [PubMed]
- Fernando, I.S.; Jayawardena, T.U.; Sanjeewa, K.A.; Wang, L.; Jeon, Y.J.; Lee, W.W. Anti-inflammatory potential of alginic acid from Sargassum horneri against urban aerosol-induced inflammatory responses in keratinocytes and macrophages. Ecotoxicol. Environ. Saf. 2018, 160, 24–31. [Google Scholar] [CrossRef]
- Balboa, E.M.; Li, Y.X.; Ahn, B.N.; Eom, S.H.; Domínguez, H.; Jiménez, C.; Rodríguez, J. Photodamage attenuation effect by a tetraprenyltoluquinol chromane meroterpenoid isolated from Sargassum muticum. J. Photochem. Photobiol. B Biol. 2015, 148, 51–58. [Google Scholar] [CrossRef]
- Heo, S.-J.; Jeon, Y.-J. Protective effect of fucoxanthin isolated from Sargassum siliquastrum on UV-B induced cell damage. J. Photochem. Photobiol. B 2009, 95, 101–107. [Google Scholar] [CrossRef]
- Seo, Y.; Park, K.E.; Kim, Y.A.; Lee, H.J.; Yoo, J.S.; Ahn, J.W.; Lee, B.J. Isolation of tetraprenyltoluquinols from the brown alga Sargassum thunbergii. Chem. Pharm. Bull. 2006, 54, 1730–1733. [Google Scholar] [CrossRef] [Green Version]
- Santos, J.P.; Torres, P.B.; dos Santos, D.Y.; Motta, L.B.; Chow, F. Seasonal effects on antioxidant and anti-HIV activities of Brazilian seaweeds. J. Appl. Phycol. 2018, 31, 1333–1341. [Google Scholar] [CrossRef]
- Velatooru, L.R.; Baggu, C.B.; Janapala, V.R. Spatane diterpinoid from the brown algae, Stoechospermum marginatum induces apoptosis via ROS induced mitochondrial mediated caspase dependent pathway in murine B16F10 melanoma cells. Mol. Carcinog. 2016, 55, 2222–2235. [Google Scholar] [CrossRef] [PubMed]
- Kelman, D.; Posner, E.K.; McDermid, K.J.; Tabandera, N.K.; Wright, P.R.; Wright, A.D. Antioxidant activity of Hawaiian marine algae. Mar. Drugs 2012, 10, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Kim, D.; Yoon, H.; Lee, W.; Lee, N.; Hyun, C. Melanogenesis inhibitory activity of Korean Undaria pinnatifida in mouse B16 melanoma cells. Interdiscip. Toxicol. 2014, 7, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.N.A.; Yoon, S.J.; Choi, J.S.; Park, N.G.; Lee, H.H.; Cho, J.Y.; Hong, Y.K. Anti-edema effects of brown seaweed (Undaria pinnatifida) extract on phorbol 12-myristate 13-acetate-induced mouse ear inflammation. Am. J. Chin. Med. 2009, 37, 373–381. [Google Scholar] [CrossRef]
- Rhimou, B.; Hassane, R.; José, M.; Nathalie, B. The antibacterial potential of the seaweeds (Rhodophyceae) of the Strait of Gibraltar and the Mediterranean Coast of Morocco. Afr. J. Biotechnol. 2010, 9, 6365–6372. [Google Scholar]
- Wang, W.J.; Li, X.L.; Zhu, J.Y.; Liang, Z.R.; Liu, F.L.; Sun, X.T.; Wang, F.J.; Shen, Z.G. Antioxidant response to salinity stress in freshwater and marine Bangia (Bangiales, Rhodophyta). Aquat. Bot. 2019, 154, 35–41. [Google Scholar] [CrossRef]
- Jaballi, I.; Sallem, I.; Feki, A.; Cherif, B.; Kallel, C.; Boudawara, O.; Jamoussi, K.; Mellouli, L.; Nasri, M.; Amara, I.B. Polysaccharide from a Tunisian red seaweed Chondrus canaliculatus: Structural characteristics, antioxidant activity and in vivo hemato-nephroprotective properties on maneb induced toxicity. Int. J. Biol. Macromol. 2019, 123, 1267–1277. [Google Scholar] [CrossRef]
- Kulshreshtha, G.; Borza, T.; Rathgeber, B.; Stratton, G.S.; Thomas, N.A.; Critchley, A.; Hafting, J.; Prithiviraj, B. Red seaweeds Sarcodiotheca gaudichaudii and Chondrus crispus down regulate virulence factors of Salmonella enteritidis and induce immune responses in Caenorhabditis elegans. Front. Microbiol. 2016, 7, 421. [Google Scholar] [CrossRef]
- Ryu, B.; Qian, Z.J.; Kim, M.M.; Nam, K.W.; Kim, S.K. Anti-photoaging activity and inhibition of matrix metalloproteinase (MMP) by marine red alga, Corallina pilulifera methanol extract. Radiat. Phys. Chem. 2009, 78, 98–105. [Google Scholar] [CrossRef]
- Francavilla, M.; Franchi, M.; Monteleone, M.; Caroppo, C. The red seaweed Gracilaria gracilis as a multi products source. Mar. Drugs 2013, 11, 3754–3776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Zhang, Z.; Wu, Y.; Sun, X.; Xu, N. Synthesized sulfated and acetylated derivatives of polysaccharide extracted from Gracilariopsis lemaneiformis and their potential antioxidant and immunological activity. Int. J. Boil. Macromol. 2019, 124, 568–572. [Google Scholar] [CrossRef] [PubMed]
- Antony, T.; Chakraborty, K. First report of antioxidative 2H-chromenyl derivatives from the intertidal red seaweed Gracilaria salicornia as potential anti-inflammatory agents. Nat. Prod. Res. 2019. [Google Scholar] [CrossRef] [PubMed]
- Moshfegh, A.; Salehzadeh, A.; Shandiz, S.A.S.; Shafaghi, M.; Naeemi, A.S.; Salehi, S. Phytochemical analysis, antioxidant, anticancer and antibacterial properties of the Caspian Sea red macroalgae, Laurencia caspica. Iran. J. Sci. Technol. Trans. A Sci. 2019, 43, 49–56. [Google Scholar] [CrossRef]
- Lajili, S.; Ammar, H.H.; Mzoughi, Z.; Amor, H.B.H.; Muller, C.D.; Majdoub, H.; Bouraoui, A. Characterization of sulfated polysaccharide from Laurencia obtusa and its apoptotic, gastroprotective and antioxidant activities. Int. J. Biol. Macromol. 2019, 126, 326–336. [Google Scholar] [CrossRef]
- De Jesus Raposo, M.; de Morais, A.; de Morais, R. Marine polysaccharides from algae with potential biomedical applications. Mar. Drugs 2015, 13, 2967–3028. [Google Scholar] [CrossRef]
- Pimentel, F.B.; Alves, R.C.; Rodrigues, F.; PP Oliveira, M.B. Macroalgae-derived ingredients for cosmetic industry—An Update. Cosmetics 2017, 5, 2. [Google Scholar] [CrossRef] [Green Version]
- Cian, R.E.; Bacchetta, C.; Rossi, A.; Cazenave, J.; Drago, S.R. Red seaweed Pyropia columbina as antioxidant supplement in feed for cultured juvenile Pacú (Piaractus mesopotamicus). J. Appl. Phycol. 2019, 31, 1455–1465. [Google Scholar] [CrossRef]
- Kim, C.R.; Kim, Y.M.; Lee, M.K.; Kim, I.H.; Choi, Y.H.; Nam, T.J. Pyropia yezoensis peptide promotes collagen synthesis by activating the TGF-β/Smad signaling pathway in the human dermal fibroblast cell line Hs27. Int. J. Mol. Med. 2017, 39, 31–38. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Li, X.M.; Gloer, J.B.; Wang, B.G. New nitrogen-containing bromophenols from the marine red alga Rhodomela confervoides and their radical scavenging activity. Food chem. 2012, 135, 868–872. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, F.; Wang, X.; Liu, X.; Hou, Y.; Zhang, Q. Extraction of the polysaccharides from five algae and their potential antioxidant activity in vitro. Carbohydr. Polym. 2010, 82, 118–121. [Google Scholar] [CrossRef]
- Premalatha, M.; Dhasarathan, P.; Theriappan, P. Phytochemical characterization and antimicrobial efficiency of seaweed samples, Ulva fasciata and Chaetomorpha antennina. Int. J. Pharm. Biol. Sci. 2011, 2, 288–293. [Google Scholar]
- Lee, C.; Park, G.H.; Ahn, E.M.; Kim, B.A.; Park, C.I.; Jang, J.H. Protective effect of Codium fragile against UVB-induced pro-inflammatory and oxidative damages in HaCaT cells and BALB/c mice. Fitoterapia 2013, 86, 54–63. [Google Scholar] [CrossRef]
- Pezeshk, F.; Babaei, S.; Abedian Kenari, A.; Hedayati, M.; Naseri, M. The effect of supplementing diets with extracts derived from three different species of macroalgae on growth, thermal stress resistance, antioxidant enzyme activities and skin colour of electric yellow cichlid (Labidochromis caeruleus). Aquac. Nutr. 2019, 25, 436–443. [Google Scholar] [CrossRef]
- Farasat, M.; Khavari-Nejad, R.A.; Nabavi, S.M.B.; Namjooyan, F. Antioxidant properties of two edible green seaweeds from northern coasts of the Persian Gulf. Jundishapur. J. Nat. Pharm. Prod. 2013, 8, 47. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, H.; Salgado, J.M.; Martins, N.; Peres, H.; Oliva-Teles, A.; Belo, I. Sequential bioprocessing of Ulva rigida to produce lignocellulolytic enzymes and to improve its nutritional value as aquaculture feed. Bioresour. Technol. 2019, 281, 277–285. [Google Scholar] [CrossRef] [Green Version]
- Adrien, A.; Bonnet, A.; Dufour, D.; Baudouin, S.; Maugard, T.; Bridiau, N. Pilot production of ulvans from Ulva sp. and their effects on hyaluronan and collagen production in cultured dermal fibroblasts. Carbohydr. Polym. 2017, 157, 1306–1314. [Google Scholar] [CrossRef]
- Mourelle, M.L.; Gómez, C.P.; Legido, J.L. The potential use of marine microalgae and cyanobacteria in cosmetics and thalassotherapy. Cosmetics 2017, 4, 46. [Google Scholar] [CrossRef] [Green Version]
- José de Andrade, C.; Maria de Andrade, L. An overview on the application of genus Chlorella in biotechnological processes. J. Adv. Res. Biotechnol. 2017, 2, 1–9. [Google Scholar] [CrossRef]
- Makpol, S.; Yeoh, T.W.; Ruslam, F.A.C.; Arifin, K.T.; Yusof, Y.A.M. Comparative effect of Piper betle, Chlorella vulgaris and tocotrienol-rich fraction on antioxidant enzymes activity in cellular ageing of human diploid fibroblasts. BMC Complement. Altern. Med. 2013, 13, 210. [Google Scholar] [CrossRef] [Green Version]
- Kang, H.; Lee, C.H.; Kim, J.R.; Kwon, J.Y.; Seo, S.G.; Han, J.G.; Kim, B.; Kim, J.; Lee, K.W. Chlorella vulgaris attenuates dermatophagoides farinae-induced atopic dermatitis-like symptoms in NC/Nga mice. Int. J. Mol. Sci. 2015, 16, 21021–21034. [Google Scholar] [CrossRef] [Green Version]
- Murthy, K.; Vanitha, A.; Rajesha, J.; Swamy, M.; Sowmya, P.; Ravishankar, G. In vivo antioxidant activity of carotenoids from Dunaliella salina—A green microalga. Life Sci. 2005, 76, 1381–1390. [Google Scholar] [CrossRef]
- Yang, D.J.; Lin, J.T.; Chen, Y.C.; Liu, S.C.; Lu, F.J.; Chang, T.J.; Wang, M.; Lin, H.W.; Chang, Y.Y. Suppressive effect of carotenoid extract of Dunaliella salina alga on production of LPS-stimulated pro-inflammatory mediators in RAW264. 7 cells via NF-κB and JNK inactivation. J. Funct. Foods 2013, 5, 607–615. [Google Scholar] [CrossRef]
- Shin, J.; Kim, J.E.; Pak, K.J.; Kang, J.I.; Kim, T.S.; Lee, S.Y.; Yeo, I.H.; Park, J.H.Y.; Kim, J.H.; Kang, N.J.; et al. A Combination of soybean and Haematococcus extract alleviates ultraviolet B-induced photoaging. Int. J. Mol. Sci. 2017, 18, 682. [Google Scholar] [CrossRef] [Green Version]
- Rao, A.R.; Sindhuja, H.N.; Dharmesh, S.M.; Sankar, K.U.; Sarada, R.; Ravishankar, G.A. Effective inhibition of skin cancer, tyrosinase, and antioxidative properties by astaxanthin and astaxanthin esters from the green alga Haematococcus pluvialis. J. Agric. Food Chem. 2013, 61, 3842–3851. [Google Scholar] [CrossRef]
- Banskota, A.H.; Sperker, S.; Stefanova, R.; McGinn, P.J.; O’Leary, S.J. Antioxidant properties and lipid composition of selected microalgae. J. Appl. Phycol. 2019, 31, 309–318. [Google Scholar] [CrossRef]
- Shen, C.T.; Chen, P.Y.; Wu, J.J.; Lee, T.M.; Hsu, S.L.; Chang, C.M.J.; Young, C.C.; Shieh, C.J. Purification of algal anti-tyrosinase zeaxanthin from Nannochloropsis oculate using supercritical anti-solvent precipitation. J. Supercrit. Fluids 2011, 55, 955–962. [Google Scholar] [CrossRef]
- Wu, H.L.; Fu, X.Y.; Cao, W.Q.; Xiang, W.Z.; Hou, Y.J.; Ma, J.K.; Wang, Y.; Fan, C.D. Induction of apoptosis in human glioma cells by fucoxanthin via triggering of ROS-mediated oxidative damage and regulation of MAPKs and PI3K-AKT pathways. J. Agric. Food Chem. 2019, 67, 2212. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, R.P.; Sonani, R.R.; Madamwar, D.; Incharoensakdi, A. Characterization and antioxidant functions of mycosporine-like amino acids in the cyanobacterium Nostoc sp. R76DM. Algal Res. 2016, 16, 110–118. [Google Scholar] [CrossRef]
- Haimeur, A.; Ulmann, L.; Mimouni, V.; Guéno, F.; Pineau-Vincent, F.; Meskini, N.; Tremblin, G. The role of Odontella aurita, a marine diatom rich in EPA, as a dietary supplement in dyslipidemia, platelet function and oxidative stress in high-fat fed rats. Lipids Health Dis. 2012, 11, 147. [Google Scholar] [CrossRef] [Green Version]
- Shannon, E.; Abu-Ghannam, N. Antibacterial derivatives of marine algae: An overview of pharmacological mechanisms and applications. Mar. Drugs 2016, 14, 81. [Google Scholar] [CrossRef] [PubMed]
- Lauritano, C.; Andersen, J.H.; Hansen, E.; Albrigtsen, M.; Escalera, L.; Esposito, F.; Helland, K.; Hanssen, K.Ø.; Romano, G.; Ianora, A. Bioactivity screening of microalgae for antioxidant, anti-inflammatory, anticancer, anti-diabetes, and antibacterial activities. Front. Mar. Sci. 2016, 3, 68. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.; Liu, L.; Miron, A.; Klímová, B.; Wan, D.; Kuča, K. The antioxidant, immunomodulatory, and anti-inflammatory activities of Spirulina: An overview. Arch. Toxicol. 2016, 90, 1817–1840. [Google Scholar] [CrossRef]
- El-Sheekh, M.M.; Daboor, S.M.; Swelim, M.A.; Mohamed, S. Production and characterization of antimicrobial active substance from Spirulina platensis. Iran. J. Microbiol. 2014, 6, 112–119. [Google Scholar]
- Reddy, S.; Comai, L. Lamin A, farnesylation and aging. Exp. Cell. Res. 2012, 318, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Zheng, Y.W.; Liu, Q.; Liu, L.P.; Luo, F.L.; Zhou, H.C.; Isoda, H.; Ohkohchi, N.; Li, Y.M. Reactive oxygen species in skin repair, regeneration, aging, and inflammation. In reactive oxygen species (ROS) in living cells. Intech Open 2017. [Google Scholar] [CrossRef] [Green Version]
- Fiers, W.; Beyaert, R.; Declercq, W.; Vandenabeele, P. More than one way to die: Apoptosis, necrosis and reactive oxygen damage. Oncogene 1999, 18, 7719–7730. [Google Scholar] [CrossRef] [Green Version]
- Kageyama, H.; Waditee-Sirisattha, R. Antioxidative, anti-inflammatory, and anti-aging properties of mycosporine-like amino acids: Molecular and cellular mechanisms in the protection of skin-aging. Mar. Drugs 2019, 17, 222. [Google Scholar] [CrossRef] [Green Version]
- Guillerme, J.B.; Couteau, C.; Coiffard, L. Applications for marine resources in cosmetics. Cosmetics 2017, 4, 35. [Google Scholar] [CrossRef] [Green Version]
- Schneider, S.L.; Lim, H.W. A review of inorganic UV filters zinc oxide and titanium dioxide. Photodermatol. Photoimmunol. Photomed. 2019, 35, 442–446. [Google Scholar] [CrossRef] [Green Version]
- Radice, M.; Manfredini, S.; Ziosi, P.; Dissette, V.; Buso, P.; Fallacara, A.; Vertuani, S. Herbal extracts, lichens and biomolecules as natural photo-protection alternatives to synthetic UV filters. A systematic review. Fitoterapia 2016, 114, 144–162. [Google Scholar] [CrossRef] [PubMed]
- Mercurio, D.G.; Wagemaker, T.A.L.; Alves, V.M.; Benevenuto, C.G.; Gaspar, L.R.; Campos, P.M. In vivo photoprotective effects of cosmetic formulations containing UV filters, vitamins, Ginkgo biloba and red algae extracts. J. Photochem. Photobiol. B Biol. 2015, 153, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Hawryluk, E.B.; Oztan, A.; Fisher, D.E. Effects of ultraviolet exposure behaviors on skin pigmentation and melanoma. J. Pigment. Disord. 2014, 1, 2. [Google Scholar] [CrossRef] [Green Version]
- Agatonovic-Kustrin, S.; Morton, D.W. Cosmeceuticals derived from bioactive substances found in marine algae. Oceanography 2013, 1, 106. [Google Scholar] [CrossRef]
- Solano, F. On the metal cofactor in the tyrosinase family. Int. J. Mol. Sci. 2018, 19, 633. [Google Scholar] [CrossRef] [Green Version]
- Parvez, S.; Kang, M.; Chung, H.S.; Bae, H. Naturally occurring tyrosinase inhibitors: Mechanism and applications in skin health, cosmetics and agriculture industries. Phytother. Res. 2007, 21, 805–816. [Google Scholar] [CrossRef]
- Hussein, M.R. Ultraviolet radiation and skin cancer: Molecular mechanisms. J. Cutan. Pathol. 2015, 32, 191–205. [Google Scholar] [CrossRef]
- Elmets, C.A.; Ledet, J.J.; Athar, M. Cyclooxygenases: Mediators of UV-induced skin cancer and potential targets for prevention. J. Investig. Dermatol. 2014, 134, 2497C2502. [Google Scholar] [CrossRef] [Green Version]
- Kunicka-Styczyńska, A.; Sikora, M.; Kalemba, D. Antimicrobial activity of lavender, tea tree and lemon oils in cosmetic preservative systems. J. Appl. Microbiol. 2009, 107, 1903–1911. [Google Scholar] [CrossRef]
- Senevirathne, W.S.M.; Kim, S.K. Cosmeceuticals from algae. In Functional Ingredients from Algae for Foods and Nutraceuticals; Dominguez, H., Ed.; Woodhead Publishing: Belihuloya, Sri Lanka, 2013; pp. 694–713. [Google Scholar]
- Burlando, B. Herbal Principles in Cosmetics; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Ribeiro, A.; Estanqueiro, M.; Oliveira, M.; Sousa Lobo, J. Main benefits and applicability of plant extracts in skin care products. Cosmetics 2015, 2, 48–65. [Google Scholar] [CrossRef] [Green Version]
- Kamei, Y.; Sueyoshi, M.; Hayashi, K.I.; Terada, R.; Nozaki, H. The novel anti-Propionibacterium acnes compound, Sargafuran, found in the marine brown alga Sargassum macrocarpum. J. Antibiot. 2009, 62, 259–263. [Google Scholar] [CrossRef]
- Klaschka, U. Natural personal care products—Analysis of ingredient lists and legal situation. Environ. Sci. Eur. 2016, 28, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fabrowska, J.; Leska, B.; Schroeder, G.; Messyasz, B.; Pikosz, M. Biomass and extracts of algae as materials for cosmetics. In Marine Algae Extracts, Processes, Products, and Applications; Kim, S., Chojnacka, K., Eds.; Wiley-VCH-Verl: Weinheim, Germany, 2015; pp. 681–706. [Google Scholar] [CrossRef]
- Olasehinde, T.A.; Olaniran, A.O.; Okoh, A.I. Therapeutic potentials of microalgae in the treatment of Alzheimer’s disease. Molecules 2017, 22, 480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, M.I.; Shin, J.H.; Kim, J.D. The promising future of microalgae: Current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb. Cell Factories 2018, 17, 36. [Google Scholar] [CrossRef] [PubMed]
- Sebök, S.; Herppich, W.B.; Hanelt, D. Outdoor cultivation of Ulva lactuca in a recently developed ring-shaped photobioreactor: Effects of elevated CO2 concentration on growth and photosynthetic performance. Botanica Mar. 2019, 62, 179–190. [Google Scholar] [CrossRef]
- Holdt, S.L.; Christensen, L.; Iversen, J.J.L. A novel closed system bubble column photobioreactor for detailed characterisation of micro-and macroalgal growth. J. Appl. Phycol. 2014, 26, 825–835. [Google Scholar] [CrossRef] [Green Version]
- Sebök, S.; Herppich, W.B.; Hanelt, D. Development of an innovative ring-shaped cultivation system for a land-based cultivation of marine macroalgae. Aquac. Eng. 2017, 77, 33–41. [Google Scholar] [CrossRef]
- Kadam, S.U.; Tiwari, B.K.; O’Donnell, C.P. Application of novel extraction technologies for bioactives from marine algae. J. Agric. Food Chem. 2013, 61, 4667–4675. [Google Scholar] [CrossRef]
- Park, P.J.; Shahidi, F.; Jeon, Y.J. Antioxidant activities of enzymatic extracts from an edible seaweed Sargassum horneri using ESR spectrometry. J. Food Lipids 2004, 11, 15–27. [Google Scholar] [CrossRef]
- Nadar, S.S.; Rao, P.; Rathod, V.K. Enzyme assisted extraction of biomolecules as an approach to novel extraction technology: A review. Food Res. Int. 2018, 108, 309–330. [Google Scholar] [CrossRef]
- Lou, H.Y.; Wang, B.; Yu, C.G.; Xu, Y.F. Optimization of microwave-assisted extraction of polyphenols from Enteromorpha prolifera by orthogonal test. Chin. Herb. Med. 2010, 2, 321–325. [Google Scholar] [CrossRef]
- Rodriguez-Jasso, R.M.; Mussatto, S.I.; Pastrana, L.; Aguilar, C.N.; Teixeira, J.A. Microwave-assisted extraction of sulfated polysaccharides (fucoidan) from brown seaweed. Carbohydr. Polym. 2011, 86, 1137–1144. [Google Scholar] [CrossRef] [Green Version]
- Cikoš, A.M.; Jokić, S.; Šubarić, D.; Jerković, I. Overview on the application of modern methods for the extraction of bioactive compounds from marine macroalgae. Mar. Drugs 2018, 16, 348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fabrowska, J.; Ibanez, E.; Leska, B.; Herrero, M. Supercritical fluid extraction as a tool to valorize underexploited freshwater green algae. Algal Res. 2016, 19, 237–245. [Google Scholar] [CrossRef]
- Sivagnanam, S.P.; Yin, S.; Choi, J.H.; Park, Y.B.; Woo, H.C.; Chun, B.S. Biological properties of fucoxanthin in oil recovered from two brown seaweeds using supercritical CO2 extraction. Mar. Drugs 2015, 13, 3422–3442. [Google Scholar] [CrossRef]
- Mittal, R.; Tavanandi, H.A.; Mantri, V.A.; Raghavarao, K.S.M.S. Ultrasound assisted methods for enhanced extraction of Phycobiliproteins from marine macro-algae, Gelidium pusillum (Rhodophyta). Ultrason. Sonochem. 2017, 38, 92–103. [Google Scholar] [CrossRef]
- Collett, M.G. Photosensitisation diseases of animals: Classification and a weight of evidence approach to primary causes. Toxicon X 2019, 3, 100012. [Google Scholar] [CrossRef]
- Patel, B.H. Natural dyes. In Handbook of Textile and Industrial Dyeing; Woodhead Publishing: Cambridge, UK, 2011; pp. 395–424. [Google Scholar]
- Takser, L.; Benachour, N.; Husk, B.; Cabana, H.; Gris, D. Cyanotoxins at low doses induce apoptosis and inflammatory effects in murine brain cells: Potential implications for neurodegenerative diseases. Toxicol. Rep. 2016, 3, 180–189. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.; Parihar, P.; Singh, M.; Bajguz, A.; Kumar, J.; Singh, S.; Singh, V.P.; Prasad, S.M. Uncovering potential applications of cyanobacteria and algal metabolites in biology, agriculture and medicine: Current status and future prospects. Front. Microbiol. 2017, 8, 515. [Google Scholar] [CrossRef] [Green Version]
- Kujbida, P.; Hatanaka, E.; Campa, A.; Colepicolo, P.; Pinto, E. Effects of microcystins on human polymorphonuclear leukocytes. Biochem. Biophys. Res. Commun. 2006, 341, 273–277. [Google Scholar] [CrossRef]





| Algae Species | Bioactive Compound/Extract | Beneficial Activity | Mechanism of Action | Experimental Model | Reference |
|---|---|---|---|---|---|
| Brown algae | |||||
| Ascophyllum nodosum | Ascophyllan | Anticancer | Inhibit MMP expression | B16 melanoma cells | [54] |
| Bifurcaria bifurcata | Eleganonal | Antioxidant | DPPH inhibition | In vitro | [55] |
| Chnoospora implexa | Ethanol extract | Antimicrobial | Bacterial growth inhibition | Staphylococcus aureus, Staphylococcus pyogenes | [56] |
| Chnoospora minima | Fucoidan | Anti-inflammation | Inhibition of LPS-induced NO production, iNOS, COX-2, and PGE2 levels | RAW macrophages | [47] |
| Cladosiphon okamuranus | Fucoxanthin | Antioxidant | DPPH inhibition | In vitro | [49] |
| Colpomenia sinuosa | Ethanol extract | Antimicrobial | Bacterial growth inhibition | S. aureus, S. pyogenes | [56] |
| Cystoseira barbata | Fat-soluble vitamin and carotenoids | Antioxidant | High fat-soluble vitamin and carotenoid content | In vitro | [57] |
| Cystoseira foeniculacea | Polyphenol | Antioxidant | DPPH inhibition (EC50 = 5.27 mg/mL) | In vitro | [58] |
| Cystoseira hakodatensis | Phenol and fucoxanthin | Antioxidant | High total phenolic and fucoxanthin content | In vitro | [59] |
| Cystoseira osmundacea | Ethanol extract | Antimicrobial | Bacterial growth inhibition | S. pyogenes | [56] |
| Dictyopteris delicatula | Ethanol extract | Antimicrobial | Bacterial growth inhibition | S. aureus, S. pyogenes | [56] |
| Dictyota dichotoma | Algae extract | Antimicrobial | Inhibit the synthesis of the peptidoglycan layer of bacterial cell walls | Penicillium purpurescens, Candida albicans, Aspergillus flavus | [60] |
| Ecklonia cava | Dieckol | Anti-inflammation | Suppression of iNOS and COX-2 | Murine BV2 microglia | [61] |
| Phlorotannin | Anti-melanogenic | Inhibit melanin production | B16F10 melanoma cells | [44] | |
| Phlorotannin | Antioxidant | ROS scavenging potential | Chinese hamster lung fibroblast (V79-4) | [62] | |
| Ecklonia kurome | Phlorotannin | Anti-inflammation | Inhibit hyaluronidase | Assay of HAase (In vitro) | [42] |
| Ecklonia Stolonifera | Phlorotannin | Anti-aging | Inhibit MMP expression | Human dermal fibroblast cell | [43] |
| Phlorofucofuroeckol A and B | Anti-inflammation | Inhibition of NO production by downregulating iNOS and prostaglandin E2 | LPS stimulated RAW 264.7 cells | [63] | |
| Eisenia arborea | Phlorotannin | Anti-inflammation | Inhibit release of histamine | Rat basophile leukemia cells (RBL-2HE) | [64] |
| Eisenia bicyclis | Phlorotannin | Anti-inflammation | Inhibit hyaluronidase | Assay of HAase (In vitro) | [42] |
| Fucus evanescens | Fucoidan | Anticancer | Inhibit cell proliferation | Human malignant melanoma cells | [45] |
| Fucus vesiculosus | Extract | Anti-aging | Stimulate collagen production | N/A | [8] |
| Fucoidan | Anti-melanogenic | Inhibit tyrosinase and melanin | B16 murine melanoma cells | [46] | |
| Fucoidan | Anticancer | Decrease melanoma growth | Mice | [65] | |
| Fucoxanthin | Antioxidant | Prevent oxidation formation | In vitro, RAW 264.7 macrophage, Mouse (ex vivo) | [66] | |
| Halopteris scoparia | Ethanol extract | Anti-inflammation | COX-2 inhibition | COX inhibitory screening assay kit | [67] |
| Himanthalia elongota | Fatty acid andPhenol | Antimicrobial | Bacterial growth inhibition | Escherichia coli, Staphylococcus aureus | [68] |
| Hizikia fusiformis | Fucosterol | Anti-aging | Inhibit MMP expression | Human dermal fibroblast | [18] |
| Ethyl acetate extract | Anti-melanogenic | Inhibit tyrosinase and melanin | B16F10 mouse melanoma cells | [69] | |
| Fucoxanthin | Antioxidant | DPPH inhibition | In vitro | [70] | |
| Hydroclathrus clathratus | Ethanol extract | Antimicrobial | Bacterial growth inhibition | S. aureus, S. pyogenes | [56] |
| Ishige foliacea | Phlorotannin | Anti-melanogenic | Downregulation of tyrosinase and melanin synthesis | B16F10 cells Zebrafish embryo | [71,72] |
| Ishige okamurae | Diphlorethohydroxycarmalol | Anti-inflammation | Down-regulation of iNOS and COX-2 expression and NF-κβ activation | Human umbilical vein endothelial cells | [73] |
| Laminaria japonica | Fucoxanthin | Anti-melanogenic | Suppress tyrosinase activity | UVB- irradiated guinea pig | [48] |
| Laminaria ochroleuca | Polyphenol | Antioxidant | High total phenolic content and antioxidant capacity | In vitro | [74] |
| Macrocystis pyrifera | Phlorotannin | Antioxidant | ROS scavenging potential | In vitro | [8] |
| Hyaluronic acid | Anti-aging | Enhance the production of syndecan-4 | N/A | [75] | |
| Padina concrescens | Ethanol extract | Antimicrobial | Bacterial growth inhibition | S. aureus, S. pyogenes | [56] |
| Padina pavonica | Polyphenol | Antimicrobial | Bacterial growth inhibition | Candida albicans and Mucor ramaniannus | [17] |
| Acetone extract | Antioxidant | Free radical scavenging activity (IC50 = 691.56 µg L−1) | In vitro | [60] | |
| Padina tetrastromatic | Diterpenes | Antioxidant | DPPH (IC50 = 1.73) & ABTS (IC50 = 2.01) inhibitions | In vitro | [76] |
| Sulfated polysaccharide | Anti-inflammation | COX-2 and iNOS inhibitions | Paw edema in rats | [77] | |
| Petalonia binghamiae | Ethanol extract | Anti-melanogenic | Inhibit tyrosinase and melanin | B16F10 murine melanoma cells | [78] |
| Aqueous extract | Antioxidant Anti-inflammation | DPPH inhibition COX-2 inhibition | In vitro In vitro | [67] | |
| Rosenvingea intrincata | Ethanol extract | Antimicrobial | Bacterial growth inhibition | S. aureus, S. pyogenes | [56] |
| Saccharina latissima | Phenol | Antioxidant | High total phenolic content, DPPH scavenging activity and FRAP | In vitro | [79] |
| Sargassum fulvellum | Fucoxanthin | Antioxidant | DPPH inhibition | In vitro | [70] |
| Sargassum furcatum | Methanol extract | Antioxidant | DPPH (EC50 = 0.461) & ABTS (EC50 = 0.266) inhibitions | In vitro | [80] |
| Sargassum hemiphyllum | Sulfated polysaccharide | Anti-inflammation | Inhibit LPS-induced inflammatory response | RAW 264.7 macrophage cells | [81] |
| Sargassum henslowianum | Sulfated polysaccharide | Anticancer | Activation of caspase-3 | B16 melanoma cells | [82] |
| Sargassum horridum | Ethanol extract | Antimicrobial | Bacterial growth inhibition | S. aureus, S. pyogenes | [56] |
| Sargassum horneri | Sargachromanol.E | Anti-aging | Inhibit MMP expression | UVA irradiated dermal fibroblast | [83] |
| Alginic acid | Anti-inflammation | Inhibit inflammatory response | HaCaT cells | [84] | |
| Sargassum muticum | Tetraprenyltoluquinol chromane meroterpenoid | Anti-aging | ROS scavenging potential | Human dermal fibroblast | [85] |
| Sargassum polycystum | Ethanol extract | Anti-melanogenic | Inhibit tyrosinase and melanin production | B16F10 melanoma cells | [39] |
| Sargassum serratifolium | Sargachromenol | Anti-melanogenic | Downregulation of microphthalmia-associated transcription factor | B16F10 melanoma cells | [39] |
| Sargassum siliquastrum | Fucoxanthin | Antioxidant | Reduced UVB-induced ROS production | Human fibroblast | [86] |
| Sargassum thunbergi | Thunbergols | Antioxidant | DPPH inhibition | In vitro | [87] |
| Sargassum vulgare | Methanol extract | Antioxidant | β-carotene bleaching activity | In vitro | [88] |
| Stoechospermum marginatum | Spatane diterpenoids | Anticancer | Cell growth inhibition | Murine B16F10 melanoma cells | [89] |
| Turbinaria conoides | Laminarin, alginate, fucoidan | Antioxidant | ROS scavenging potential | N/A | [33] |
| Turbinaria ornata | Fucoxanthin | Antioxidant | High FRAP value (>10 µM/µg of extract) | In vitro | [90] |
| Undaria pinnatifida | Fucoxanthin | Anti-aging | MMP expression reduction, VEGF | Mouse | [50] |
| Ethyl acetate extract | Anti-melanogenic | Down regulate melanin and inhibit tyrosinase | Mouse B16 melanoma cells | [91] | |
| Polyunsaturated fatty acid | Anti-inflammation | N/A | Mouse ear edema and erythema | [92] | |
| Fucoxanthin | Antioxidant | DPPH inhibition | In vitro | [70] | |
| Red algae | |||||
| Alsidium corallinum | Methanol extract | Antimicrobial | Bacterial growth inhibition | Escherichia coli, Klebsiella pneumoniae, Staphylococcus aureus | [93] |
| Bangia | Algae extract | Antioxidant | Induce peroxidase and superoxide dismutase to reduce oxidative stress | In vitro | [94] |
| Bryothamnion triquetrum | Methanol extract | Antioxidant | DPPH (EC50 = 0.357) & ABTS (EC50 = 0.370) inhibitions | In vitro | [80] |
| Ceramium rubrum | Methanol extract | Antimicrobial | Bacterial growth inhibition | Escherichia coli, Enterococcus faecalis, Staphylococcus aureus | [93] |
| Chondrocanthus acicularis | Methanol extract | Antimicrobial | Bacterial growth inhibition | E. coli, K. pneumoniae, E. faecalis, S. aureus | [93] |
| Chondrus canaliculatus | Polysaccharide | Antioxidant | DPPH inhibition | In vitro | [95] |
| Chondrus crispus | Aqueous extract | Antimicrobial | Bacterial growth inhibition | Salmonella Enteritidis | [96] |
| Corallina pilulifera | Methanol extract | Anti-aging Antioxidant | Reduce the expression of gelatinase Inhibit free radical oxidation | Human dermal fibroblast Human fibrosarcoma (HT-1080) | [97] |
| Corallina vancouverensis | Ethanol extract | Antimicrobial | Bacterial growth inhibition | S. aureus, S. pyogenes | [56] |
| Ganonema farinosum | Ethanol extract | Antimicrobial | Bacterial growth inhibition | S. aureus, S. pyogenes | [56] |
| Gelidium crinaale | Fat-soluble vitamin and carotenoids | Antioxidant | High fat-soluble vitamin and carotenoid content | In vitro | [57] |
| Gelidium robustum | Ethanol extract | Antimicrobial | Bacterial growth inhibition | S. aureus, S. pyogenes | [56] |
| Gracilaria gracilis | Phenol | Antioxidant | ROS scavenging potential | In vitro | [98] |
| Gracilariopsis lemaneiformis | Sulfated polysaccharide | Antioxidant | DPPH, Superoxide radical assay, hydroxyl radical assay (EC50 = 2.45 mg/mL) | In vitro | [99] |
| Gracilaria salicornia | 2H- chromenyl | Antioxidant Anti-inflammation | DPPH and ABTS inhibitions COX-1 inhibition | In vitro | [100] |
| Jania rubens | Glycosaminoglycan | Anti-aging | Collagen synthesis | Unknown | [75] |
| Laurencia caspica | Phenol Ethanol extract | Antioxidant Antimicrobial | DPPH inhibition Bacterial growth inhibition | In vitro Klebsiella pneumonia, Pseudomonas aeroginosa | [101] |
| Laurencia luzonensis | Sesquiterpenes | Antimicrobial | Bacterial growth inhibition | Bacillus megaterium | [12] |
| Laurenicia obtusa | Polysaccharide | Antioxidant | DPPH (IC50 = 24 µg/mL), FRAP (IC50 = 92 µg/mL), Hydroxyl radical scavenging activity (IC50 = 113 µg/mL) | In vitro | [102] |
| Laurenicia pacifica | Laurinterol | Antimicrobial | Bacterial growth inhibition | Staphylococcus aureus | [9] |
| Laurencia rigida | Sesquiterpenes | Antimicrobial | Bacterial growth inhibition | Bacillus megaterium | [12] |
| Meristotheca dakarensis | Glycosaminoglycan | Anti-aging | Collagen synthesis | Unknown | [75] |
| Osmundaria obtusilo | Methanol extract | Antioxidant | DPPH (EC50 = 0.041 mg/mL), ABTS (EC50 = 0.031 mg/mL), Metal chelating (EC50 = 0.1 mg/mL), folin ciocalteu (EC50 = 0.128 mg/mL) | In vitro | [80] |
| Palisada flagellifera | Methanol extract | Antioxidant | β-carotene bleaching activity | In vitro | [88] |
| Palmaria palmata | MAA | Anti-aging | Collagenase inhibition | Clostridium histolyticum | [53] |
| Polysiphonia howei | Fucoxanthin | Antioxidant | High FRAP value (>5 µM/µg of extract) | In vitro | [90] |
| Porphyra haitanensis | Sulfated Polysaccharide | Antioxidant | ROS scavenging potential | Mice | [103] |
| Porphyra umbilicalis | MAA | Anti-aging | Control expression of MMP | Human dermal fibroblast | [16] |
| Porphyra sp. | MAA | Anti-aging | Collagenases inhibition | Clostridium histolyticum | [53] |
| Porphyra yezoensis | MAA Polyphenol Phycoerythrin | Antioxidant Anticancer Anti-inflammation | ROS scavenging potential and MMP expression Induce apoptosis Suppression of mast cells | Human skin fibroblast HaCaT cells Rat | [51] |
| Pterocladia capillacea | Sulfated polysaccharide | Antimicrobial | N/A | Staphylococcus aureus Escherichia coli | [104] |
| Pyropia columbia | Phenol | Antioxidant | DPPH, β-carotene bleaching and ABTS inhibitions | Piaractus mesopotamicus | [105] |
| Pyropia yezoensis | Polysaccharide | Anti-aging | Promote collagen synthesis | Human dermal fibroblast | [106] |
| Rhodomela confervoides | Polyphenol | Antimicrobial | Bacterial growth inhibition | Candida albicans, Mucor ramaniannus | [17] |
| Bromophenol | Antioxidant | DPPH inhibition | In vitro | [107] | |
| Schizymenia dubyi | Phenol | Anti-melanogenic | Inhibit tyrosinase activity | In vitro | [39] |
| Green algae | |||||
| Bryopsis plumose | Polysaccharide | Antioxidant | ROS scavenging potential | In vitro | [108] |
| Chaetomorpha antennia | Fucoxanthin | Antioxidant | DPPH inhibition (63.77%) | In vitro | [109] |
| Chlamydomo-nas hedleyi | MAA | Antioxidant Anti-aging Anti-inflammation | ROS scavenging potential Increase UV-suppressed genes (procollagen C proteinase enhancer and elastin) expression Reduce COX-2 and involucrin expression | In vitro HaCaT cells HaCaT cells | [52] |
| Cladophora sp. | Ethanol extract | Antimicrobial | Bacterial growth inhibition | S. aureus, S. pyogenes | [56] |
| Codium amplivesicula-tum | Ethanol extract | Antimicrobial | Bacterial growth inhibition | S. aureus, S. pyogenes | [56] |
| Codium cuneatum | Ethanol extract | Antimicrobial | Bacterial growth inhibition | S. aureus, S. pyogenes | [56] |
| Codium fragile | Sterol | Anti-inflammation | Reduces the expression of COX-2, iNOS, and TNF-α | Mice | [110] |
| Codium simulans | Ethanol extract | Antimicrobial | Bacterial growth inhibition | S. aureus, S. pyogenes | [56] |
| Entromorpha intestinalis | Chloroform and methanol extract | Antioxidant | SOD activity is reduced | Labidochromis caeruleus | [111] |
| Enteromorpha linza | Polysaccharide | Antioxidant | ROS scavenging potential | In vitro | [108] |
| Gayralia oxysperma | Fucoxanthin | Antioxidant | High FRAP value (>6 µM/µg of extract) | In vitro | [90] |
| Ulva dactilifera | Ethanol extract | Antimicrobial | Bacterial growth inhibition | S. aureus, Streptococcus pyogenes | [56] |
| Ulva fasciata | Fucoxanthin | Antioxidant | DPPH inhibition (83.95%) | In vitro | [109] |
| Ulva lactuca | Phycocolloids | Anti-inflammation | N/A | N/A | [75] |
| Ulva pertusa | Polysaccharide | Antioxidant | ROS scavenging potential | In vitro | [108] |
| Ulva prolifera | Phenol and flavonoid | Antioxidant | DPPH inhibition, high phenolic and flavonoid contents | In vitro | [112] |
| Ulva rigida | Phenol | Antioxidant | DPPH inhibition | In vitro | [113] |
| Ulva sp. | Sulfated polysaccharide | Anti-aging | Increase hyaluronan production | Human dermal fibroblast | [114] |
| Microalgae/Cyanobacteria | |||||
| Anabaena vaginicola | Lycopene | Antioxidant Anti-aging | N/A | In vitro | [115] |
| Arthrospira platensis | Methanol extracts of exopolysaccharides | Antioxidant | N/A | In vitro | [115] |
| Chlorella fusca | Sporopollenin | Anti-aging | Protect cells from UV radiation | N/A | [116] |
| Chlorella minutissima | MAA | Anti-aging | Protect cells from UV radiation | N/A | [116] |
| Chlorella sorokiniana | MAA | Anti-aging | Protect cells from UV radiation | N/A | [116] |
| Lutein | Anti-aging | Reduce UV induced damage | N/A | [33] | |
| Chlorella vulgaris | Hot water extract | Anti-aging | Reduced activity of SOD | Human diploid fibroblast | [117] |
| Anti-inflammation | Downregulated mRNA expression levels of IL-4 and IFN-γ | NC/Nga mice | [118] | ||
| Dunaliella salina | β-carotene | Antioxidant | Protect against oxidative stress | Rat | [119] |
| β-cryptoxanthin | Anti-inflammation | Reduced the production of IL-1β, IL-6, TNF-α, the protein expression of iNOS and COX-2 | LPS-stimulated RAW 264.7 cells | [120] | |
| Haematococcus pluvialis | Astaxanthin (carotenoid) | Anti-aging | Inhibit MMP expression | Mice and human dermal fibroblasts | [121] |
| Anticancer | ROS scavenging potential | Mice | [122] | ||
| Nannochloropsis granulata | Carotenoid | Antioxidant | DPPH inhibition | In vitro | [123] |
| Nannochloropsis oculata | Zeaxanthin | Anti-melanogenic | Inhibit tyrosinase | In vitro | [124] |
| Nitzschia sp. | Fucoxanthin | Antioxidant | Reduced oxidative stress | Human Glioma Cells | [125] |
| Nostoc sp. | MAA | Antioxidant | ROS scavenging potential | In vitro | [126] |
| Odontella aurita | EPA | Antioxidant | Reduce oxidative stress | Rat | [127] |
| Planktochlorella nurekis | Fatty acid | Antimicrobial | Bacterial growth inhibition | Campylobacter jejuni, E. coli, Salmonella enterica var. | [128] |
| Porphyridium sp. | Sulfated polysaccharide | Anti-inflammation Antioxidant | Inhibit proinflammatory modulator Inhibited oxidative damage | Unknown 3T3 cells | [103] |
| Rhodella reticulata | Sulfated polysaccharide | Antioxidant | ROS scavenging potential | In vitro | [103] |
| Skeletonema marinoi | Polyunsaturated aldehyde and fatty acid | Anticancer | Inhibit cell proliferation | Human melanoma cells (A2058) | [129] |
| Spirulina platensis | β-carotene and phycocyanin | Antioxidant Anti-inflammatio | Inhibit lipid peroxidation Inhibit TNF-α and IL-6 expressions | MouseHuman dermal fibroblast cells (CCD-986sk) | [130] |
| Ethanol extract | Antimicrobial | Bacterial growth inhibition | E. coli, Pseudomonas aeruginosa, Bacillus subtilis, and Aspergillus niger | [131] | |
| Synechocystis spp. | Fatty acids and phenols | Antimicrobial | Bacterial growth inhibition | E. coli S. aureus | [68] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thiyagarasaiyar, K.; Goh, B.-H.; Jeon, Y.-J.; Yow, Y.-Y. Algae Metabolites in Cosmeceutical: An Overview of Current Applications and Challenges. Mar. Drugs 2020, 18, 323. https://doi.org/10.3390/md18060323
Thiyagarasaiyar K, Goh B-H, Jeon Y-J, Yow Y-Y. Algae Metabolites in Cosmeceutical: An Overview of Current Applications and Challenges. Marine Drugs. 2020; 18(6):323. https://doi.org/10.3390/md18060323
Chicago/Turabian StyleThiyagarasaiyar, Krishnapriya, Bey-Hing Goh, You-Jin Jeon, and Yoon-Yen Yow. 2020. "Algae Metabolites in Cosmeceutical: An Overview of Current Applications and Challenges" Marine Drugs 18, no. 6: 323. https://doi.org/10.3390/md18060323
APA StyleThiyagarasaiyar, K., Goh, B. -H., Jeon, Y. -J., & Yow, Y. -Y. (2020). Algae Metabolites in Cosmeceutical: An Overview of Current Applications and Challenges. Marine Drugs, 18(6), 323. https://doi.org/10.3390/md18060323