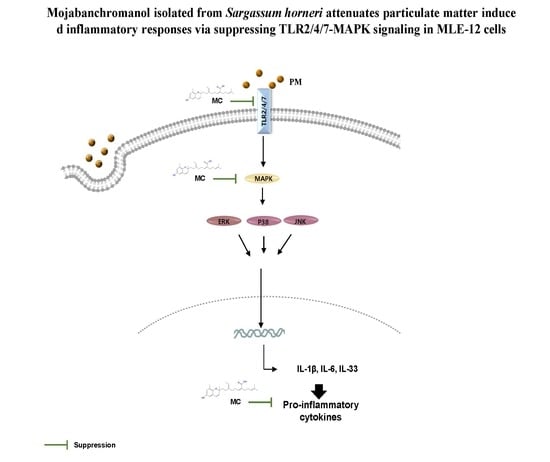
Mojabanchromanol Isolated from Sargassum horneri Attenuates Particulate Matter Induced Inflammatory Responses via Suppressing TLR2/4/7-MAPK Signaling in MLE-12 Cells
Abstract
:1. Introduction
2. Results
2.1. Low Concentration MC Is Not Cytotoxic and Does Not Affect Cell Proliferation of MLE-12 Cells
2.2. MC Attenuates PM-Triggered Cytotoxicity of MLE-12 Cells
2.3. MC Suppresses the Lipid Peroxidation in PM-Exposed MLE-12 Cells
2.4. MC Attenuates the PM-Triggered Activation of MAPK Pathway in MLE-12 Cells
2.5. MC Suppresses the Secretion of Pro-inflammatory Cytokines in PM-Exposed MLE-12 Cells
2.6. MC Attenuates the PM-Triggered Activation of TLR2/4/7 in MLE-12 Cells
3. Discussion
4. Materials and Methods
4.1. Reagents and Kits
4.2. Particulate Matter
4.3. Preparation of Seaweed and Isolation of MC
4.4. Cell Culture
4.5. LDH Release Assay
4.6. 3H-thymidine Incorporation Assay
4.7. Immunocytochemistry Analysis for 8-OHdG
4.8. ELISA
4.9. Western Blot Analysis for MAPK Signaling Pathway Activation
4.10. RNA Isolation, cDNA Preparation, and qPCR Analysis
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xing, Y.-F.; Xu, Y.-H.; Shi, M.-H.; Lian, Y.-X. The impact of PM2.5 on the human respiratory system. J. Thorac. Dis. 2016, 8, E69–E74. [Google Scholar] [CrossRef] [PubMed]
- Farina, F.; Sancini, G.; Battaglia, C.; Tinaglia, V.; Mantecca, P.; Camatini, M.; Palestini, P. Milano Summer Particulate Matter (PM10) Triggers Lung Inflammation and Extra Pulmonary Adverse Events in Mice. PLoS ONE 2013, 8, e56636. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Hu, R.; Chen, Z.; Li, Q.; Huang, S.; Zhu, Z.; Zhou, L.F. Fine particulate matter (PM2.5): The culprit for chronic lung diseases in China. Chronic. Dis. Transl. Med. 2018, 4, 176–186. [Google Scholar] [CrossRef]
- Perrone, M.; Gualtierm, M.; Consonni, V.; Ferrero, L.; Sangiorgi, G.; Longhin, E.; Ballabio, D.; Bolzacchini, E.; Camatini, M. Particle size, chemical composition, seasons of the year and urban, rural or remote site origins as determinants of biological effects of particulate matter on pulmonary cells. Environ. Pollut. 2013, 176, 215–227. [Google Scholar] [CrossRef]
- Huang, X.-F.; He, L.-Y.; Hu, M.; Zhang, Y.-H. Annual variation of particulate organic compounds in PM2.5 in the urban atmosphere of Beijing. Atmos. Environ. 2006, 40, 2449–2458. [Google Scholar] [CrossRef]
- Pu, X.-J.; Li, J.; Zhou, Q.-L.; Pan, W.; Li, Y.-Q.; Zhang, Y.; Wang, J.; Jiao, Z. Rosiglitazone inhibits PM2.5-induced cytotoxicity in human lung epithelial A549 cells. Ann. Transl. Med. 2018, 6, 152. [Google Scholar] [CrossRef] [PubMed]
- Kannan, S.; Huang, H.; Seeger, D.; Audet, A.; Chen, Y.; Huang, C.; Gao, H.; Li, S.; Wu, M. Alveolar epithelial type II cells activate alveolar macrophages and mitigate P. Aeruginosa infection. PLoS ONE 2009, 4, e4891. [Google Scholar] [CrossRef] [Green Version]
- Upadhyay, D.; Panduri, V.; Ghio, A.; Kamp, D.W. Particulate matter induces alveolar epithelial cell DNA damage and apoptosis: Role of free radicals and the mitochondria. Am. J. Resp. Cell Mol. 2003, 29, 180–187. [Google Scholar] [CrossRef] [Green Version]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, K.; Loridas, S. Pulmonary oxidative stress, inflammation and cancer: Respirable particulate matter, fibrous dusts and ozone as major causes of lung carcinogenesis through reactive oxygen species mechanisms. Int. J. Environ. Res. Public Health 2013, 10, 3886–3907. [Google Scholar] [CrossRef]
- Zhang, Z.G.; Niu, X.Y.; He, X.J.; Shu, J. Ginsenoside Rg1 reduces toxicity of fine particulate matter on human alveolar epithelial cells: A preliminary observation. Mol. Med. Rep. 2014, 9, 989–992. [Google Scholar] [CrossRef]
- Canakci, C.F.; Cicek, Y.; Yildirim, A.; Sezer, U.; Canakci, V. Increased levels of 8-hydroxydeoxyguanosine and malondialdehyde and its relationship with antioxidant enzymes in saliva of periodontitis patients. Eur. J. Dent. 2009, 3, 100–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, Y.; Fan, J.; Chen, H.-w.; Liu, E.-q. Trametes orientalis polysaccharide alleviates PM2.5-induced lung injury in mice through its antioxidant and anti-inflammatory activities. Food Funct. 2019, 10, 8005–8015. [Google Scholar] [CrossRef] [PubMed]
- Duecker, R.; Baer, P.; Eickmeier, O.; Strecker, M.; Kurz, J.; Schaible, A.; Henrich, D.; Zielen, S.; Schubert, R. Oxidative stress-driven pulmonary inflammation and fibrosis in a mouse model of human ataxia-telangiectasia. Redox Biol. 2018, 14, 645–655. [Google Scholar] [CrossRef]
- Yan, Z.; Jin, Y.; An, Z.; Liu, Y.; Samet, J.M.; Wu, W. Inflammatory cell signaling following exposures to particulate matter and ozone. BBA-Gen. Subj. 2016, 1860, 2826–2834. [Google Scholar] [CrossRef]
- Ma, A.-C.; Chen, Z.; Wang, T.; Song, N.; Yan, Q.; Fang, Y.-C.; Guan, H.-S.; Liu, H.-B. Isolation of the molecular species of monogalactosyldiacylglycerols from brown edible seaweed Sargassum horneri and their inhibitory effects on triglyceride accumulation in 3T3-L1 adipocytes. J. Agric. Food Chem. 2014, 62, 11157–11162. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Heinrich, M.; Myers, S.; Dworjanyn, S.A. Towards a better understanding of medicinal uses of the brown seaweed Sargassum in Traditional Chinese Medicine: A phytochemical and pharmacological review. J. Ethnopharmacol. 2012, 142, 591–619. [Google Scholar] [CrossRef]
- Sanjeewa, K.K.A.; Jayawardena, T.U.; Kim, S.-Y.; Lee, H.G.; Je, J.-G.; Jee, Y.; Jeon, Y.-J. Sargassum horneri (Turner) inhibit urban particulate matter-induced inflammation in MH-S lung macrophages via blocking TLRs mediated NF-κB and MAPK activation. J. Ethnopharmacol. 2020, 249, 112363. [Google Scholar] [CrossRef]
- Cho, S.; Cho, J.; Kang, S.; Hong, Y.; Ahn, D. Antioxidant activity of mojabanchromanol, a novel chromene, isolated from brown alga Sargassum siliquastrum. J. Environ. Biol. 2008, 29, 479–484. [Google Scholar]
- Jang, K.H.; Lee, B.H.; Choi, B.W.; Lee, H.S.; Shin, J. Chromenes from the brown alga Sargassum siliquastrum. J. Nat. Prod. 2005, 68, 716–723. [Google Scholar] [CrossRef]
- Kim, H.-S.; Han, E.J.; Fernando, I.P.S.; Sanjeewa, K.K.A.; Jayawardena, T.U.; Kim, H.-J.; Jee, Y.; Kang, S.-H.; Jang, J.-H.; Jang, J.-P.; et al. Anti-allergy effect of mojabanchromanol isolated from Sargassum horneri in bone marrow-derived cultured mast cells. Algal Res. 2020, 48, 101898. [Google Scholar] [CrossRef]
- Herath, K.H.I.N.M.; Kim, H.J.; Mihindukulasooriya, S.P.; Kim, A.; Kim, H.J.; Jeon, Y.-J.; Jee, Y. Sargassum horneri extract containing mojabanchromanol attenuates the particulate matter exacerbated allergic asthma through reduction of Th2 and Th17 response in mice. Environ. Pollut. 2020. [Google Scholar] [CrossRef]
- Morales, M.; Cabello, C.; Nepomuceno, F.; Peralta, L.; Maqueda, E.; Zavala, M. Parainfluenza Virus Type 1 Induces Epithelial IL-8 Production via p38-MAPK Signalling. J. Immunol. Res. 2014, 2014, 515984. [Google Scholar] [CrossRef] [Green Version]
- Cooper, D.M.; Loxham, M. Particulate matter and the airway epithelium: The special case of the underground? Eur. Respir. Rev. 2019, 28. [Google Scholar] [CrossRef] [Green Version]
- Cheng, G.; Zielonka, J.; McAllister, D.M.; Mackinnon, A.C., Jr.; Joseph, J.; Dwinell, M.B.; Kalyanaraman, B. Mitochondria-targeted vitamin E analogs inhibit breast cancer cell energy metabolism and promote cell death. BMC Cancer 2013, 13, 285. [Google Scholar] [CrossRef] [Green Version]
- Dai, P.; Shen, D.; Shen, J.; Tang, Q.; Xi, M.; Li, Y.; Li, C. The roles of Nrf2 and autophagy in modulating inflammation mediated by TLR4 - NFκB in A549 cell exposed to layer house particulate matter 2.5 (PM2.5). Chemosphere 2019, 235, 1134–1145. [Google Scholar] [CrossRef] [PubMed]
- Gehling, W.; Dellinger, B. Environmentally Persistent Free Radicals and Their Lifetimes in PM2.5. Environ. Sci. Technol. 2013, 47, 8172–8178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bae, S.; Pan, X.-C.; Kim, S.-Y.; Park, K.; Kim, Y.-H.; Kim, H.; Hong, Y.-C. Exposures to particulate matter and polycyclic aromatic hydrocarbons and oxidative stress in schoolchildren. Environ. Health Perspect. 2010, 118, 579–583. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, M.; Takano, H.; Fujitani, Y.; Hirano, S.; Ichinose, T.; Shimada, A.; Inoue, K.-I. Effects of exposure to nanoparticle-rich diesel exhaust on 8-OHdG synthesis in the mouse asthmatic lung. Exp. Ther. Med. 2013, 6, 703–706. [Google Scholar] [CrossRef]
- Ahmadi, K.; Kumalaningsih, S.; Wijana, S.; Santoso, I. Antioxidative effect of tocotrienol rich fraction from palm fatty acid distillate on oxidative stress. Food Public Health 2013, 3, 130–136. [Google Scholar]
- Wang, J.; Huang, J.; Wang, L.; Chen, C.; Yang, D.; Jin, M.; Bai, C.; Song, Y. Urban particulate matter triggers lung inflammation via the ROS-MAPK-NF-kappaB signaling pathway. J. Thorac. Dis. 2017, 9, 4398–4412. [Google Scholar] [CrossRef] [Green Version]
- Yoon, W.-J.; Heo, S.-J.; Han, S.-C.; Lee, H.-J.; Kang, G.-J.; Kang, H.-K.; Hyun, J.-W.; Koh, Y.-S.; Yoo, E.-S. Anti-inflammatory effect of sargachromanol G isolated from Sargassum siliquastrum in RAW 264.7 cells. Arch. Pharm. Res. 2012, 35, 1421–1430. [Google Scholar] [CrossRef]
- Lee, J.H.; Ko, J.Y.; Samarakoon, K.; Oh, J.Y.; Heo, S.J.; Kim, C.Y.; Nah, J.W.; Jang, M.K.; Lee, J.S.; Jeon, Y.J. Preparative isolation of sargachromanol E from Sargassum siliquastrum by centrifugal partition chromatography and its anti-inflammatory activity. Food Chem. Toxicol. 2013, 62, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.J.; Paek, H.J.; Yu, J. Oxidative stress by layered double hydroxide nanoparticles via an SFK-JNK and p38-NF-kappaB signaling pathway mediates induction of interleukin-6 and interleukin-8 in human lung epithelial cells. Int. J. Nanomed. 2015, 10, 3217–3229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dou, C.; Zhang, J.; Qi, C. Cooking oil fume-derived PM2.5 induces apoptosis in A549 cells and MAPK/NF-small ka, CyrillicB/STAT1 pathway activation. Environ. Sci. Pollut. Res. Int. 2018, 25, 9940–9948. [Google Scholar] [CrossRef]
- Fang, L.; Sun, Q.; Roth, M. Immunologic and Non-Immunologic Mechanisms Leading to Airway Remodeling in Asthma. Int. J. Mol. Sci. 2020, 21, 757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Budinger, G.R.; McKell, J.L.; Urich, D.; Foiles, N.; Weiss, I.; Chiarella, S.E.; Gonzalez, A.; Soberanes, S.; Ghio, A.J.; Nigdelioglu, R.; et al. Particulate matter-induced lung inflammation increases systemic levels of PAI-1 and activates coagulation through distinct mechanisms. PLoS ONE 2011, 6, e18525. [Google Scholar] [CrossRef] [Green Version]
- Yu, M.; Zheng, X.; Witschi, H.; Pinkerton, K.E. The Role of Interleukin-6 in Pulmonary Inflammation and Injury Induced by Exposure to Environmental Air Pollutants. Toxicol. Sci. 2002, 68, 488–497. [Google Scholar] [CrossRef]
- Lappalainen, U.; Whitsett, J.; Wert, S.; Tichelaar, J.; Bry, K. Interleukin-1β Causes Pulmonary Inflammation, Emphysema, and Airway Remodeling in the Adult Murine Lung. Am. J. Resp. Cell Mol. Biol. 2005, 32, 311–318. [Google Scholar] [CrossRef]
- Jackson, D.J.; Makrinioti, H.; Rana, B.M.; Shamji, B.W.; Trujillo-Torralbo, M.B.; Footitt, J.; Jerico, D.-R.; Telcian, A.G.; Nikonova, A.; Zhu, J.; et al. IL-33-dependent type 2 inflammation during rhinovirus-induced asthma exacerbations in vivo. Am. J. Respir. Crit. Care Med. 2014, 190, 1373–1382. [Google Scholar] [CrossRef] [Green Version]
- Hajmousa, G.; Vogelaar, P.; Brouwer, L.A.; van der Graaf, A.C.; Henning, R.H.; Krenning, G. The 6-chromanol derivate SUL-109 enables prolonged hypothermic storage of adipose tissue-derived stem cells. Biomaterials 2017, 119, 43–52. [Google Scholar] [CrossRef]
- He, M.; Ichinose, T.; Yoshida, Y.; Arashidani, K.; Yoshida, S.; Takano, H.; Sun, G.; Shibamoto, T. Urban PM2.5 exacerbates allergic inflammation in the murine lung via a TLR2/TLR4/MyD88-signaling pathway. Sci. Rep. 2017, 7, 11027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiang, M.; Fan, J.; Fan, J. Association of Toll-like receptor signaling and reactive oxygen species: A potential therapeutic target for posttrauma acute lung injury. Mediat. Inflamm. 2010, 2010. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Deng, S.-L.; Lian, Z.-X.; Yu, K. Roles of Toll-Like Receptors in Nitroxidative Stress in Mammals. Cells 2019, 8, 576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shoenfelt, J.; Mitkus, R.J.; Zeisler, R.; Spatz, R.O.; Powell, J.; Fenton, M.J.; Squibb, K.A.; Medvedev, A.E. Involvement of TLR2 and TLR4 in inflammatory immune responses induced by fine and coarse ambient air particulate matter. J. Leukoc. Biol. 2009, 86, 303–312. [Google Scholar] [CrossRef] [Green Version]
- Jayawardena, T.U.; Asanka Sanjeewa, K.K.; Shanura Fernando, I.P.; Ryu, B.M.; Kang, M.-C.; Jee, Y.; Lee, W.W.; Jeon, Y.-J. Sargassum horneri (Turner) C. Agardh ethanol extract inhibits the fine dust inflammation response via activating Nrf2/HO-1 signaling in RAW 264.7 cells. BMC Complement. Altern. Med. 2018, 18, 249. [Google Scholar] [CrossRef] [Green Version]
- Herath, K.H.I.N.M.; Cho, J.; Kim, A.; Kim, H.-S.; Han, E.J.; Kim, H.J.; Kim, M.S.; Ahn, G.; Jeon, Y.-J.; Jee, Y. Differential modulation of immune response and cytokine profiles of Sargassum horneri ethanol extract in murine spleen with or without Concanavalin A stimulation. Biomed. Pharmacother. 2019, 110, 930–942. [Google Scholar] [CrossRef]
- Herath, K.M.; Lee, J.H.; Cho, J.; Kim, A.; Shin, S.M.; Kim, B.; Jeon, Y.J.; Jee, Y. Immunostimulatory effect of pepsin enzymatic extract from Porphyra yezoensis on murine splenocytes. J. Sci. Food Agric. 2018, 98, 3400–3408. [Google Scholar] [CrossRef]








| Gene | Sequence | |
|---|---|---|
| Forward (5′-3′) | Reverse (5′-3′) | |
| TLR1 | GTT GTC ACT GAT GTC TTC AGC | CTG TAC CTT AGA GAA TTC TG |
| TLR2 | CAG CTTA AAG GGC GGG TCA GAG | TGG AGA CGC CAG CTC TGG CTCA |
| TLR3 | GAA GCA GGC GTC CTT GGA CTT | TGT GCT GAA TTC CGA GAT CCA |
| TLR4 | AGT GGG TCA AGG AAC AGA AGC A | CTT TAC CAG CTC ATT TCT CAC C |
| TLR5 | GAA TTC CTT AAG CGA CGT AA | GAG AAG ATA AAG CCG TGC GA |
| TLR6 | AGT GCT GCC AAG TTC CGA CA | AGC AAA CAC CGA GTA TAG CG |
| TLR7 | CCT GTT CTA CTG GGG TCC AA | GCC TCA AGG CTC AGA AGA TG |
| TLR8 | GGC ACA ACT CCC TTG TGA TT | CAT TTG GGT GCT GTT GTT TG |
| TLR9 | CCA GAC GCT CTT CGA GAA CC | GTT ATA GAA GTG GCG GTT GT |
| GAPDH | AAC GAC CCC TTC ATT GAC C | TCA GAT GCC TGC TTC ACC C |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herath, K.H.I.N.M.; Kim, H.J.; Jang, J.-H.; Kim, H.-S.; Kim, H.J.; Jeon, Y.-J.; Jee, Y. Mojabanchromanol Isolated from Sargassum horneri Attenuates Particulate Matter Induced Inflammatory Responses via Suppressing TLR2/4/7-MAPK Signaling in MLE-12 Cells. Mar. Drugs 2020, 18, 355. https://doi.org/10.3390/md18070355
Herath KHINM, Kim HJ, Jang J-H, Kim H-S, Kim HJ, Jeon Y-J, Jee Y. Mojabanchromanol Isolated from Sargassum horneri Attenuates Particulate Matter Induced Inflammatory Responses via Suppressing TLR2/4/7-MAPK Signaling in MLE-12 Cells. Marine Drugs. 2020; 18(7):355. https://doi.org/10.3390/md18070355
Chicago/Turabian StyleHerath, Kalahe Hewage Iresha Nadeeka Madushani, Hyo Jin Kim, Jae-Hyuk Jang, Hyun-Soo Kim, Hyun Jung Kim, You-Jin Jeon, and Youngheun Jee. 2020. "Mojabanchromanol Isolated from Sargassum horneri Attenuates Particulate Matter Induced Inflammatory Responses via Suppressing TLR2/4/7-MAPK Signaling in MLE-12 Cells" Marine Drugs 18, no. 7: 355. https://doi.org/10.3390/md18070355
APA StyleHerath, K. H. I. N. M., Kim, H. J., Jang, J. -H., Kim, H. -S., Kim, H. J., Jeon, Y. -J., & Jee, Y. (2020). Mojabanchromanol Isolated from Sargassum horneri Attenuates Particulate Matter Induced Inflammatory Responses via Suppressing TLR2/4/7-MAPK Signaling in MLE-12 Cells. Marine Drugs, 18(7), 355. https://doi.org/10.3390/md18070355