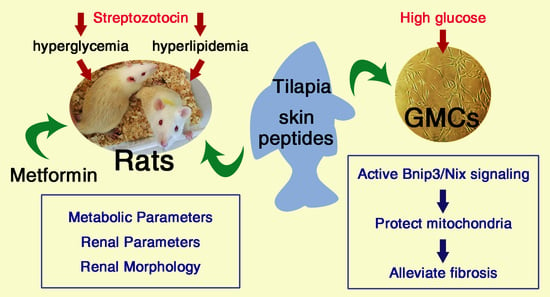
Tilapia Skin Peptides Ameliorate Diabetic Nephropathy in STZ-Induced Diabetic Rats and HG-Induced GMCs by Improving Mitochondrial Dysfunction
Abstract
:1. Introduction
2. Results
2.1. Effect of TSPs on Metabolic and Renal Parameters in Streptozotocin (STZ)-Induced Diabetic Rats
2.2. TSPs Improve the Renal Morphology and ECM Accumulation in STZ-Induced Diabetic Rats
2.3. TSPs Prevent the Increased Protein Expressions of FN, Collagen IV and ICAM-1 in GMCs Challenged with HG
2.4. TSPs Ameliorate the Excessive Mitochondrial Fragmentation, Decreased Mitochondrial Membrane Potential (MMP), Increased Production of Mitochondrial Superoxide and Cellular ROS in HG-Induced GMCs
2.5. Bnip3/Nix Signaling is Enhanced by TSPs Treatment in HG-Induced GMCs
3. Discussion
4. Materials and Methods
4.1. Reagents and Materials
4.2. Animal Experiment
4.3. Biochemical and Morphological Studies
4.4. Cell Culture and Treatment
4.5. Western Blot Assay
4.6. Immunofluorescence
4.7. Assessment of Mitochondrial Morphology
4.8. Measurement of Mitochondrial Superoxide
4.9. Measurement of ROS
4.10. Measurement of MMP
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Huang, K.; Chen, C.; Hao, J.; Huang, J.; Wang, S.; Liu, P.; Huang, H. Polydatin promotes Nrf2-ARE anti-oxidative pathway through activating Sirt1 to resist AGEs-induced upregulation of fibronetin and transforming growth factor-beta1 in rat glomerular messangial cells. Mol. Cell. Endocrinol. 2015, 399, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Shah, I.M.; Mackay, S.P.; McKay, G.A. Therapeutic strategies in the treatment of diabetic nephropathy—A translational medicine approach. Curr. Med. Chem. 2009, 16, 997–1016. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Xie, X.; Huang, J.; Gong, W.; Zhu, X.; Chen, Q.; Huang, J.; Huang, H. Connexin43 regulates high glucose-induced expression of fibronectin, ICAM-1 and TGF-β1 via Nrf2/ARE pathway in glomerular mesangial cells. Free Radic. Biol. Med. 2017, 102, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Robotham, J.L.; Yoon, Y. Increased production of reactive oxygen species in hyperglycemic conditions requires dynamic change of mitochondrial morphology. Proc. Natl. Acad. Sci. USA 2006, 103, 2653–2658. [Google Scholar] [CrossRef] [Green Version]
- Xie, X.; Chen, Q.; Tao, J. Astaxanthin Promotes Nrf2/ARE Signaling to Inhibit HG-Induced Renal Fibrosis in GMCs. Mar. Drugs 2018, 16, 117. [Google Scholar] [CrossRef] [Green Version]
- Higgins, G.C.; Coughlan, M.T. Mitochondrial dysfunction and mitophagy: The beginning and end to diabetic nephropathy? Br. J. Pharmacol. 2014, 171, 1917–1942. [Google Scholar] [CrossRef] [Green Version]
- Kroemer, G.; Marino, G.; Levine, B. Autophagy and the integrated stress response. Mol. Cell 2010, 40, 280–293. [Google Scholar] [CrossRef] [Green Version]
- Xiao, L.; Xu, X.; Zhang, F.; Wang, M.; Xu, Y.; Tang, D.; Wang, J.; Qin, Y.; Liu, Y.; Tang, C.; et al. The mitochondria-targeted antioxidant MitoQ ameliorated tubular injury mediated by mitophagy in diabetic kidney disease via Nrf2/PINK1. Redox Biol. 2017, 11, 297–311. [Google Scholar] [CrossRef]
- Sun, J.; Zhu, H.; Wang, X.; Gao, Q.; Li, Z.; Huang, H. CoQ10 ameliorates mitochondrial dysfunction in diabetic nephropathy through mitophagy. J. Endocrinol. 2019, 240, 445–465. [Google Scholar] [CrossRef]
- Zhan, M.; Usman, I.M.; Sun, L.; Kanwar, Y.S. Disruption of renal tubular mitochondrial quality control by Myo-inositol oxygenase in diabetic kidney disease. J. Am. Soc. Nephrol. 2015, 26, 1304–1321. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Chen, Z.; Tao, Y.; Zhu, J.; Yang, H.; Liang, W.; Ding, G. Increased mitochondrial fission of glomerular podocytes in diabetic nephropathy. Endocr. Connect. 2019, 8, 1206–1212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Czajka, A.; Ajaz, S.; Gnudi, L.; Parsade, C.K.; Jones, P.; Reid, F.; Malik, A.N. Altered Mitochondrial Function, Mitochondrial DNA and Reduced Metabolic Flexibility in Patients with Diabetic Nephropathy. EBioMedicine 2015, 2, 499–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novak, I.; Kirkin, V.; McEwan, D.G.; Zhang, J.; Wild, P.; Rozenknop, A.; Rogov, V.; Löhr, F.; Popovic, D.; Occhipinti, A.; et al. Nix is a selective autophagy receptor for mitochondrial clearance. EMBO Rep. 2010, 11, 45–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsushima, M.; Fujiwara, T.; Takahashi, E.; Minaguchi, T.; Eguchi, Y.; Tsujimoto, Y.; Suzumori, K.; Nakamura, Y. Isolation, mapping, and functional analysis of a novel human cDNA (BNIP3L) encoding a protein homologous to human NIP3. Gene Chromosome Cancer 1998, 21, 230–235. [Google Scholar] [CrossRef]
- Dale, H.F.; Madsen, L.; Lied, G.A. Fish–derived proteins and their potential to improve human health. Nutr. Rev. 2019, 77, 572–583. [Google Scholar] [CrossRef] [Green Version]
- Xia, E.Q.; Zhu, S.S.; He, M.J.; Luo, F.; Fu, C.Z.; Zou, T.B. Marine Peptides as Potential Agents for the Management of Type 2 Diabetes Mellitus—A Prospect. Mar. Drugs 2017, 15, 88. [Google Scholar] [CrossRef] [Green Version]
- Zhu, C.F.; Li, G.Z.; Peng, H.B.; Zhang, F.; Chen, Y.; Li, Y. Therapeutic effects of marine collagen peptides on Chinese patients with type 2 diabetes mellitus and primary hypertension. Am. J. Med. Sci. 2010, 340, 360–366. [Google Scholar] [CrossRef]
- Le Gouic, A.V.; Harnedy, P.A.; FitzGerald, R.J. Bioactive peptides from fish protein by-products. In Bioactive Molecules in Food; Mérillon, J.M., Ramawat, K.G., Eds.; Springer: Cham, Switzerland, 2019; pp. 355–388. [Google Scholar] [CrossRef]
- Jha, V.; Garcia-Garcia, G.; Iseki, K.; Li, Z.; Naicker, S.; Plattner, B.; Saran, R.; Wang, A.Y.; Yang, C.W. Chronic kidney disease: Global dimension and perspectives. Lancet 2013, 382, 260–272. [Google Scholar] [CrossRef]
- Alberti, K.G.; Zimmet, P. Global burden of disease—Where does diabetes mellitus fit in? Nat. Rev. Endocrinol. 2013, 9, 258–260. [Google Scholar] [CrossRef]
- Dong, L.; Li, Y.; Zhang, D.; Zhang, H.; Han, J.; Wang, Z.; Zhou, J.; Lu, C.; Su, X. Dietary Apostichopus japonicus alleviates diabetes symptoms and modulates genes expression in Kidney Tissues of db/db Mice. J. Agric. Food Chem. 2018, 66, 154–162. [Google Scholar] [CrossRef]
- Gul, K.; Singh, A.K.; Jabeen, R. Nutraceuticals and functional foods: The foods for the future world. Crit. Rev. Food Sci. Nutr. 2016, 56, 2617–2627. [Google Scholar] [CrossRef] [PubMed]
- Alkhatib, A.; Tsang, C.; Tiss, A.; Bahorun, T.; Arefanian, H.; Barake, R.; Khadir, A.; Tuomilehto, J. Functional foods and lifestyle approaches for diabetes prevention and management. Nutrients 2017, 9, 1310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.; Yan, Z.; Bu, L.; An, C.; Wang, D.; Liu, X.; Zhang, J.; Yang, W.; Deng, B.; Xie, J.; et al. Rapeseed protein-derived antioxidant peptide RAP alleviates renal fibrosis through MAPK/NF-κB signaling pathways in diabetic nephropathy. Drug Des. Dev. Ther. 2018, 12, 1255–1268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, X.; Sun, L.; Lai, X.; Xiang, L.; Li, Q.; Zhang, W.; Zhang, L.; Sun, S. Tea polypeptide ameliorates diabetic nephropathy through RAGE and NF-κB signaling pathway in type 2 diabetes mice. J. Agric. Food Chem. 2018, 66, 11957–11967. [Google Scholar] [CrossRef] [PubMed]
- Pangestuti, R.; Kim, S.K. Bioactive peptide of marine origin for the prevention and treatment of non-communicable diseases. Mar. Drugs 2017, 15, 67. [Google Scholar] [CrossRef]
- Lin, H.C.; Alashi, A.M.; Aluko, R.E.; Sun Pan, B.; Chang, Y.W. Antihypertensive properties of tilapia (Oreochromis spp.) frame and skin enzymatic protein hydrolysates. Food Nutr. Res. 2017, 61, 1391666. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Z.; Liang, P.; Chen, J.; Chen, M.F.; Gong, F.; Li, C.; Zhou, C.; Hong, P.; Yang, P.; Qian, Z.J. A peptide YGDEY from Tilapia gelatin Hydrolysates inhibits UVB-mediated skin Photoaging by regulating MMP-1 and MMP-9 expression in HaCaT cells. Photochem. Photobiol. 2019, 95, 1424–1432. [Google Scholar] [CrossRef]
- Mei, F.; Liu, J.; Wu, J.; Duan, Z.; Chen, M.; Meng, K.; Chen, S.; Shen, X.; Xia, G.; Zhao, M. Collagen peptides isolated from Salmo salar and Tilapia nilotica skin accelerate wound healing by altering cutaneous Microbiome colonization via upregulated NOD2 and BD14. J. Agric. Food Chem. 2020, 68, 1621–1633. [Google Scholar] [CrossRef]
- Li, D.D.; Li, W.J.; Kong, S.Z.; Li, S.D.; Guo, J.Q.; Guo, M.H.; Cai, T.T.; Li, N.; Chen, R.Z.; Luo, R.Q.; et al. Protective effects of collagen polypeptide from tilapia skin against injuries to the liver and kidneys of mice induced by d-galactose. Biomed. Pharmacother. 2019, 117, 109204. [Google Scholar] [CrossRef]
- Xiong, X.; Liang, J.; Xu, Y.; Liu, J.; Liu, Y. The wound healing effects of the Tilapia collagen peptide mixture TY001 in streptozotocin diabetic mice. J. Sci. Food Agric. 2020, 100, 2848–2858. [Google Scholar] [CrossRef] [PubMed]
- Sacks, F.M.; Hermans, M.P.; Fioretto, P.; Valensi, P.; Davis, T.; Horton, E.; Wanner, C.; Al-Rubeaan, K.; Aronson, R.; Barzon, I.; et al. Association between plasma triglycerides and high-density lipoprotein cholesterol and microvascular kidney disease and retinopathy in type 2 diabetes mellitus: A global case-control study in 13 countries. Circulation 2014, 129, 999–1008. [Google Scholar] [CrossRef] [PubMed]
- Kreisberg, R.A. Diabetic dyslipidemia. Am. J. Cardiol. 1998, 82 (Suppl. 1), 67U–73U. [Google Scholar] [CrossRef]
- Tak, Y.J.; Kim, Y.J.; Lee, J.G.; Yi, Y.-H.; Cho, Y.H.; Kang, G.H.; Lee, S.Y. Effect of oral ingestion of low-molecular collagen peptides derived from skate (Raja Kenojei) skin on body fat in overweight adults: A randomized, double-blind, placebo-controlled trial. Mar. Drugs 2019, 17, 157. [Google Scholar] [CrossRef] [Green Version]
- Woo, M.; Song, Y.O.; Kang, K.H.; Noh, J.S. Anti-obesity effects of collagen peptide derived from skate (Raja kenojei) skin through regulation of lipid metabolism. Mar. Drugs 2018, 16, 306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolset, S.O.; Reinholt, F.P.; Jenssen, T. Diabetic nephropathy and extracellular matrix. J. Histochem. Cytochem. 2012, 60, 976–986. [Google Scholar] [CrossRef] [Green Version]
- Donate-Correa, J.; Luis-Rodríguez, D.; Martín-Núñez, E.; Tagua, V.G.; Hernández-Carballo, C.; Ferri, C.; Rodríguez-Rodríguez, A.E.; Mora-Fernández, C.; Navarro-González, J.F. Inflammatory targets in diabetic nephropathy. J. Clin. Med. 2020, 9, 458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, H.; Zhang, Z.; Huang, H.; Wang, Y.; Lin, B.; Wu, S.; Ma, J.; Chen, B.; He, Z.; Wu, J.; et al. Inhibition of bleomycin-induced pulmonary fibrosis in mice by the novel peptide EZY-1 purified from Eucheuma. Food Funct. 2019, 10, 3198–3208. [Google Scholar] [CrossRef] [PubMed]
- Porporato, P.E.; Payen, V.L.; Baselet, B.; Sonveaux, P. Metabolic changes associated with tumor metastasis, part 2: Mitochondria, lipid and amino acid metabolism. Cell. Mol. Life Sci. 2016, 73, 1349–1363. [Google Scholar] [CrossRef]
- Coughlan, M.T.; Thorburn, D.R.; Penfold, S.A.; Laskowski, A.; Harcourt, B.E.; Sourris, K.C.; Tan, A.L.; Fukami, K.; Thallas-Bonke, V.; Nawroth, P.P.; et al. RAGE-induced cytosolic ROS promote mitochondrial superoxide generation in diabetes. J. Am. Soc. Nephrol. 2009, 20, 742–752. [Google Scholar] [CrossRef] [Green Version]
- Rosca, M.G.; Mustata, T.G.; Kinter, M.T.; Ozdemir, A.M.; Kern, T.S.; Szweda, L.I.; Brownlee, M.; Monnier, V.M.; Weiss, M.F. Glycation of mitochondrial proteins from diabetic rat kidney is associated with excess superoxide formation. Am. J. Physiol.-Ren. Physiol. 2005, 289, F420–F430. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Ji, L.; Tang, X.; Chen, X.; Li, Z.; Mi, X.; Yang, L. Inhibition to DRP1 translocation can mitigate p38 MAPK-signaling pathway activation in GMC induced by hyperglycemia. Ren. Fail. 2015, 37, 903–910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayanga, B.A.; Badal, S.S.; Wang, Y.; Galvan, D.L.; Chang, B.H.; Schumacker, P.T.; Danesh, F.R. Dynamin-related protein 1 deficiency improves mitochondrial fitness and protects against progression of diabetic nephropathy. J. Am. Soc. Nephrol. 2016, 27, 2733–2747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, F.; Wang, J.; Yu, F.; Tang, Y.; Ding, G.; Yang, Z.; Sun, Y. Protective effect of Meretrix meretrix Oligopeptides on high-fat-diet-induced non-alcoholic fatty liver disease in mice. Mar. Drugs 2018, 16, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, D.; Li, L.; Xu, T.; Wang, T.; Ren, J.; Liu, X.; Li, Y. Effect of low molecular weight Oligopeptides isolated from sea cucumber on diabetic wound healing in db/db mice. Mar. Drugs 2018, 16, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nasri, R.; Abdelhedi, O.; Jemil, I.; Daoued, I.; Hamden, K.; Kallel, C.; Elfeki, A.; Lamri-Senhadji, M.; Boualga, A.; Nasri, M.; et al. Ameliorating effects of goby fish protein hydrolysates on high-fat-high-fructose diet-induced hyperglycemia, oxidative stress and deterioration of kidney function in rats. Chem. Biol. Interact. 2015, 242, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Han, H.; Liu, Z.; Liu, Y.; Yin, L.; Cai, J.; He, L.; Liu, Y.; Chen, G.; Zhang, Z.; et al. Activation of BNIP3-mediated mitophagy protects against renal ischemia-reperfusion injury. Cell Death Dis. 2019, 10, 677. [Google Scholar] [CrossRef]
- Tang, Q.; Zheng, G.; Feng, Z.; Chen, Y.; Lou, Y.; Wang, C.; Zhang, X.; Zhang, Y.; Xu, H.; Shang, P.; et al. Trehalose ameliorates oxidative stress-mediated mitochondrial dysfunction and ER stress via selective autophagy stimulation and autophagic flux restoration in osteoarthritis development. Cell Death Dis. 2017, 8, e3081. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Xin, T.; Li, D.; Wang, C.; Zhu, H.; Zhou, H. Therapeutic effect of Sirtuin 3 on ameliorating nonalcoholic fatty liver disease: The role of the ERK-CREB pathway and Bnip3-mediated mitophagy. Redox Biol. 2018, 18, 229–243. [Google Scholar] [CrossRef]
- Liang, X.; Yang, Y.; Huang, Z.; Zhou, J.; Li, Y.; Zhong, X. Panax notoginseng saponins mitigate cisplatin induced nephrotoxicity by inducing mitophagy via HIF-1α. Oncotarget 2017, 8, 102989–103003. [Google Scholar] [CrossRef] [Green Version]
- Xu, D.; Chen, P.; Wang, B.; Wang, Y.; Miao, N.; Yin, F.; Cheng, Q.; Zhou, Z.; Xie, H.; Zhou, L.; et al. NIX-mediated mitophagy protects against proteinuria-induced tubular cell apoptosis and renal injury. Am. J. Physiol.-Ren. Physiol. 2019, 316, F382–F395. [Google Scholar] [CrossRef]
- Zhu, C.F.; Peng, H.B.; Liu, G.Q.; Zhang, F.; Li, Y. Beneficial effects of oligopeptides from marine salmon skin in a rat model of type 2 diabetes. Nutrition 2010, 26, 1014–1020. [Google Scholar] [CrossRef] [PubMed]





| Parameters | Control (n = 8) | STZ (n = 8) | STZ + TSPs (n = 8) | STZ + Met (n = 8) |
|---|---|---|---|---|
| KW/BW (%) | 0.40 ± 0.02 # | 0.80 ± 0.67 * | 0.69 ± 0.09 *,# | 0.69 ± 0.10 *,# |
| FBG (mM) | 4.27 ± 0.15 # | 22.18 ± 2.48 * | 19.63 ± 1.43 * | 8.83 ± 2.08 *,# |
| BUN (mM) | 8.39 ± 0.33 # | 22.06 ± 1.22 * | 18.34 ± 1.05 *,# | 16.77 ± 4.33 *,# |
| Cr (μM) | 21.84 ± 8.70 # | 89.66 ± 12.45 * | 65.64 ± 10.77 *,# | 50.98 ± 20.23 *,# |
| UP 24 h (mg) | 9.65 ± 3.98 # | 29.62 ± 8.53 * | 14.04 ± 4.94 # | 12.77 ± 6.46 # |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, L.; Zheng, D.; Yang, G.; Li, W.; Yang, H.; Jiang, Q.; Chen, Y.; Zhang, Y.; Xie, X. Tilapia Skin Peptides Ameliorate Diabetic Nephropathy in STZ-Induced Diabetic Rats and HG-Induced GMCs by Improving Mitochondrial Dysfunction. Mar. Drugs 2020, 18, 363. https://doi.org/10.3390/md18070363
Jin L, Zheng D, Yang G, Li W, Yang H, Jiang Q, Chen Y, Zhang Y, Xie X. Tilapia Skin Peptides Ameliorate Diabetic Nephropathy in STZ-Induced Diabetic Rats and HG-Induced GMCs by Improving Mitochondrial Dysfunction. Marine Drugs. 2020; 18(7):363. https://doi.org/10.3390/md18070363
Chicago/Turabian StyleJin, Lin, Dongxiao Zheng, Guanyu Yang, Wei Li, Huan Yang, Qian Jiang, Yongjun Chen, Yingxia Zhang, and Xi Xie. 2020. "Tilapia Skin Peptides Ameliorate Diabetic Nephropathy in STZ-Induced Diabetic Rats and HG-Induced GMCs by Improving Mitochondrial Dysfunction" Marine Drugs 18, no. 7: 363. https://doi.org/10.3390/md18070363
APA StyleJin, L., Zheng, D., Yang, G., Li, W., Yang, H., Jiang, Q., Chen, Y., Zhang, Y., & Xie, X. (2020). Tilapia Skin Peptides Ameliorate Diabetic Nephropathy in STZ-Induced Diabetic Rats and HG-Induced GMCs by Improving Mitochondrial Dysfunction. Marine Drugs, 18(7), 363. https://doi.org/10.3390/md18070363