Physicochemical Properties of Collagen from Acaudina Molpadioides and Its Protective Effects against H2O2-Induced Injury in RAW264.7 Cells
Abstract
:1. Introduction
2. Results and Discussion
2.1. Ultraviolet (UV) Absorption Spectrum
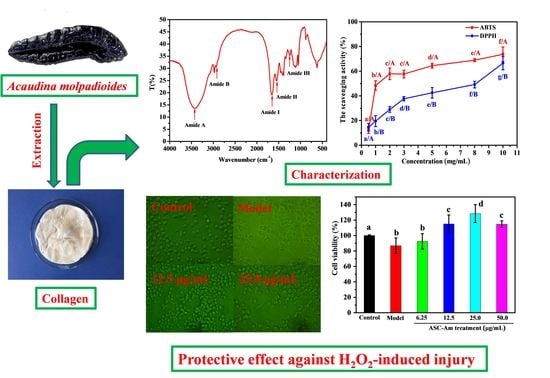
2.2. Fourier Transform Infrared Spectroscopy (FTIR)
2.3. Amino Acid Analysis
2.4. Zeta Potential
2.5. Antioxidant Activity
2.6. Protective Effect against H2O2-induced Injury on RAW264.7 Macrophage Cells
3. Materials and Methods
3.1. Materials
3.2. Extraction of Collagen from A. Molpadioides
3.3. UV Absorption Spectrum
3.4. FTIR Spectrum Analysis
3.5. Amino Acid Analysis
3.6. Zeta Potential
3.7. Antioxidant Activity
3.7.1. DPPH Radical Scavenging Activity
3.7.2. ABTS Radical Scavenging Activity
3.8. Cell Morphology Observation and Measurement of Cell Viability
3.9. Assays for the Levels of MDA, SOD, and GSH-Px
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, J.; Duan, R.; Huang, L.; Song, Y.; Regenstein, J.M. Characterisation of acid-soluble and pepsin-solubilised collagen from jellyfish (Cyanea nozakii Kishinouye). Food Chem. 2014, 150, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Zhong, M.; Chen, T.; Hu, C.; Ren, C. Isolation and Characterization of Collagen from the Body Wall of Sea CucumberStichopus monotuberculatus. J. Food Sci. 2015, 80, C671–C679. [Google Scholar] [CrossRef] [PubMed]
- Herod, T.W.; Chambers, N.C.; Veres, S.P. Collagen fibrils in functionally distinct tendons have differing structural responses to tendon rupture and fatigue loading. Acta Biomater. 2016, 42, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ma, D.; Wang, Y.; Qin, W. A comparative study of the properties and self-aggregation behavior of collagens from the scales and skin of grass carp (Ctenopharyngodon idella). Int. J. Boil. Macromol. 2018, 106, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Dhaka, V.; Gulia, N.; Ahlawat, K.S.; Khatkar, B.S. Trans fats—Sources, health risks and alternative approach—A review. J. Food Sci. Technol. 2011, 48, 534–541. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Wang, Y.-M.; Chi, C.-F.; Luo, H.Y.; Deng, S.-G.; Ma, J.-Y. Isolation and Characterization of Collagen and Antioxidant Collagen Peptides from Scales of Croceine Croaker (Pseudosciaena crocea). Mar. Drugs 2013, 11, 4641–4661. [Google Scholar] [CrossRef]
- Yang, Y.; Li, C.; Song, W.; Wang, W.; Qian, G. Purification, optimization and physicochemical properties of collagen from soft-shelled turtle calipash. Int. J. Boil. Macromol. 2016, 89, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.-H.; Li, H.; Sun, K.; Wang, B. Preparation, Physicochemical and Antioxidant Properties of Acid- and Pepsin-Soluble Collagens from the Swim Bladders of Miiuy Croaker (Miichthys miiuy). Mar. Drugs 2018, 16, 161. [Google Scholar] [CrossRef] [Green Version]
- Rigby, B.J. Amino-acid Composition and Thermal Stability of the Skin Collagen of the Antarctic Ice-fish. Nature 1968, 219, 166–167. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Chi, C.-F.; Wang, B.; Ding, G.-F.; Li, Z.-R. Characterization of acid-and pepsin-soluble collagens from spines and skulls of skipjack tuna (Katsuwonus pelamis). Chin. J. Nat. Med. 2014, 12, 712–720. [Google Scholar] [CrossRef]
- Yu, F.; Zong, C.; Jin, S.; Zheng, J.; Chen, N.; Huang, J.; Chen, Y.; Huang, F.; Yang, Z.; Tang, Y.; et al. Optimization of Extraction Conditions and Characterization of Pepsin-Solubilised Collagen from Skin of Giant Croaker (Nibea japonica). Mar. Drugs 2018, 16, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bordbar, S.; Anwar, F.; Saari, N. High-value components and bioactives from sea cucumbers for functional foods—A review. Mar. Drugs 2011, 9, 1761–1805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Oliveira, A.C.; Su, Y.C. Purification and characterization of pepsin-solubilized collagen from skin and connective tissue of giant red sea cucumber (Parastichopus californicus). J. Agric. Food Chem. 2010, 58, 1270–1274. [Google Scholar] [CrossRef] [PubMed]
- Abedin, M.Z.; Karim, A.A.; Ahmed, F.; Latiff, A.A.; Gan, C.Y.; Che Ghazali, F.; Islam Sarker, M.Z. Isolation and characterization of pepsin-solubilized collagen from the integument of sea cucumber (Stichopus vastus). J. Sci. Food Agric. 2013, 93, 1083–1088. [Google Scholar] [CrossRef]
- Adibzadeh, N.; Aminzadeh, S.; Jamili, S.; Karkhane, A.A.; Farrokhi, N. Purification and characterization of pepsin-solubilized collagen from skin of sea cucumber Holothuria parva. Appl. Biochem. Biotechnol. 2014, 173, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.J.; Zhan, C.L.; Cai, Q.F.; Weng, L.; Du, C.H.; Liu, G.M.; Su, W.J.; Cao, M.J. Purification, characterization, cDNA cloning and in vitro expression of a serine proteinase from the intestinal tract of sea cucumber (Stichopus japonicus) with collagen degradation activity. J. Agric. Food Chem. 2014, 62, 4769–4777. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chang, Y.; Wu, F.; Xu, X.; Xue, C. Fucosylated chondroitin sulfate is covalently associated with collagen fibrils in sea cucumber Apostichopus japonicus body wall. Carbohydr. Polym. 2018, 186, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.J.; Xue, Y.P.; San, E.L.; Keong, T.C.; Chen, L.F.; Zheng, Y.G. Extraction and Characterization of Pepsin Soluble Collagen from the Body Wall of Sea Cucumber Acaudina leucoprocta. J. Aquat. Food Prod. Technol. 2017, 26, 502–515. [Google Scholar] [CrossRef]
- Park, S.Y.; Lim, H.K.; Lee, S.; Hwang, H.C.; Cho, S.K.; Cho, M. Pepsin-solubilised collagen (PSC) from Red Sea cucumber (Stichopus japonicus) regulates cell cycle and the fibronectin synthesis in HaCaT cell migration. Food Chem. 2012, 132, 487–492. [Google Scholar] [CrossRef]
- Zhong, C.; Sun, L.C.; Yan, L.J.; Lin, Y.C.; Liu, G.M.; Cao, M.J. Production, optimisation and characterisation of angiotensin converting enzyme inhibitory peptides from sea cucumber (Stichopus japonicus) gonad. Food Funct. 2018, 9, 594–603. [Google Scholar] [CrossRef]
- Ye, J.; Shen, C.; Huang, Y.; Zhang, X.; Xiao, M. Anti-fatigue activity of sea cucumber peptides prepared from Stichopus japonicus in an endurance swimming rat model. J. Sci. Food Agric. 2017, 97, 4548–4556. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, J.; Lin, S.J.; Yang, Z.S.; Jin, H.X. Preparation of Antioxidant Peptide by Microwave- Assisted Hydrolysis of Collagen and Its Protective Effect Against H2O2-Induced Damage of RAW264.7 Cells. Mar. Drugs 2019, 17, 642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, F.X.; Xue, C.H.; Li, Z.J.; Zhang, Y.Q.; Dong, P.; Fu, X.Y.; Gao, X. Characterization and subunit composition of collagen from the body wall of sea cucumber Stichopus japonicus. Food Chem. 2007, 100, 1120–1125. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, W.T.; LI, G.Y. Isolation and characterisation of collagens from the skin of largefin longbarbel catfish (Mystus macropterus). Food Chem. 2009, 115, 826–831. [Google Scholar] [CrossRef]
- Doyle, B.B.; Bendit, E.G.; Blout, E.R. Infrared spectroscopy of collagen and collagen-like polypeptides. Biopolymers 1975, 14, 937–957. [Google Scholar] [CrossRef]
- Li, L.Y.; Zhao, Y.Q.; He, Y.; Chi, C.F.; Wang, B. Physicochemical and Antioxidant Properties of Acid- and Pepsin-Soluble Collagens from the Scales of Miiuy Croaker (Miichthys Miiuy). Mar. Drugs 2018, 16, 394. [Google Scholar] [CrossRef] [Green Version]
- Tan, Y.; Chang, S.K.C. Isolation and characterization of collagen extracted from channel catfish (Ictalurus punctatus) skin. Food Chem. 2018, 242, 147–155. [Google Scholar] [CrossRef]
- Ferraro, V.; Gaillard-Martinie, B.; Sayd, T.; Chambon, C.; Anton, M.; Sante-Lhoutellier, V. Collagen type I from bovine bone. Effect of animal age, bone anatomy and drying methodology on extraction yield, self-assembly, thermal behaviour and electrokinetic potential. Int. J. Biol. Macromol. 2017, 97, 55–66. [Google Scholar] [CrossRef]
- Ahmad, M.; Benjakul, S.; Nalinanon, S. Compositional and physicochemical characteristics of acid solubilized collagen extracted from the skin of unicorn leatherjacket (Aluterus monoceros). Food Hydrocolloid 2010, 24, 588–594. [Google Scholar] [CrossRef]
- Chen, J.; Li, L.; Yi, R.; Xu, N.; Gao, R.; Hong, B. Extraction and characterization of acid-soluble collagen from scales and skin of tilapia (Oreochromis niloticus). LWT Food Sci. Technol. 2016, 66, 453–459. [Google Scholar] [CrossRef]
- Kittiphattanabawon, P.; Benjakul, S.; Visessanguan, W.; Shahidi, F. Comparative study on characteristics of gelatin from the skins of brownbanded bamboo shark and blacktip shark as affected by extraction conditions. Food Hydrocolloid 2010, 24, 164171. [Google Scholar] [CrossRef]
- Sinthusamran, S.; Benjakul, S.; Kishimura, H. Comparative study on molecular characteristics of acid soluble collagens from skin and swim bladder of seabass (Lates calcarifer). Food Chem. 2013, 138, 2435–2441. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.W.; Dong, X.P.; Zhou, D.Y.; Gao, Y.; Yang, J.F.; Li, D.M.; Zhao, X.K.; Ren, T.T.; Ye, W.X.; Tan, H.; et al. Physicochemical properties and radical scavenging capacities of pepsin-solubilized collagen from sea cucumber Stichopus japonicus. Food Hydrocolloid 2012, 28, 182–188. [Google Scholar] [CrossRef]
- Pal, G.K.; Suresh, P.V. Physico-chemical characteristics and fibril-forming capacity of carp swim bladder collagens and exploration of their potential bioactive peptides by in silico approaches. Int. J. Biol. Macromol. 2017, 101, 304–313. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, F.; Li, Q.; Chen, H.; Zhang, W.; Yu, P.; Zhao, T.; Mao, G.; Feng, W.; Yang, L.; et al. Structure characterization of one polysaccharide from Lepidium meyenii Walp., and its antioxidant activity and protective effect against H2O2-induced injury RAW264.7 cells. Int. J. Biol. Macromol. 2018, 118, 816–833. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Guillén, M.C.; Giménez, B.; López-Caballero, M.E.; Montero, M.P. Functional and bioactive properties of collagen and gelatin from alternative sources: A review. Food Hydrocoll. 2011, 25, 1813–1827. [Google Scholar] [CrossRef] [Green Version]
- Holmstrom, K.M.; Finkel, T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat. Reviews. Mol. Cell Biol. 2014, 15, 411–421. [Google Scholar] [CrossRef]
- Qin, T.; Ren, Z.; Liu, X.; Luo, Y.; Long, Y.; Peng, S.; Chen, S.; Zhang, J.; Ma, Y.; Li, J.; et al. Study of the selenizing Codonopsis pilosula polysaccharides protects RAW264.7 cells from hydrogen peroxide-induced injury. Int. J. Biol. Macromol. 2019, 125, 534–543. [Google Scholar] [CrossRef]
- Bedoya-Ramirez, D.; Cilla, A.; Contreras-Calderon, J.; Alegria-Toran, A. Evaluation of the antioxidant capacity, furan compounds and cytoprotective/cytotoxic effects upon Caco-2 cells of commercial Colombian coffee. Food Chem. 2017, 219, 364–372. [Google Scholar] [CrossRef]
- Niki, E. Assessment of antioxidant capacity in vitro and in vivo. Free Radic. Biol. Med. 2010, 49, 503–515. [Google Scholar] [CrossRef]
- Gheldof, N.; Wang, X.H.; Engeseth, N.J. Buckwheat honey increases serum antioxidant capacity in humans. J. Agric. Food Chem. 2003, 51, 1500–1505. [Google Scholar] [CrossRef] [PubMed]
- Chi, C.F.; Wang, B.; Li, Z.R.; Luo, H.Y.; Ding, G.F. Characterization of acid-soluble collagens from the cartilages of scalloped hammerhead (Sphyrna lewini), red stingray (Dasyatis akajei), and skate (Raja porosa). Food Sci. Biotechnol 2013, 22, 909–916. [Google Scholar] [CrossRef]







| Amino Acid | A. Molpadioides | S. Monotuberculatus | H. Parva | Calf Skin |
|---|---|---|---|---|
| Aspartic acid (Asp) | 63.7 | 70 | 50 | 45 |
| Threonine (Thr) | 40.8 | 34 | ND 1 | ND 1 |
| Serine (Ser) | 40.7 | 26 | 20 | 39 |
| Glutamic acid (Glu) | 100.8 | 127 | 74 | 75 |
| Glycine (Gly) | 336.9 | 320 | 270 | 330 |
| Alanine (Ala) | 127.9 | 92 | 91 | 119 |
| Valine (Val) | 21.1 | 21 | 18 | 21 |
| Methionine (Met) | 5.7 | 6 | 5 | 6 |
| Isoleucine (Ile) | 7.1 | 15 | 4 | 11 |
| Leucine (Leu) | 19.6 | 20 | 16 | 23 |
| Tyrosine (Tyr) | 10.5 | 6 | 4 | 3 |
| Phenylalanine (Phe) | 8.0 | 10 | 6 | 3 |
| Lysine (Lys) | 4.1 | 6 | 7 | 26 |
| Histidine (His) | 4.6 | 9 | ND 1 | 5 |
| Arginine (Arg) | 45.1 | 71 | 49 | 50 |
| Proline (Pro) | 98.5 | 84 | 96 | 121 |
| Hydroxyproline (Hyp) | 57.5 | 67 | 62 | 94 |
| Imino Acid | 156 | 151 | 158 | 215 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Li, Y.; Li, Y.; Yang, Z.; Jin, H. Physicochemical Properties of Collagen from Acaudina Molpadioides and Its Protective Effects against H2O2-Induced Injury in RAW264.7 Cells. Mar. Drugs 2020, 18, 370. https://doi.org/10.3390/md18070370
Li J, Li Y, Li Y, Yang Z, Jin H. Physicochemical Properties of Collagen from Acaudina Molpadioides and Its Protective Effects against H2O2-Induced Injury in RAW264.7 Cells. Marine Drugs. 2020; 18(7):370. https://doi.org/10.3390/md18070370
Chicago/Turabian StyleLi, Jie, Yan Li, Yuyao Li, Zuisu Yang, and Huoxi Jin. 2020. "Physicochemical Properties of Collagen from Acaudina Molpadioides and Its Protective Effects against H2O2-Induced Injury in RAW264.7 Cells" Marine Drugs 18, no. 7: 370. https://doi.org/10.3390/md18070370
APA StyleLi, J., Li, Y., Li, Y., Yang, Z., & Jin, H. (2020). Physicochemical Properties of Collagen from Acaudina Molpadioides and Its Protective Effects against H2O2-Induced Injury in RAW264.7 Cells. Marine Drugs, 18(7), 370. https://doi.org/10.3390/md18070370