Neuroprotective Effect of Carotenoid-Rich Enteromorpha prolifera Extract via TrkB/Akt Pathway against Oxidative Stress in Hippocampal Neuronal Cells
Abstract
:1. Introduction
2. Results
2.1. Neuroprotective Effect of EAEP on Glutamate-Induced Oxidative Stress in HT-22 Cells
2.2. Inhibitory Effect of EAEP on Oxidative Stress-Induced Apoptosis in HT-22 Cells
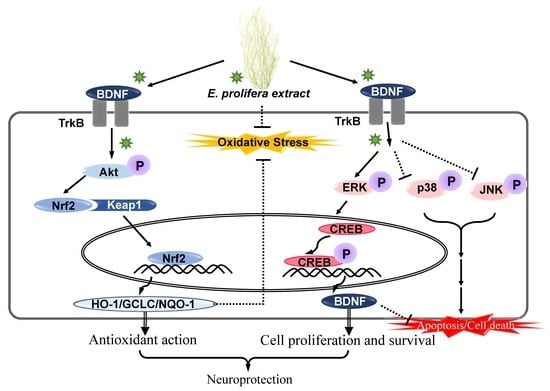
2.3. EAEP Activates the Expression of Antioxidant Enzymes via Akt/Nrf2 Pathway
2.4. Effect of Activated EAEP on the Expression of BDNF Mediated via ERK/CREB/TrkB Pathway
2.5. K252a and MK2206 Inhibit the Neuroprotective Effects of EAEP
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Preparation of EAEP Extract
4.3. Cell Viability Assay
4.4. Measurement of Intracellular ROS Level
4.5. Flow Cytometry Analysis
4.6. Protein Determination
4.7. Extraction of Nuclear and Cytosolic Protein
4.8. Western Blot Analysis
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Akt | protein kinase B |
| BDNF | brain-derived neurotrophic factor |
| CREB | cAMP response element-binding protein |
| EAEP | ethyl acetate with Enteromorpha prolifera |
| GCLC | glutamate–cysteine ligase catalytic subunit |
| HO-1 | heme oxygenase-1 |
| NQO-1 | NAD(P)H quinine oxidoreductase-1 |
| Nrf2 | NF-E2-related factor-2 |
| ROS | reactive oxygen species |
| TrkB | Tropomyosin-related kinase receptor B |
References
- Hou, Y.; Dan, X.; Babbar, M.; Wei, Y.; Hasselbalch, S.G.; Croteau, D.L.; Bohr, V.A. Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol. 2019, 15, 565–581. [Google Scholar] [CrossRef] [PubMed]
- Chin-Chan, M.; Navarro-Yepes, J.; Quintanilla-Vega, B. Environmental pollutants as risk factors for neurodegenerative disorders: Alzheimer and Parkinson diseases. Front. Cell. Neurosci. 2015, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simon, H.-U.; Haj-Yehia, A.; Levi-Schaffer, F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis 2000, 5, 415–418. [Google Scholar] [CrossRef]
- Uttara, B.; Singh, A.V.; Zamboni, P.; Mahajan, R.T. Oxidative Stress and Neurodegenerative Diseases: A Review of Upstream and Downstream Antioxidant Therapeutic Options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef] [Green Version]
- Lee, B.D.; Yoo, J.-M.; Baek, S.Y.; Li, F.Y.; Sok, D.-E.; Kim, M.R. 3,3′-Diindolylmethane Promotes BDNF and Antioxidant Enzyme Formation via TrkB/Akt Pathway Activation for Neuroprotection against Oxidative Stress-Induced Apoptosis in Hippocampal Neuronal Cells. Antioxidants 2019, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, J.; Deng, G.; Huang, H. The activation of BDNF reduced inflammation in a spinal cord injury model by TrkB/p38 MAPK signaling. Exp. Ther. Med. 2019, 17, 1688–1696. [Google Scholar] [CrossRef] [Green Version]
- Numakawa, T.; Suzuki, S.; Kumamaru, E.; Adachi, N.; Richards, M.; Kunugi, H. BDNF function and intracellular signaling in neurons. Histol. Histopathol. 2010, 25, 2. [Google Scholar]
- Zhang, F.; Kang, Z.; Li, W.; Xiao, Z.-C.; Zhou, X.-F. Roles of brain-Derived neurotrophic factor/tropomyosin-Related kinase B (BDNF/TrkB) signalling in Alzheimer’s disease. J. Clin. Neurosci. 2012, 19, 946–949. [Google Scholar] [CrossRef]
- Shin, S.K.; Yoo, J.-M.; Li, F.Y.; Baek, S.Y.; Kim, M.-R. Mulberry fruit improves memory in scopolamine-treated mice: Role of cholinergic function, antioxidant system, and TrkB/Akt signaling. Nutr. Neurosci. 2019, 3, 1–11. [Google Scholar] [CrossRef]
- Jeong, E.J.; Hwang, L.; Lee, M.; Lee, K.Y.; Ahn, M.-J.; Sung, S.H. Neuroprotective biflavonoids of Chamaecyparis obtusa leaves against glutamate-Induced oxidative stress in HT22 hippocampal cells. Food Chem. Toxicol. 2014, 64, 397–402. [Google Scholar] [CrossRef]
- Olasehinde, T.A.; Olaniran, A.O.; Okoh, A.I. Neuroprotective effects of some seaweeds against Zn-Induced neuronal damage in HT-22 cells via modulation of redox imbalance, inhibition of apoptosis and acetylcholinesterase activity. Metab. Brain Dis. 2019, 34, 1615–1627. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Rios, J.; Pérez-Gálvez, A.; Roca, M. Comprehensive chlorophyll composition in the main edible seaweeds. Food Chem. 2017, 228, 625–633. [Google Scholar] [CrossRef] [Green Version]
- Alves, C.; Silva, J.; Pinteus, S.; Gaspar, H.; Alpoim, M.C.M.D.C.; Botana, L.M.; Pedrosa, R. From Marine Origin to Therapeutics: The Antitumor Potential of Marine Algae-Derived Compounds. Front. Pharmacol. 2018, 9. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Guzmán, M.; Rodríguez-Nogales, A.; Algieri, F.; Gálvez, J. Potential Role of Seaweed Polyphenols in Cardiovascular-Associated Disorders. Mar. Drugs 2018, 16. [Google Scholar] [CrossRef] [Green Version]
- Eismann, A.I.; Reis, R.P.; Da Silva, A.F.; Cavalcanti, D.N. Ulva spp. carotenoids: Responses to environmental conditions. Algal Res. 2020, 48. [Google Scholar] [CrossRef]
- Lin, W.; Wang, W.; Liao, D.; Chen, D.; Zhu, P.; Cai, G.; Kiyoshi, A. Polysaccharides from Enteromorpha proliferaImprove Glucose Metabolism in Diabetic Rats. J. Diabetes Res. 2015, 2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, J.; Wang, S.; Liu, G.; Pei, D.; Liu, Y.; Liu, Y.; Di, D. Polysaccharides from Enteromorpha prolifera enhance the immunity of normal mice. Int. J. Biol. Macromol. 2014, 64, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Zhuang, X.; Han, M.; Lin, W. Polysaccharides from Enteromorpha prolifera protect against carbon tetrachloride-induced acute liver injury in mice via activation of Nrf2/HO-1 signaling, and suppression of oxidative stress, inflammation and apoptosis. Food Funct. 2020, 11, 4485–4498. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.-J.; Wei, X.; Xu, J.; Chen, B.-J.; Zhao, D.-Y.; Cui, S.; Zhou, T. Carboxymethylated degraded polysaccharides from Enteromorpha prolifera: Preparation and in vitro antioxidant activity. Food Chem. 2017, 215, 76–83. [Google Scholar] [CrossRef]
- Zhong, R.; Wan, X.; Wang, D.; Zhao, C.; Liu, D.; Gao, L.; Wang, M.; Wu, C.; Nabavi, S.F.; Daglia, M.; et al. Polysaccharides from Marine Enteromorpha: Structure and function. Trends Food Sci. Technol. 2020, 99, 11–20. [Google Scholar] [CrossRef]
- Baek, S.Y.; Kim, M.R. Comparison of Quality Characteristic and Antioxidant Activity of Enteromorpha prolifera from Seosan and Muan in Korea. J. Korean Soc. Food Sci. Nutr. 2019, 48, 1070–1078. [Google Scholar] [CrossRef]
- Cho, M.; Lee, H.-S.; Kang, I.J.; Won, M.H.; You, S. Antioxidant properties of extract and fractions from Enteromorpha prolifera, A type of green seaweed. Food Chem. 2011, 127, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.Y.; Kim, D.H.; Kim, S.J.; Kim, M.R. Phytochemicals and Antioxidant Properties of Enteromorpha prolifera Extract in Korea. J. Korean Soc. Food Sci. Nutr. 2020, 49, 462–472. [Google Scholar] [CrossRef]
- Lin, G.; Liu, X.; Yan, X.; Liu, D.; Yang, C.; Liu, B.; Huang, Y.; Zhao, C. Role of Green Macroalgae Enteromorpha Prolifera Polyphenols in the Modulation of Gene Expression and Intestinal Microflora Profiles in Type 2 Diabetic Mice. Int. J. Mol. Sci. 2018, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, X.; Yang, C.; Lin, G.; Chen, Y.; Miao, S.; Liu, B.; Zhao, C. Antidiabetic Potential of Green Seaweed Enteromorpha prolifera Flavonoids Regulating Insulin Signaling Pathway and Gut Microbiota in Type 2 Diabetic Mice. J. Food Sci. 2018, 84, 165–173. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.; Huang, L.; Yu, J.; Xiang, S.; Wang, J.; Zhang, J.; Yan, X.; Cui, W.; He, S.; Wang, Q. Fucoxanthin, A Marine Carotenoid, Reverses Scopolamine-Induced Cognitive Impairments in Mice and Inhibits Acetylcholinesterase in Vitro. Mar. Drugs 2016, 14. [Google Scholar] [CrossRef] [Green Version]
- Das, M.; Prakash, S.; Nayak, C.; Thangavel, N.; Singh, S.K.; Manisankar, P.; Devi, K.P. Dihydroactinidiolide, a natural product against Aβ25-35 induced toxicity in Neuro2a cells: Synthesis, in silico and in vitro studies. Bioorganic Chem. 2018, 81, 340–349. [Google Scholar] [CrossRef]
- Chan, K.-C.; Mong, M.-C.; Yin, M.-C. Antioxidative and Anti-Inflammatory Neuroprotective Effects of Astaxanthin and Canthaxanthin in Nerve Growth Factor Differentiated PC12 Cells. J. Food Sci. 2009, 74, H225–H231. [Google Scholar] [CrossRef]
- Pangestuti, R.; Kim, S.-K. Neuroprotective Effects of Marine Algae. Mar. Drugs 2011, 9, 803–818. [Google Scholar] [CrossRef] [PubMed]
- Fukui, M.; Song, J.-H.; Choi, J.; Choi, H.J.; Zhu, B.T. Mechanism of glutamate-Induced neurotoxicity in HT22 mouse hippocampal cells. Eur. J. Pharmacol. 2009, 617, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.E.; Park, J. Can Co-Activation of Nrf2 and Neurotrophic Signaling Pathway Slow Alzheimer’s Disease? Int. J. Mol. Sci. 2017, 18. [Google Scholar] [CrossRef] [Green Version]
- Yoo, J.-M.; Lee, B.D.; Sok, D.-E.; Ma, J.Y.; Kim, M.R. Neuroprotective action of N-acetyl serotonin in oxidative stress-induced apoptosis through the activation of both TrkB/CREB/BDNF pathway and Akt/Nrf2/Antioxidant enzyme in neuronal cells. Redox Biol. 2017, 11, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Xu, X.; He, Z.; Wang, S.; Zhao, L.; Qiu, J.; Wang, D.; Gong, Z.; Qiu, X.; Huang, H. Antidepressant-Like Effects and Cognitive Enhancement of Coadministration of Chaihu Shugan San and Fluoxetine: Dependent on the BDNF-ERK-CREB Signaling Pathway in the Hippocampus and Frontal Cortex. BioMed. Res. Int. 2020, 2020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoo, J.-M.; Lee, B.D.; Lee, S.J.; Ma, J.Y.; Kim, M.R. Anti-Apoptotic Effect ofN-Palmitoyl Serotonin on Glutamate-Mediated Apoptosis Through Secretion of BDNF and Activation of TrkB/CREB Pathway in HT-22 Cells. Eur. J. Lipid Sci. Technol. 2017, 120. [Google Scholar] [CrossRef]
- Mao, X.-Y.; Cao, Y.-G.; Ji, Z.; Zhou, H.-H.; Liu, Z.-Q.; Sun, H. Topiramate protects against glutamate excitotoxicity via activating BDNF/TrkB-dependent ERK pathway in rodent hippocampal neurons. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2015, 60, 11–17. [Google Scholar] [CrossRef]
- Giacobbo, B.L.; Doorduin, J.; Klein, H.; Dierckx, R.A.J.O.; Bromberg, E.; De Vries, E. Brain-Derived Neurotrophic Factor in Brain Disorders: Focus on Neuroinflammation. Mol. Neurobiol. 2018, 56, 3295–3312. [Google Scholar] [CrossRef] [Green Version]
- Park, C.-H.; Song, J.H.; Kim, S.-N.; Lee, J.H.; Lee, H.-J.; Kang, K.; Lim, H.-H. Neuroprotective Effects of Tetrahydrocurcumin against Glutamate-Induced Oxidative Stress in Hippocampal HT22 Cells. Molecules 2019, 25. [Google Scholar] [CrossRef] [Green Version]
- Hannan, A.; Dash, R.; Haque, N.; Mohibbullah, M.; Sohag, A.; Rahman, A.; Uddin, J.; Alam, M.; Moon, I.S. Neuroprotective Potentials of Marine Algae and Their Bioactive Metabolites: Pharmacological Insights and Therapeutic Advances. Mar. Drugs 2020, 10. [Google Scholar] [CrossRef]
- Suh, H.-W.; Kang, S.; Kwon, K.-S. Curcumin attenuates glutamate-Induced HT22 cell death by suppressing MAP kinase signaling. Mol. Cell. Biochem. 2006, 298, 187–194. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-Dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baek, S.Y.; Kim, M.R. Neuroprotective Effect of Carotenoid-Rich Enteromorpha prolifera Extract via TrkB/Akt Pathway against Oxidative Stress in Hippocampal Neuronal Cells. Mar. Drugs 2020, 18, 372. https://doi.org/10.3390/md18070372
Baek SY, Kim MR. Neuroprotective Effect of Carotenoid-Rich Enteromorpha prolifera Extract via TrkB/Akt Pathway against Oxidative Stress in Hippocampal Neuronal Cells. Marine Drugs. 2020; 18(7):372. https://doi.org/10.3390/md18070372
Chicago/Turabian StyleBaek, Seung Yeon, and Mee Ree Kim. 2020. "Neuroprotective Effect of Carotenoid-Rich Enteromorpha prolifera Extract via TrkB/Akt Pathway against Oxidative Stress in Hippocampal Neuronal Cells" Marine Drugs 18, no. 7: 372. https://doi.org/10.3390/md18070372
APA StyleBaek, S. Y., & Kim, M. R. (2020). Neuroprotective Effect of Carotenoid-Rich Enteromorpha prolifera Extract via TrkB/Akt Pathway against Oxidative Stress in Hippocampal Neuronal Cells. Marine Drugs, 18(7), 372. https://doi.org/10.3390/md18070372