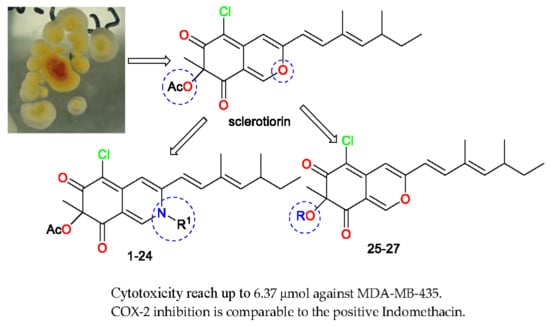
3.1.4. General Procedure for Synthesis of Sclerotioramine Derivatives 1–24
A mixture of sclerotiorin (39 mg, 0.10 mmol) and varied amines (0.12 mmol) in anhydrous dichloromethane (2 mL) was stirred at room temperature until the sclerotiorin was disappeared. The resulting mixture was extracted with EtOAc (3 × 5 mL) and the organic phase was washed with saturated brine (3 × 15 mL). Then the organic phase was dried with MgSO
4 and concentrated in vacuo. The crude product was purified by column chromatography with eluent of EA/PE (5:1,
v/
v) to give the pure compound. All the synthesized compounds were identified by MS and NMR spectra, please see
Figures S1–S79 in the supplementary materials.
Compound 1
(R)-5-chloro-3-((S, 1E, 3E)-3,5-dimethylhepta-1, 3-dien-1-yl)-7-methyl-6, 8-dioxo-2-propyl-2, 6, 7, 8-tetrahydroisoquinolin-7-yl acetate. Red solid, the yield of 90%; m.p. 230.1–232.2 °C. +13.2 (c 0.02, MeOH); UV (MeOH) λmax (log ε): 234 (3.13), 376 (3.03), 488 (1.68) nm; IR (KBr) νmax: 2956, 2922, 1743, 1708, 1590, 1491, 1368, 1251, 1190, 1092, 853 cm−1. 1H NMR (600 MHz, CDCl3) δH 7.75 (s, 1H), 7.02 (s, 1H), 6.96(d, J = 15.4 Hz, 1H), 6.12 (d, J = 15.4 Hz, 1H), 5.70 (d, J = 9.7 Hz, 1H), 3.89–3.72 (m, 2H), 2.59–2.34 (m, 1H), 2.16 (s, 3H), 1.85 (s, 3H), 1.80 (dd, J = 14.9, 7.4 Hz, 2H), 1.55 (s, 3H), 1.48–1.40 (m, 1H), 1.39–1.31 (m, 1H), 1.01 (dd, J = 15.3 7.2 Hz, 6H), 0.88 (t, J = 7.4 Hz, 3H). 13C NMR (150 MHz, CDCl3) δC 193.9, 184.2, 170.1, 148.2, 147.8, 145.0, 144.6, 141.1, 131.6, 114.6, 114.5, 111.7, 102.2, 84.8, 55.8, 35.1, 30.0, 23.6, 23.2, 20.3, 20.2, 12.6, 12.0, 10.9. LRMS (EI) m/z 432 [M]+.
Compound 2
(R)-2-butyl-5-chloro-3-((S, 1E, 3E)-3,5-dimethylhepta-1, 3-dien-1-yl)-7-methyl-6, 8-dioxo-2, 6, 7, 8-tetrahydroisoquinolin-7-yl acetate. Red solid, the yield of 92%; m.p. 216.3–218.5 °C. +13.6 (c 0.02, MeOH); UV (MeOH) λmax (log ε): 238 (3.10), 378 (3.09), 485 (1.78) nm; IR (KBr) νmax: 2953, 2921, 1743, 1708, 1590, 1493, 1368, 1252, 1190, 1092, 851 cm−1. 1H NMR (600 MHz, CDCl3) δH 7.74 (s, 1H), 7.02 (s, 1H), 6.96 (d, J = 15.4 Hz, 1H), 6.13 (d, J = 15.4 Hz, 1H), 5.70 (d, J = 9.7 Hz, 1H), 3.94–3.76 (m, 2H), 2.59–2.39 (m, 1H), 2.16 (s, 3H), 1.84 (s, 3H) 1.74 (s, 2H), 1.54 (s, 3H), 1.48–1.31 (m, 4H), 1.02 (d, J = 6.6 Hz, 3H), 0.98 (t, J = 7.4 Hz, 3H), 0.88 (t, J = 7.4 Hz, 3H). 13C NMR (150 MHz, CDCl3) δC 194.1, 184.3, 170.2, 148.3, 147.9, 145.1, 144.7, 141.2, 131.7, 114.8, 114.6, 111.8, 102.3, 84.9, 54.2, 35.2, 32.3, 30.2, 23.4, 20.4, 20.4, 19.8, 13.7, 12.7, 12.1. LRMS (EI) m/z 446 [M]+.
Compound 3
(R)-5-chloro-2-(2-(cyclohex-1-en-1-yl)ethyl)-3-((S, 1E, 3E)-3,5-dimethylhepta-1, 3-dien-1-yl)-7-methyl-6, 8-dioxo-2, 6, 7, 8-tetrahydroisoquinolin-7-yl acetate. Red solid, the yield of 92%; m.p. 180.6–182.8 °C. +14.2 (c 0.02, MeOH); UV (MeOH) λmax (log ε): 235 (3.11), 382 (3.28), 491 (1.66) nm; IR (KBr) νmax: 2965, 2920, 1743, 1703, 1600, 1491, 1368, 1288, 1248, 1180, 1089, 953 cm−1. 1H NMR (600 MHz, CDCl3) δH 7.64 (s, 1H), 7.01 (s, 1H), 6.96 (d, J = 15.4 Hz, 1H), 6.16 (d, J = 15.4 Hz, 1H), 5.70 (d, J = 9.7 Hz, 1H), 5.41 (s, 1H), 4.05–3.74 (m, 2H), 2.56–2.38 (m, 1H), 2.31 (t, J = 7.1 Hz, 2H), 2.16 (s, 3H), 2.00–1.87 (m, 4H), 1.85 (s, 3H), 1.63 (dd, J = 10.1, 4.1 Hz, 2H), 1.56–1.48 (m, 5H), 1.47–1.40 (m, 1H), 1.35 (dd, J = 14.3, 6.9, 1H), 1.02 (d, J = 6.6 Hz, 3H), 0.88 (t, J = 7.4 Hz, 3H). 13C NMR (150 MHz, CDCl3) δC 194.0, 184.3, 170.1, 148.2, 147.8, 145.1, 144.7, 141.5, 131.9, 131.7, 127.0, 114.7, 114.4, 111.7, 102.1, 84.9, 53.1, 38.6, 35.2, 30.2, 28.4, 25.4, 23.4, 22.7, 22.0, 20.4, 20.4, 12.7, 12.1. HRMS (ESI) for [M + H]+: calcd for C29H37ClNO4: 498.23332. Found: 498.24115.
Compound 4
(7R)-5-chloro-2-(2, 3-dihydroxypropyl)-3-((S, 1E, 3E)-3,5-dimethylhepta-1, 3-dien-1-yl)-7-methyl-6, 8-dioxo-2, 6, 7, 8-tetrahydroisoquinolin-7-yl acetate. Red solid, the yield of 88%; m.p. 152.8–154.3 °C. +14.5 (c 0.02, MeOH); UV (MeOH) λmax (log ε): 236 (3.09), 379 (3.10), 488 (1.64) nm; IR (KBr) νmax: 3537, 2958, 2926, 1733, 1701, 1581, 1489, 1242, 1154, 858 cm−1. 1H NMR (600 MHz, CD3OD) δH 8.15 (s, 1H), 7.20 (s, 1H), 7.11 (d, J = 15.4 Hz, 1H), 6.64 (d, J = 15.4 Hz, 1H), 5.79 (d, J = 9.7 Hz, 1H), 4.46 (dd, J = 14.4, 2.5 Hz, 1H), 3.94 (dd, J = 14.4, 9.2 Hz, 1H), 3.91–3.84 (m, 1H), 3.63 (dd, J = 11.1, 4.7 Hz, 1H), 3.53 (dd, J = 11.1, 6.7 Hz, 1H), 2.56–2.38 (m, 1H), 2.13 (d, J = 20 Hz, 3H), 1.92 (d, J = 0.9 Hz, 3H), 1.51 (d, J = 5.2 Hz, 3H), 1.50–1.46 (m, 1H), 1.41–1.34 (m, 1H), 1.05 (dd, J = 6.7, 2.1 Hz, 3H), 0.91 (t, J = 7.4 Hz, 3H). 13C NMR (150 MHz, CD3OD) δC 195.1, 185.5, 171.6, 151.8, 148.7, 148.3, 146.5, 144.9, 134.0, 117.4, 116.1, 112.3, 101.2, 86.2, 71.7, 64.5, 58.3, 36.2, 31.2, 23.8, 20.6, 20.2, 12.8, 12.4. HRMS (ESI) for [M − H]−: calcd for C24H29ClNO6: 462.17617. Found: 462.16899.
Compound 5
(R)-5-chloro-3-((S, 1E, 3E)-3,5-dimethylhepta-1, 3-dien-1-yl)-2-(furan-2-ylmethyl)-7-methyl-6, 8-dioxo-2, 6, 7, 8-tetrahydroisoquinolin-7-yl acetate. Red solid, the yield of 96%; m.p. 160.0–161.5 °C. +16.6 (c 0.02, MeOH); UV (MeOH) λmax (log ε): 240 (3.16), 380 (3.06), 496 (1.66) nm; IR (KBr) νmax: 2960, 2928, 1735, 1703, 1595, 1497, 1371, 1256, 1144, 1104, 1083, 860 cm−1. 1H NMR (600 MHz, CDCl3) δH 7.84 (s, 1H), 7.45–7.41 (m, 1H), 6.97 (s, 1H), 6.90 (d, J = 15.3Hz, 1H), 6.42–6.37 (m, 2H), 6.33 (d, J = 15.4 Hz, 1H), 5.67 (d, J = 9.8 Hz, 1H), 4.97 (d, J = 4.8 Hz, 2H), 2.52–2.40 (m, 1H), 2.14 (s, 3H), 1.84 (d, J = 0.9 Hz, 3H), 1.53 (s, 3H), 1.46–1.40 (m, 1H), 1.36–1.28 (m, 1H), 1.01 (d, J = 6.7 Hz, 3H), 0.87 (t, J = 7.4 Hz, 3H). 13C NMR (150 MHz, CDCl3) δC 193.8, 184.5, 170.2, 148.3, 148.1, 147.0, 145.1, 144.5, 144.2, 141.1, 131.9, 115.1, 115.0, 111.6, 111.1, 110.6, 102.7, 85.0, 50.3, 35.1, 30.1, 23.3, 20.4, 20.3, 12.7, 12.1. HRMS (ESI) for [M + H]+: calcd for C26H29ClNO5: 470.16560. Found: 470.17326.
Compound 6
(R)-2-(benzyloxy)-5-chloro-3-((S, 1E, 3E)-3,5-dimethylhepta-1, 3-dien-1-yl)-7-methyl-6, 8-dioxo-2, 6, 7, 8-tetrahydroisoquinolin-7-yl acetate. Red solid, the yield of 96%; m.p. 166.6–168.3 °C. +14.3 (c 0.02, MeOH); UV (MeOH) λmax (log ε): 248 (3.09), 386 (3.06), 491 (1.67) nm; IR (KBr) νmax: 3080, 2964, 2929, 1723, 1703, 1611, 1487, 1375, 1252, 1221, 1148, 1080, 952, 781 cm−1. 1H NMR (600 MHz, CDCl3) δH 7.82 (s, 1H), 7.44 (d, J = 7.1 Hz, 3H), 7.36 (dd, J = 7.6, 1.7 Hz, 2H), 7.08 (d, J = 15.9 Hz, 1H), 7.00 (s, 1H), 6.29 (d, J = 15.9 Hz, 1H), 5.74 (d, J = 9.7 Hz, 1H), 5.06 (dd, J = 25.6, 10.1 Hz, 2H), 258–2.43 (m, 1H), 2.17 (s, 3H), 1.83 (d, J = 0.7 Hz, 3H), 1.53 (s, 3H), 1.48–1.40 (m, 1H), 1.38–1.34 (m, 1H), 1.03 (d, J = 6.6 Hz, 3H), 0.90 (d, J = 7.4 Hz, 3H). 13C NMR (150 MHz, CDCl3) δC 193.15, 184.41, 170.22, 149.11, 145.86, 144.88, 143.54, 137.20, 132.12, 131.62, 130.58, 130.27, 129.37, 128.98, 113.77, 112.08, 109.43, 103.14, 84.74, 81.96, 65.71, 35.28, 30.17, 29.83, 23.29, 20.35, 12.63, 12.13. HRMS (ESI) for [M + H]+: calcd for C28H31ClNO5: 496.18125. Found: 496.18889.
Compound 7
(R)-5-chloro-3-((S, 1E, 3E)-3,5-dimethylhepta-1, 3-dien-1-yl)-7-methyl-6, 8-dioxo-2-(2-(pyrrolidin-1-yl)ethyl)-2, 6, 7, 8-tetrahydroisoquinolin-7-yl acetate. Red solid, the yield of 96%; m.p. 145.5–147.6 °C. +16.1 (c 0.02, MeOH); UV (MeOH) λmax (log ε): 246 (3.09), 379 (3.00), 488 (1.64) nm; IR (KBr) νmax: 2958, 2922, 1748, 1708, 1591, 1491, 1365, 1252, 1190, 1092, 860 cm−1. 1H NMR (600 MHz, CDCl3) δH 7.78 (s, 1H), 7.00 (s, 1H), 6.95 (d, J = 15.3 Hz, 1H), 6.23 (d, J = 15.3 Hz, 1H), 5.70 (d, J = 9.8 Hz, 1H), 3.98 (s, 2H), 2.84 (s, 2H), 2.61 (s, 4H), 2.53–2.43 (m, 1H), 2.17 (s, 3H), 1.86 (s, 3H), 1.81 (s, 4H), 1.54 (s, 3H), 1.49–1.40 (m, 1H), 1.38–1.34 (m, 1H), 1.03 (d, J = 6.6 Hz, 3H), 0.90 (d, J = 7.4 Hz, 3H). 13C NMR (150 MHz, CDCl3) δC 194.0, 184.4, 170.2, 149.2, 147.6, 145.0, 144.5, 136.5, 131.6, 115.4, 115.3, 112.7, 102.3, 84.9, 58.8, 35.1, 30.1, 30.0, 23.3, 20.9, 20.7, 20.4, 20.3, 12.7, 12.1, 10.8, 10.8. HRMS (ESI) for [M + H]+: calcd for C27H36ClN2O4: 487.22854. Found: 487.23595.
Compound 8
(7R)-2-(sec-butyl)-5-chloro-3-((S, 1E, 3E)-3,5-dimethylhepta-1, 3-dien-1-yl)-7-methyl-6, 8-dioxo-2, 6, 7, 8-tetrahydroisoquinolin-7-yl acetate. Red solid, the yield of 93%; m.p. 164.4–466.8 °C. +16.8 (c 0.02, MeOH); UV (MeOH) λmax (log ε): 256 (3.05), 386 (2.98), 498 (1.53) nm; IR (KBr) νmax: 2964, 2920, 1736, 1701, 1599, 1497, 1370, 1252, 1200, 1141, 1084, 861 cm−1. 1H NMR (600 MHz, CDCl3) δH 7.83 (s, 1H), 6.95 (s, 1H), 6.87 (d J = 15.3 Hz, 1H), 6.13 (d, J = 15.3 Hz, 1H), 5.67 (d, J = 9.7 Hz, 1H), 4.23 (dd, J = 13.8, 6.9 Hz, 1H), 2.53–2.41 (m, 1H), 2.17 (s, 3H), 1.85 (s,3H), 1.80 (dd, J = 14.8, 7.4 Hz, 2H), 1.56 (s, 3H), 1.47–1.42 (m, 4H), 1.38–1.33 (m, 1H), 1.02 (d, J = 6.6 Hz, 3H), 0.93 (t, J = 7.3 Hz, 3H), 0.88 (t, J = 7.4 Hz, 3H). 13C NMR (150 MHz, CDCl3) δC 194.0, 184.5, 170.2, 148.4, 147.9, 145.1, 144.6, 142.2, 131.7, 114.6, 114.4, 111.7, 102.3, 84.9, 54.0, 53.4, 51.5, 35.2, 30.1, 29.8, 23.4, 20.4, 20.3, 12.8, 12.1. HRMS (ESI) for [M + H]+: calcd for C25H33ClNO4: 446.20199. Found: 446.20947.
Compound 9
(R)-5-chloro-3-((S, 1E, 3E)-3, 5-dimethylhepta-1, 3-dien-1-yl)-2-(2-hydroxyethyl)-7-methyl-6, 8-dioxo-2, 6, 7, 8-tetrahydroisoquinolin-7-yl acetate. Red solid, the yield of 88%; m.p. 180.2–182.8 °C. +15.8 (c 0.02, MeOH); UV (MeOH) λmax (log ε): 233 (3.06), 389 (3.18), 498 (1.64) nm; IR (KBr) νmax: 3535, 2950, 2926, 1733, 1706, 1581, 1484, 1242, 1155, 866 cm−1. 1H NMR (600 MHz, CDCl3) δH 7.93 (s, 1H), 7.05 (s, 1H), 6.94 (d, J = 15.3 Hz, 1H), 6.28 (d, J = 15.4 Hz, 1H), 5.70 (d, J = 9.7 Hz, 1H), 4.04 (d, J = 5.4 Hz, 2H), 3.91 (d, J = 4.6 Hz, 2H), 2.46 (d, J = 8.6 Hz, 1H), 2.14 (s, 3H), 1.84 (s, 3H), 1.53 (s, 3H), 1.46–1.42 (m, 1H), 1.36–1.30 (m, 1H), 1.01 (d, J = 6.6 Hz, 3H), 0.87 (t, J = 7.4 Hz, 3H). LRMS (EI) m/z 434 [M]+.
Compound 10
(R)-5-chloro-3-((S, 1E, 3E)-3, 5-dimethylhepta-1, 3-dien-1-yl)-2-heptyl-7-methyl-6, 8-dioxo-2, 6, 7, 8-tetrahydroisoquinolin-7-yl acetate. Red solid, the yield of 93%; m.p. 95.6–97.3 °C. +13.9 (c 0.02, MeOH); UV (MeOH) λmax (log ε): 246 (3.05), 389 (3.20), 488 (1.64) nm; IR (KBr) νmax: 2966, 2928, 1748, 1705, 1591, 1493, 1368, 1252, 1190, 1093, 862 cm−1. 1H NMR (600 MHz, CDCl3) δH 7.74 (s, 1H), 7.01 (s, 1H), 6.95 (d, J = 15.3 Hz, 1H), 6.12 (d, J = 15.4 Hz, 1H), 5.70 (d, J = 9.8 Hz, 1H), 3.82 (td, J = 7.2, 2.9 Hz, 2H), 2.52–2.37 (m, 1H), 2.16 (s, 3H), 1.84 (s, 3H), 1.77–1.72 (m, 2H), 1.54 (s, 3H), 1.46–1.42 (m, 1H), 1.37–1.25 (m, 9H), 1.02 (d, J = 6.7 Hz, 3H), 088 (t, J = 7.3 Hz, 6H). 13C NMR (150 MHz, CDCl3) δC 194.1, 184.4, 170.2, 148.2, 147.9, 145.0, 144.7, 141.1, 131.7, 114.8, 114.7, 111.8, 102.2, 84.9, 54.5, 35.2, 31.6, 30.3, 30.1, 28.8, 26.4, 23.4, 22.6, 20.4, 20.3, 14.1, 12.7, 12.1. HRMS (ESI) for [M + H]+: calcd for C28H39ClNO4: 488.24894. Found: 488.25679.
Compound 11
(R)-5-chloro-3-((S, 1E, 3E)-3, 5-dimethylhepta-1, 3-dien-1-yl)-2-(2-(furan-2-yl)ethyl)-7-methyl-6, 8-dioxo-2, 6, 7, 8-tetrahydroisoquinolin-7-yl acetate. Red solid, the yield of 90%; m.p. 130.3–132.8 °C. +15.9 (c 0.02, MeOH); UV (MeOH) λmax (log ε): 256 (3.03), 379 (3.16), 493 (1.54) nm; IR (KBr) νmax: 2966, 2923, 1738, 1703, 1595, 1497, 1372, 1256, 1148, 1104, 1083, 869 cm−1. 1H NMR (600 MHz, CDCl3) δH 7.59 (s, 1H), 7.33 (d, J = 1.6 Hz, 1H), 6.96 (d, J = 15.3 Hz, 1H), 6.91 (d, J = 15.3 Hz, 1H), 6.28 (dd, J = 2.9, 2.0 Hz, 1H), 6.09 (d, J = 3.1 Hz, 1H), 5.98 (d, J = 15.3 Hz, 1H), 5.68 (d, J = 9.7 Hz, 1H), 4.17–4.05 (m, 2H), 3.22–2.98 (m, 2H), 2.47 (dt, J = 8.0, 7.3 Hz, 1H), 2.16 (s, 3H), 1.83 (s, 3H), 1.53 (s, 3H), 1.46–1.42 (m, 1H), 1.37–1.31 (m, 1H), 1.02 (d, J = 6.6 Hz, 3H), 0.88 (t, J = 7.4 Hz, 3H). 13C NMR (150 MHz, CDCl3) δC 193.8, 184.5, 170.2, 149.4, 148.1, 148.0, 145.2, 144.5, 142.7, 141.0, 131.8, 114.9, 114.5, 111.5, 110.9, 108.6, 102.5, 84.9, 52.8, 35.2, 30.2, 29.0, 23.3, 20.4, 20.4, 12.7, 12.1. HRMS (ESI) for [M + H]+: calcd for C27H31ClNO5: 484.18125. Found: 484.18897.
Compound 12
(R)-5-chloro-2-cyclopropyl-3-((S, 1E, 3E)-3, 5-dimethylhepta-1, 3-dien-1-yl)-7-methyl-6, 8-dioxo-2, 6, 7, 8-tetrahydroisoquinolin-7-yl acetate. Red solid, the yield of 91%; m.p. 172.3–174.4 °C. +15.6 (c 0.02, MeOH); UV (MeOH) λmax (log ε): 251 (3.01), 388 (3.31), 489 (1.66) nm; IR (KBr) νmax: 2968, 2921, 1743, 1708, 1610, 1491, 1369, 1288, 1245, 1180, 1089, 955 cm−1. 1H NMR (600 MHz, CDCl3) δH 7.90 (s, 1H), 7.07 (s, 1H), 6.98 (d, J = 15.6 Hz, 1H), 6.55 (d, J = 15.6 Hz, 1H), 5.72 (d, J = 9.8 Hz, 1H), 3.27 (dd, J = 6.8, 3.3 Hz, 1H), 2.68–2.39 (m, 1H), 2.16 (s, 3H), 1.86 (s, 3H), 1.53 (s, 3H), 1.48–1.40 (m, 1H), 1.37–1.31 (m, 1H), 1.22 (d, J = 6.8 Hz, 2H), 1.05 (dd, J = 15.1, 2.5 Hz, 2H), 1.02 (d, J = 6.7 Hz, 3H), 0.88 (t, J = 7.4 Hz, 3H). LRMS (EI) m/z 431 [M]+.
Compound 13
(R)-5-chloro-3-((S, 1E, 3E)-3, 5-dimethylhepta-1, 3-dien-1-yl)-7-methyl-6, 8-dioxo-2-(thiophen-2-ylmethyl)-2, 6, 7, 8-tetrahydroisoquinolin-7-yl acetate. Red solid, the yield of 89%; m.p. 106.5–108.4 °C. +13.3 (c 0.02, MeOH); UV (MeOH) λmax (log ε): 244 (3.00), 381 (3.10), 497 (1.68) nm; IR (KBr) νmax: 2960, 2924, 1735, 1704, 1591, 1499, 1371, 1256, 1140, 1080, 1008, 860 cm−1. 1H NMR (600 MHz, CDCl3) δH 7.86 (s, 1H), 7.35 (dd, J = 3.9, 2.4 Hz, 1H), 7.10–6.98 (m, 3H), 6.92 (d, J = 15.3 Hz, 1H), 6.22 (dd, J = 15.3 Hz, 1H), 5.68 (d, J = 9.8 Hz, 1H), 5.17 (q, J = 16.2 Hz, 2H), 2.54–2.41 (m, 1H), 2.15 (s, 3H), 1.80 (d, J = 10 Hz, 3H), 1.54 (s, 3H), 1.46–1.40 (m, 1H), 1.37–1.29 (m, 1H), 1.01 (d, J = 6.7 Hz, 3H), 0.87 (t, J = 7.4 Hz, 3H). 13C NMR (150 MHz, CDCl3) δC 193.8, 184.6, 170.3, 148.3, 148.0, 145.3, 144.4, 140.9, 136.3, 131.9, 127.8, 127.5, 127.2, 115.1, 114.9, 111.7, 103.0, 85.0, 52.6, 35.2, 30.1, 23.3, 20.4, 20.3, 12.7, 12.1. HRMS (ESI) for [M + H]+: calcd for C26H29ClNO5S: 486.14276. Found: 486.15086.
Compound 14
(R)-5-chloro-3-((S, 1E, 3E)-3,5-dimethylhepta-1, 3-dien-1-yl)-7-methyl-6, 8-dioxo-2-(2-(thiophen-2-yl)ethyl)-2, 6, 7, 8-tetrahydroisoquinolin-7-yl acetate. Red solid, the yield of 91%; m.p. 100.2–102.0 °C. +16.2 (c 0.02, MeOH); UV (MeOH) λmax (log ε): 251 (3.09), 389 (3.00), 501 (1.55) nm; IR (KBr) νmax: 2966, 2920, 1735, 1708, 1591, 1489, 1371, 1258, 1140, 1088, 1008, 849 cm−1. 1H NMR (600 MHz, CDCl3) δH 7.59 (s, 1H), 7.19 (dd, J = 5.1, 1.1 Hz, 1H), 6.97 (s, 1H), 6.95–6.88 (m, 2H), 6.78 (d, J = 3.3 Hz, 1H), 5.98 (d, J = 15.3 Hz, 1H), 5.68 (d, J = 9.7 Hz, 1H), 4.08 (ddt, J = 78.5, 14.6, 7.2 Hz, 2H), 3.34–3.13 (m, 2H), 2.52–2.39 (m, 1H), 2.16 (s, 3H), 1.82 (d, J = 1.0 Hz, 3H), 1.52 (s, 3H), 1.49–1.39 (m, 1H), 1.38–1.31 (m,1H), 1.02 (d, J = 6.7 Hz, 3H), 0.88 (t, J = 7.4 Hz, 3H). 13C NMR (150 MHz, CDCl3) δC 193.8, 184.4, 170.2, 148.3, 147.9, 145.2, 144.4, 140.9, 137.4, 131.8, 127.8, 126.9, 125.4, 114.8, 114.5, 111.6, 102.6, 85.0, 55.5, 35.2, 30.6, 30.2, 23.4, 20.4, 20.4, 12.7, 12.1. HRMS (ESI) for [M + H]+: calcd for C27H31ClNO5S: 500.15841. Found: 500.16563.
Compound 15
(R)-5-chloro-3-((S, 1E, 3E)-3, 5-dimethylhepta-1, 3-dien-1-yl)-7-methyl-6, 8-dioxo-2-phenyl-2, 6, 7, 8-tetrahydroisoquinolin-7-yl acetate. Red solid, the yield of 93%; m.p. 190.2–192.1 °C. +16.5 (c 0.02, MeOH); UV (MeOH) λmax (log ε): 241 (3.19), 379 (3.12), 506 (1.53) nm; IR (KBr) νmax: 3088, 2964, 2916, 1731, 1707, 1611, 1591, 1495, 1363, 1275, 1204, 1130, 1084, 1020, 856, 773 cm−1. 1H NMR (600 MHz, CDCl3) δH 7.83 (s, 3H), 7.59–7.47 (m, 3H), 7.32–7.27 (m, 2H), 7.15 (s, 1H), 6.96 (d, J = 15.6 Hz), 5.65 (d, J = 9.7 Hz, 1H), 5.58 (d, J = 15.6 Hz, 1H), 2.46–2.30 (m, 1H), 2.17 (s, 3H), 1.58 (s, 3H), 1.49 (d, J = 0.9 Hz, 3H), 1.45–1.36 (m, 1H), 1.33–1.28 (m, 1H), 0.97 (d, J = 6.6 Hz, 3H), 0.84 (t, 7.4 Hz, 3H). 13C NMR (150 MHz, CDCl3) δC 193.9, 184.8, 170.3, 148.0, 147.6, 144.2, 143.3, 141.3, 140.5, 131.9, 130.3, 130.3, 130.2, 126.7, 126.7, 116.3, 114.5, 109.9, 103.4, 84.9, 35.1, 30.1, 23.3, 20.4, 20.3, 12.3, 12.1. LRMS (EI) m/z 467 [M]+.
Compound 16
(R)-5-chloro-3-((S, 1E, 3E)-3,5-dimethylhepta-1, 3-dien-1-yl)-7-methyl-6, 8-dioxo-2-phenethyl-2, 6, 7, 8-tetrahydroisoquinolin-7-yl acetate. Red solid, the yield of 91%; m.p. 180.2–181.0 °C. +15.3 (c 0.02, MeOH); UV (MeOH) λmax (log ε): 243 (3.06), 386 (3.14), 498 (1.55) nm; IR (KBr) νmax: 3080, 2962, 2916, 1731, 1715, 1611, 1588, 1495, 1366, 1275, 1212, 1130, 1084, 1022, 856, 780 cm−1. 1H NMR (600 MHz, CDCl3) δH 7.51 (s, 1H), 7.30 (dd, J = 10.2, 4.6 Hz, 2H), 7.26–7.22 (m, 1H), 7.15- 7.01 (m, 2H), 6.94 (s, 1H), 6.88 (d, J = 15.2 Hz, 1H), 5.99 (d, J = 15.4 Hz, 1H), 5.67 (d, J = 9.7 Hz, 1H), 4.32–3.89 (m, 2H), 3.28–2.84 (m, 2H), 2.53–2.40 (m, 1H), 2.14 (s, 3H), 1.80 (d, J = 1.0 Hz, 3H), 1.50 (s, 3H), 1.48–1.40 (m, 1H), 1.38–1.31 (m, 1H), 1.02 (d, J = 6.7 Hz, 3H), 0.88 (t, J = 7.4 Hz, 3H). 13C NMR (150 MHz, CDCl3) δC 193.8, 184.4, 170.1, 148.1, 148.0, 145.1, 144.5, 141.1, 135.9, 131.8, 129.3, 129.3, 128.9, 128.9, 127.8, 114.7, 114.7, 111.5, 102.3, 85.0, 55.6, 36.7, 35.1, 30.1, 23.4, 20.4, 20.3, 12.7, 12.1. LRMS (EI) m/z 495 [M]+.
Compound 17
(R)-2-benzyl-5-chloro-3-((S, 1E, 3E)-3,5-dimethylhepta-1, 3-dien-1-yl)-7-methyl-6, 8-dioxo-2, 6, 7, 8-tetrahydroisoquinolin-7-yl acetate. Red solid, the yield of 92%; m.p. 188.8–190.2 °C. +13.9 (c 0.02, MeOH); UV (MeOH) λmax (log ε): 259 (3.06), 399 (3.10), 499 (1.65) nm; IR (KBr) νmax: 3082, 2964, 2918, 1731, 1710, 1611, 1591, 1495, 1363, 1276, 1204, 1130, 1084, 1020, 858, 773 cm−1. 1H NMR (600 MHz, CDCl3) δH 7.86 (s, 1H), 7.45–7.33 (m, 3H), 7.18 (d, J = 7.2 Hz, 2H), 7.04 (s, 1H), 6.89 (d, J = 15.4 Hz, 1H), 6.02 (d, J = 15.4 Hz, 1H), 5.64 (d, J = 9.8 Hz, 1H), 5.19–4.88 (m, 2H), 2.54–2.30 (m, 1H), 2.16 (s, 3H), 1.66 (d, J = 1.0 Hz, 3H), 1.57 (s, 3H), 1.46–1.40 (m, 1H), 1.34–1.28 (s, 1H), 0.98 (d, J = 6.7 Hz, 3H), 0.84 (t, J = 6.7 Hz, 3H). 13C NMR (150 MHz, CDCl3) δC 194.0, 184.6, 170.3, 148.4, 148.2, 145.0, 144.5, 141.7, 134.2, 131.8, 129.7, 129.7, 129.1, 126.6, 126.6, 115.2, 114.9, 111.7, 102.9, 84.9, 57.9, 35.1, 30.1, 23.4, 20.4, 20.3, 12.6, 12.1. HRMS (ESI) for [M + H]+: calcd for C28H31ClNO4: 480.1863. Found: 480.1935.
Compound 18
(R)-5-chloro-3-((S, 1E, 3E)-3, 5-dimethylhepta-1, 3-dien-1-yl)-7-methyl-2-(3-morpholinopropyl) -6, 8-dioxo-2, 6, 7, 8-tetrahydroisoquinolin-7-yl acetate. Red solid, the yield of 96%; m.p. 110.2–112.6 °C. +16.6 (c 0.02, MeOH); UV (MeOH) λmax (log ε): 245 (3.19), 376 (3.00), 505 (1.63) nm; IR (KBr) νmax: 2960, 2920, 1727, 1706, 1595, 1499, 1371, 1248, 1208, 1148, 1080, 864 cm−1. 1H NMR (600 MHz, CDCl3) δH 7.93 (s, 1H), 7.01 (s, 1H), 6.96 (d, J = 15.4 Hz, 1H), 6.18 (d, J = 15.4 Hz, 1H), 5.71 (d, J = 9.7 Hz, 1H), 4.01 (s, 2H), 3.75 (s, 4H), 2.68–2.24 (m, 7H), 2.16 (s, 3H), 1.98 (dd, J = 28.3, 21.6 Hz, 2H), 1.86 (s, 3H), 1.54 (s, 3H), 1.46–1.40 (m, 1H), 1.37–1.32 (m, 1H), 1.02 (d, J = 6.6 Hz, 3H), 0.88 (t, J = 7.4 Hz, 3H). 13C NMR (150 MHz, CDCl3) δC 194.1, 184.5, 170.2, 148.4, 147.9, 145.1, 144.6, 142.2, 131.7, 114.6, 114.4, 111.7, 102.3, 84.9, 54.0, 53.4, 51.5, 35.2, 30.1, 29.8, 23.4, 20.4, 20.3, 12.8, 12.1. 10.9, 10.8. HRMS (ESI) for [M + H]+: calcd for C28H38ClN2O5: 517.22345. Found:517.24647.
Compound 19
(R)-5-chloro-2-(2-(dimethylamino)ethyl)-3-((S, 1E, 3E)-3,5-dimethylhepta-1, 3-dien-1-yl)-7-methyl-6, 8-dioxo-2, 6, 7, 8-tetrahydroisoquinolin-7-yl acetate. Red solid, the yield of 92%; m.p. 185.6–187.2 °C. +14.8 (c 0.02, MeOH); UV (MeOH) λmax (log ε): 236 (3.01), 371 (3.23), 487 (1.45) nm; IR (KBr) νmax: 2960, 2923, 1731, 1706, 1595, 1499, 1371, 1251, 1209, 1140, 1080, 866 cm−1. 1H NMR (600 MHz, CDCl3) δH 7.77 (s, 1H), 7.00 (s, 1H), 6.94 (d, J = 15.3 Hz, 1H), 6.21 (d, J = 15.4 Hz, 1H), 5.70 (d, J = 9.70 Hz, 1H), 3.93 (s, 2H), 2.64 (s, 2H), 2.53–2.41 (m, 1H), 2.31 (s, 6H), 2.16 (s, 3H), 1.85 (d, J = 0.8 Hz, 3H), 1.54 (s, 3H), 1.46–1.40 (m, 1H), 1.39–1.31 (m, 1H), 1.02 (d, J = 6.6 Hz, 3H), 0.88 (t, J = 7.4 Hz, 3H). 13C NMR (150 MHz, CDCl3) δC 193.9, 184.4, 170.1, 148.1, 148.0, 145.1, 144.5, 141.5, 131.7, 114.8, 114.6, 111.6, 102.3, 84.9, 58.5, 51.8, 45.6, 35.1, 30.0, 29.3, 23.3, 20.3, 20.2, 12.6, 12.0. HRMS (ESI) for [M + H]+: calcd for C25H34ClN2O4: 461.26289. Found: 461.22027.
Compound 20
(R)-2-(2-(benzylamino)ethyl)-5-chloro-3-((S, 1E, 3E)-3, 5-dimethylhepta-1, 3-dien-1-yl)-7-methyl-6, 8-dioxo-2, 6, 7, 8-tetrahydroisoquinolin-7-yl acetate. Red solid, the yield of 91%; m.p. 160.1–161.8. +14.3 (c 0.02, MeOH); UV (MeOH) λmax (log ε): 251 (3.03), 381 (3.26), 497 (1.55) nm; IR (KBr) νmax: 3412, 2963, 2924, 1737, 1703, 1589, 1495, 1369, 1249, 1145, 1082, 1007, 965, 861 cm−1. 1H NMR (600 MHz, CDCl3) δH 7.81 (s, 1H), 7.32–7.27 (m, 2H), 7.24 (td, J = 7.6, 2.1 Hz, 3H), 6.94 (s, 1H), 6.83 (d, J = 15.3 Hz, 1H), 6.16 (d, J = 15.4 Hz, 1H), 5.66 (d, J = 9.7 Hz, 1H), 4.09–3.80 (m, 2H), 3.79 (s, 2H), 3.12–2.77 (m, 2H), 2.53–2.37 (m, 1H), 2.16 (s, 3H), 1.80 (d, J = 1.0 Hz, 3H), 1.55 (s, 3H), 1.49–1.41 (m, 1H), 1.37–1.32 (m, 1H), 1.01 (d, J = 6.7 Hz, 3H), 0.88 (t, J = 7.4 Hz, 3H). 13C NMR (150 MHz, CDCl3) δC 194.1, 184.5, 170.2, 148.1, 148.0, 145.1, 144.8, 142.1, 131.8, 128.8, 128.8, 128.8, 128.3, 128.3, 127.6, 114.9, 114.4, 111.8, 102.2, 85.0, 54.1, 53.6, 47.5, 35.13, 30.2, 23.4, 20.5, 20.4, 12.7, 12.1. HRMS (ESI) for [M + H]+: calcd for C30H36ClN2O5: 523.22854. Found: 523.23594.
Compound 21
(R)-5-chloro-2-cyclopentyl-3-((S, 1E, 3E)-3, 5-dimethylhepta-1, 3-dien-1-yl)-7-methyl-6, 8-dioxo-2, 6, 7, 8-tetrahydroisoquinolin-7-yl acetate. Red solid, the yield of 95%; m.p. 132.3–133.5 °C. +15.3 (c 0.02, MeOH); UV (MeOH) λmax (log ε): 233 (3.09), 369 (3.13), 499 (1.65) nm; IR (KBr) νmax: 2971, 2925, 1743, 1700, 1600, 1491, 1368, 1288, 1246, 1180, 1089, 955 cm−1. 1H NMR (600 MHz, CDCl3) δH 7.88 (s, 1H), 6.95 (s, 1H), 6.87 (d, J = 15.3 Hz, 1H), 6.19 (d, J = 15.3 Hz, 1H), 5.67 (d, J = 9.7 Hz, 1H), 4.53 (p, J = 7.4 Hz, 1H), 2.55–2.38 (m, 1H), 2.29–2.12 (m, 5H), 1.91 (ddd, J = 16.4, 8.5, 5.0 Hz, 2H), 1.85 (d, J = 1.0 Hz, 3H), 1.84–1.70 (m, 4H), 1.54 (s, 3H), 1.46–1.39 (m,1H), 1.38–1.30 (m, 1H), 1.01 (d, J = 6.7 Hz, 3H), 0.88 (t, J = 7.4 Hz, 3H). 13C NMR (150 MHz, CDCl3) δC 194.1, 184.4, 170.2, 149.1, 147.7, 144.9, 144.5, 136.9, 131.7, 115.6, 115.3, 112.7, 102.2, 84.9, 62.7, 35.1, 33.3, 33.2, 30.1, 24.3, 24.3, 23.4, 20.4, 20.4, 12.8, 12.1. HRMS (ESI) for [M + H]+: calcd for C26H33ClNO4: 458.20199. Found: 458.20981.
Compound 22
(R)-5-chloro-2-cyclohexyl-3-((S, 1E, 3E)-3, 5-dimethylhepta-1, 3-dien-1-yl)-7-methyl-6, 8-dioxo-2, 6, 7, 8-tetrahydroisoquinolin-7-yl acetate. Red solid, the yield of 95%; m.p. 136.2–138.1 °C. +16.5 (c 0.02, MeOH); UV (MeOH) λmax (log ε): 248 (3.08), 379 (3.16), 503 (1.51) nm; IR (KBr) νmax: 2955, 2918, 1743, 1705, 1600, 1491, 1368, 1286, 1248, 1180, 1089, 923 cm−1. 1H NMR (600 MHz, CDCl3) δH 7.90 (s, 1H), 6.96 (s, 1H), 6.88 (d, J = 15.2 Hz, 1H), 6.14 (d, J = 15.3 Hz, 1H), 5.68 (d, J = 9.7 Hz, 1H), 3.97 (tt, J = 12.0, 3.2 Hz, 1H), 2.65–2.34 (m, 1H), 2.16 (s, 3H), 1.98 (dd, J = 23.3, 12.1 Hz, 5H), 1.85 (d, J = 1.0 Hz, 3H), 1.77 (d, J = 13.3 Hz, 1H), 1.64 (tt, 12.4, 6.3 Hz, 2H), 1.47–1.31 (m, 4H), 1.29–1.16 (m, 3H), 1.02 (d, J = 6.7 Hz, 3H), 0.88 (t, J = 7.4 Hz, 3H). 13C NMR (150 MHz, CDCl3) δC 194.1, 184.4, 170.2, 148.6, 147.7, 145.1, 144.6, 136.9, 131.6, 115.1, 115.0, 112.9, 102.1, 84.9, 61.2, 35.1, 33.3, 33.1, 30.1, 25.8, 25.8, 25.0, 23.4, 20.4, 20.4, 12.7, 12.1. LRMS (EI) m/z 472 [M]+.
Compound 23
(R)-2-(4-bromophenyl)-5-chloro-3-((S, 1E, 3E)-3, 5-dimethylhepta-1, 3-dien-1-yl)-7-methyl-6, 8-dioxo-2, 6, 7, 8-tetrahydroisoquinolin-7-yl acetate. Red solid, the yield of 91%; m.p. 190.0–192.3 °C. +16.8 (c 0.02, MeOH); UV (MeOH) λmax (log ε): 236 (3.03), 377 (3.25), 493 (1.48) nm; IR (KBr) νmax: 3101, 2959, 2919, 1735, 1707, 1601, 1508, 1487, 1365, 1268, 1250, 1207, 1129, 1080, 960 cm−1. 1H NMR (600 MHz, CDCl3) δH 7.76 (s, 1H), 7.73–7.63 (m, 2H), 7.24–7.15 (m, 2H), 7.12 (s, 1H), 6.96 (d, J = 15.5 Hz, 1H), 5.67 (d, J = 9.7 Hz, 1H), 5.55 (d, J = 15.5 Hz, 1H), 2.53–2.32 (m, 1H), 2.17 (s, 3H), 1.63–1.49 (m, 6H), 1.46–1.40 (m, 1H), 1.39–1.31 (m, 1H), 0.99 (d, J = 6.6 Hz, 3H), 0.85 (t, J = 7.4 Hz, 3H). 13C NMR (150 MHz, CDCl3) δC 193.7, 184.9, 170.3, 148.3, 147.2, 143.8, 143.6, 140.9, 139.5, 133.6, 133.6, 131.9, 128.3, 128.3, 124.3, 116.0, 114.7, 110.1, 104.0, 84.8, 35.1, 30.1, 23.2, 20.4, 20.3, 12.5, 12.1. HRMS (ESI) for [M + H]+: calcd for C27H28ClBrNO4: 544.08120. Found: 544.08855.
Compound 24
(R)-5-chloro-3-((S, 1E, 3E)-3, 5-dimethylhepta-1, 3-dien-1-yl)-2-ethyl-7-methyl-6, 8-dioxo-2, 6, 7, 8-tetrahydroisoquinolin-7-yl acetate. Red solid, the yield of 91%; m.p. 250.0–251.1 °C. +15.5 (c 0.02, MeOH); UV (MeOH) λmax (log ε): 238 (3.12), 385 (3.33), 499 (1.47) nm; IR (KBr) νmax: 2955, 2922, 1748, 1712, 1591, 1491, 1368, 1252, 1190, 1093, 853 cm−1. 1H NMR (600 MHz, CDCl3) δH 7.78 (s, 1H), 6.99 (s, 1H), 6.95 (d, J = 15.3 Hz, 1H), 6.14 (d, J = 15.4 Hz, 1H), 5.69 (d, J = 9.7 Hz, 1H), 3.92 (q, J = 7.3 Hz, 2H), 2.52–2.36 (m, 1H), 2.14 (s, 3H), 1.84 (d, J = 0.9 Hz, 3H), 1.52 (s, 3H), 1.46–1.36 (m, 4H), 1.36–1.27 (m, 1H), 1.00 (d, J = 6.7 Hz, 3H), 0.86 (t, J = 7.4 Hz, 3H). 13C NMR (150 MHz, CDCl3) δC 193.9, 184.3, 170.2, 148.2, 147.9, 145.1, 144.8, 140.8, 131.7, 115.0, 114.5, 111.7, 102.0, 84.9, 49.5, 35.1, 30.1, 23.2, 20.4, 20.3, 15.7, 12.7, 12.1. HRMS (ESI) for [M + H]+: calcd for C23H29ClNO4: 418.17069. Found: 418.17845.