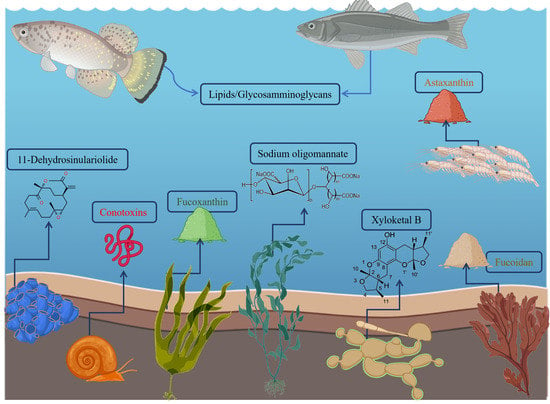
Benefits under the Sea: The Role of Marine Compounds in Neurodegenerative Disorders
Abstract
:1. Introduction
2. Neurodegenerative Diseases
3. Classification of Marine Compounds
3.1. Principal Methods of Extraction, Separation, Isolation, and Identification
3.2. Anti-Oxidant, Anti-Inflammatory, and Anti-Apoptotic Effects: How do Marine Drugs Help US?
3.3. The Effects of Marine Compounds on CNS
4. Marine Drugs in Parkinson’s Disease
4.1. Fucoidan
4.2. Xyloketal B
4.3. Seaweeds
4.4. Astaxanthin
5. Marine Drugs in Alzheimer’s Disease
5.1. Fucoxanthin
5.2. Cerebrosides
5.3. Methyl-Fascaplysin
5.4. Sodium Oligomannate
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Voultsiadou, E. Therapeutic Properties and Uses of Marine Invertebrates in the Ancient Greek World and Early Byzantium. J. Ethnopharmacol. 2010, 130, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.-M.; Zhang, M.-Q.; Shao, C.-L.; Li, G.-Q.; Bai, H.; Dai, G.-L.; Chen, Q.-W.; Kong, W.; Fu, X.-J.; Wang, C.-Y. Chinese Marine Materia Medica Resources: Status and Potential. Marine. Drugs. 2016, 14, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerwick, W.H.; Moore, B.S. Lessons from the Past and Charting the Future of Marine Natural Products Drug Discovery and Chemical Biology. Chem. Biol. 2012, 19, 85–98. [Google Scholar] [CrossRef] [Green Version]
- Udwary, D.W.; Zeigler, L.; Asolkar, R.N.; Singan, V.; Lapidus, A.; Fenical, W.; Jensen, P.R.; Moore, B.S. Genome Sequencing Reveals Complex Secondary Metabolome in the Marine Actinomycete Salinispora Tropica. Proc. Natl. Acad. Sci. USA 2007, 104, 10376–10381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pohnert, G. Chemical Defense Strategies of Marine Organisms. In The Chemistry of Pheromones and Other Semiochemicals I; Schulz, S., Ed.; Topics in Current Chemistry; Springer: Berlin/Heidelberg, Germany, 2004; Volume 239, pp. 179–219. ISBN 978-3-540-20828-0. [Google Scholar]
- Avila, C.; Angulo-Preckler, C. Bioactive Compounds from Marine Heterobranchs. Mar. Drugs 2020, 18, 657. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, K.; Gruber, S.H. Shark Repellent Lipophilic Constituents in the Defense Secretion of the Moses Sole (Pardachirus Marmoratus). Toxicon 1988, 26, 839–853. [Google Scholar] [CrossRef]
- Petersen, L.-E.; Kellermann, M.Y.; Schupp, P.J. Secondary Metabolites of Marine Microbes: From Natural Products Chemistry to Chemical Ecology. In YOUMARES 9—The Oceans: Our Research, Our Future: Proceedings of the 2018 conference for Young Marine Researcher in Oldenburg, Germany; Jungblut, S., Liebich, V., Bode-Dalby, M., Jungblut, S., Liebich, V., Bode-Dalby, M., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 159–180. ISBN 978-3-030-20389-4. [Google Scholar]
- Morris, J.J.; Kirkegaard, R.; Szul, M.J.; Johnson, Z.I.; Zinser, E.R. Facilitation of Robust Growth of Prochlorococcus Colonies and Dilute Liquid Cultures by “Helper” Heterotrophic Bacteria. Appl. Environ. Microbiol. 2008, 74, 4530–4534. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.S.; Kwon, K.K.; Kang, S.G.; Cha, S.-S.; Kim, S.-J.; Lee, J.-H. Approaches for Novel Enzyme Discovery from Marine Environments. Curr. Opin. Biotechnol. 2010, 21, 353–357. [Google Scholar] [CrossRef]
- Nalini, S.; Sandy Richard, D.; Mohammed Riyaz, S.U.; Kavitha, G.; Inbakandan, D. Antibacterial Macro Molecules from Marine Organisms. Int. J. Biol. Macromol. 2018, 115, 696–710. [Google Scholar] [CrossRef]
- Khalifa, S.A.M.; Elias, N.; Farag, M.A.; Chen, L.; Saeed, A.; Hegazy, M.-E.F.; Moustafa, M.S.; Abd El-Wahed, A.; Al-Mousawi, S.M.; Musharraf, S.G.; et al. Marine Natural Products: A Source of Novel Anticancer Drugs. Mar. Drugs 2019, 17, 491. [Google Scholar] [CrossRef] [Green Version]
- Sagar, S.; Kaur, M.; Minneman, K.P. Antiviral Lead Compounds from Marine Sponges. Mar. Drugs 2010, 8, 2619–2638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, D.; Kwon, S.-H.; Chun, Y.S.; Gu, M.-Y.; Yang, H.O. Anti-Neuroinflammatory Effects of Fucoxanthin via Inhibition of Akt/NF-ΚB and MAPKs/AP-1 Pathways and Activation of PKA/CREB Pathway in Lipopolysaccharide-Activated BV-2 Microglial Cells. Neurochem. Res. 2017, 42, 667–677. [Google Scholar] [CrossRef]
- Unnikrishnan, P.S.; Jayasri, M.A. Marine Algae As A Prospective Source For Antidiabetic Compounds—A Brief Review. Curr. Diabetes Rev. 2018, 14, 237–245. [Google Scholar] [CrossRef]
- Abachi, S.; Bazinet, L.; Beaulieu, L. Antihypertensive and Angiotensin-I-Converting Enzyme (ACE)-Inhibitory Peptides from Fish as Potential Cardioprotective Compounds. Mar. Drugs 2019, 17, 613. [Google Scholar] [CrossRef] [Green Version]
- Luo, L.; Wu, M.; Xu, L.; Lian, W.; Xiang, J.; Lu, F.; Gao, N.; Xiao, C.; Wang, S.; Zhao, J. Comparison of Physicochemical Characteristics and Anticoagulant Activities of Polysaccharides from Three Sea Cucumbers. Mar. Drugs 2013, 11, 399–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Guo, C.; Kong, J. Oxidative Stress in Neurodegenerative Diseases. Neural Regen. Res. 2012, 7, 376–385. [Google Scholar] [CrossRef]
- Mullaart, E.; Boerrigter, M.E.T.I.; Ravid, R.; Swaab, D.F.; Vijg, J. Increased Levels of DNA Breaks in Cerebral Cortex of Alzheimer’s Disease Patients. Neurobiol. Aging 1990, 11, 169–173. [Google Scholar] [CrossRef] [Green Version]
- Talmard, C.; Guilloreau, L.; Coppel, Y.; Mazarguil, H.; Faller, P. Amyloid-Beta Peptide Forms Monomeric Complexes with Cu(II) and Zn(II) Prior to Aggregation. ChemBioChem 2007, 8, 163–165. [Google Scholar] [CrossRef]
- Nakamura, M.; Shishido, N.; Nunomura, A.; Smith, M.A.; Perry, G.; Hayashi, Y.; Nakayama, K.; Hayashi, T. Three Histidine Residues of Amyloid-Beta Peptide Control the Redox Activity of Copper and Iron. Biochemistry. 2007, 46, 12737–12743. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.H.; Kim, J.E.; Yoon, S.J.R. and S. The Role of Oxidative Stress in Neurodegenerative Diseases. Exp. Neurobio. 2015, 24, 325–340. [Google Scholar] [CrossRef] [PubMed]
- Hwang, O. Role of Oxidative Stress in Parkinson’s Disease. Exp. Neurobiol. 2013, 22, 11–17. [Google Scholar] [CrossRef] [Green Version]
- Meng, F.; Yao, D.; Shi, Y.; Kabakoff, J.; Wu, W.; Reicher, J.; Ma, Y.; Moosmann, B.; Masliah, E.; Lipton, S.A.; et al. Oxidation of the Cysteine-Rich Regions of Parkin Perturbs Its E3 Ligase Activity and Contributes to Protein Aggregation. Mol. Neurodegener. 2011, 6, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, D.; Gu, Z.; Nakamura, T.; Shi, Z.-Q.; Ma, Y.; Gaston, B.; Palmer, L.A.; Rockenstein, E.M.; Zhang, Z.; Masliah, E.; et al. Nitrosative Stress Linked to Sporadic Parkinson’s Disease: S-Nitrosylation of Parkin Regulates Its E3 Ubiquitin Ligase Activity. Proc. Natl. Acad. Sci. USA 2004, 101, 10810–10814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashimoto, M.; Hsu, L.J.; Xia, Y.; Takeda, A.; Sisk, A.; Sundsmo, M.; Masliah, E. Oxidative Stress Induces Amyloid-like Aggregate Formation of NACP/Alpha-Synuclein In Vitro. Neuroreport 1999, 10, 717–721. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, H.-L.; Yang, C.-M. Role of Redox Signaling in Neuroinflammation and Neurodegenerative Diseases. Available online: https://www.hindawi.com/journals/bmri/2013/484613/ (accessed on 22 December 2020).
- Mishra, A.; Kim, H.J.; Shin, A.H.; Thayer, S.A. Synapse Loss Induced by Interleukin-1β Requires Pre- and Post-Synaptic Mechanisms. J. Neuroimmune Pharmacol. 2012, 7, 571–578. [Google Scholar] [CrossRef] [Green Version]
- Grosso, C.; Valentão, P.; Ferreres, F.; Andrade, P.B. Bioactive Marine Drugs and Marine Biomaterials for Brain Diseases. Mar. Drugs 2014, 12, 2539–2589. [Google Scholar] [CrossRef] [Green Version]
- Chai, H.-J.; Wu, C.-J.; Yang, S.-H.; Li, T.-L.; Sun Pan, B. Peptides from Hydrolysate of Lantern Fish (Benthosema Pterotum) Proved Neuroprotective In Vitro and In Vivo. J. Funct. Foods 2016, 24, 438–449. [Google Scholar] [CrossRef]
- Lin, L.; Yang, K.; Zheng, L.; Zhao, M.; Sun, W.; Zhu, Q.; Liu, S. Anti-Aging Effect of Sea Cucumber (Cucumaria Frondosa) Hydrolysate on Fruit Flies and d-Galactose-Induced Aging Mice. J. Funct. Foods 2018, 47, 11–18. [Google Scholar] [CrossRef]
- Miljanich, G.P. Ziconotide: Neuronal Calcium Channel Blocker for Treating Severe Chronic Pain. Curr. Med. Chem. 2004, 11, 3029–3040. [Google Scholar] [CrossRef]
- Livett, B.G.; Sandall, D.W.; Keays, D.; Down, J.; Gayler, K.R.; Satkunanathan, N.; Khalil, Z. Therapeutic Applications of Conotoxins That Target the Neuronal Nicotinic Acetylcholine Receptor. Toxicon 2006, 48, 810–829. [Google Scholar] [CrossRef]
- Jimenez, E.C.; Donevan, S.; Walker, C.; Zhou, L.-M.; Nielsen, J.; Cruz, L.J.; Armstrong, H.; White, H.S.; Olivera, B.M. Conantokin-L, a New NMDA Receptor Antagonist: Determinants for Anticonvulsant Potency. Epilepsy Res. 2002, 51, 73–80. [Google Scholar] [CrossRef]
- Pangestuti, R.; Ryu, B.; Himaya, S.W.A.; Kim, S.-K. Optimization of Hydrolysis Conditions, Isolation, and Identification of Neuroprotective Peptides Derived from Seahorse Hippocampus Trimaculatus. Amino Acids 2013, 45, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Vanderlei, E.S.O.; Patoilo, K.K.N.R.; Lima, N.A.; Lima, A.P.S.; Rodrigues, J.A.G.; Silva, L.M.C.M.; Lima, M.E.P.; Lima, V.; Benevides, N.M.B. Antinociceptive and Anti-Inflammatory Activities of Lectin from the Marine Green Alga Caulerpa Cupressoides. Int. Immunopharmacol. 2010, 10, 1113–1118. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Shibata, T.; Hisaka, S.; Osawa, T. Astaxanthin Inhibits Reactive Oxygen Species-Mediated Cellular Toxicity in Dopaminergic SH-SY5Y Cells via Mitochondria-Targeted Protective Mechanism. Brain Res. 2009, 1254, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Fakhri, S.; Yosifova Aneva, I.; Farzaei, M.H.; Sobarzo-Sánchez, E. The Neuroprotective Effects of Astaxanthin: Therapeutic Targets and Clinical Perspective. Molecules 2019, 24, 2640. [Google Scholar] [CrossRef] [Green Version]
- Ikeda, Y.; Tsuji, S.; Satoh, A.; Ishikura, M.; Shirasawa, T.; Shimizu, T. Protective Effects of Astaxanthin on 6-Hydroxydopamine-Induced Apoptosis in Human Neuroblastoma SH-SY5Y Cells. J. Neurochem. 2008, 107, 1730–1740. [Google Scholar] [CrossRef]
- Mohibbullah, M.; Haque, M.N.; Khan, M.N.A.; Park, I.-S.; Moon, I.S.; Hong, Y.-K. Neuroprotective Effects of Fucoxanthin and Its Derivative Fucoxanthinol from the Phaeophyte Undaria Pinnatifida Attenuate Oxidative Stress in Hippocampal Neurons. J. Appl. Phycol. 2018, 30, 3243–3252. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, H.; Fan, Y.; Gao, Y.; Li, X.; Hu, Z.; Ding, K.; Wang, Y.; Wang, X. Fucoxanthin Provides Neuroprotection in Models of Traumatic Brain Injury via the Nrf2-ARE and Nrf2-Autophagy Pathways. Sci. Rep. 2017, 7, 46763. [Google Scholar] [CrossRef] [Green Version]
- Maoka, T.; Nishino, A.; Yasui, H.; Yamano, Y.; Wada, A. Anti-Oxidative Activity of Mytiloxanthin, a Metabolite of Fucoxanthin in Shellfish and Tunicates. Mar. Drugs 2016, 14, 93. [Google Scholar] [CrossRef] [Green Version]
- El Shatshat, A.; Pham, A.T.; Rao, P.P.N. Interactions of Polyunsaturated Fatty Acids with Amyloid Peptides Aβ40 and Aβ42. Arch. Biochem. Biophys. 2019, 663, 34–43. [Google Scholar] [CrossRef]
- Li, Q.; Che, H.-X.; Wang, C.-C.; Zhang, L.-Y.; Ding, L.; Xue, C.-H.; Zhang, T.-T.; Wang, Y.-M. Cerebrosides from Sea Cucumber Improved Aβ1–42-Induced Cognitive Deficiency in a Rat Model of Alzheimer’s Disease. Mol. Nutr. Food Res. 2019, 63, 1800707. [Google Scholar] [CrossRef]
- Ye, Q.; Hai, K.; Liu, W.; Wang, Y.; Zhou, X.; Ye, Z.; Liu, X. Investigation of the Protective Effect of Heparin Pre-Treatment on Cerebral Ischaemia in Gerbils. Pharm. Biol. 2019, 57, 519–528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khelif, Y.; Toutain, J.; Quittet, M.-S.; Chantepie, S.; Laffray, X.; Valable, S.; Divoux, D.; Sineriz, F.; Pascolo-Rebouillat, E.; Papy-Garcia, D.; et al. A Heparan Sulfate-Based Matrix Therapy Reduces Brain Damage and Enhances Functional Recovery Following Stroke. Theranostics 2018, 8, 5814–5827. [Google Scholar] [CrossRef] [PubMed]
- Kushchayev, S.V.; Giers, M.B.; Hom Eng, D.; Martirosyan, N.L.; Eschbacher, J.M.; Mortazavi, M.M.; Theodore, N.; Panitch, A.; Preul, M.C. Hyaluronic Acid Scaffold Has a Neuroprotective Effect in Hemisection Spinal Cord Injury. J. Neurosurg. Spine 2016, 25, 114–124. [Google Scholar] [CrossRef]
- Cañas, N.; Valero, T.; Villarroya, M.; Montell, E.; Vergés, J.; García, A.G.; López, M.G. Chondroitin Sulfate Protects SH-SY5Y Cells from Oxidative Stress by Inducing Heme Oxygenase-1 via Phosphatidylinositol 3-Kinase/Akt. J. Pharmacol. Exp. Ther. 2007, 323, 946–953. [Google Scholar] [CrossRef] [Green Version]
- Ye, P.; Li, P.; Yang, W.; Zhao, Y.; Zhao, Y.; Sun, K.; Wang, B.; Chen, Y. Structure and Neuroprotective Effect of Polysaccharide from Viscera Autolysates of Squid Ommastrephes Bartrami. Mar. Drugs. 2019, 17, 188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alghazwi, M.; Smid, S.; Karpiniec, S.; Zhang, W. Comparative Study on Neuroprotective Activities of Fucoidans from Fucus Vesiculosus and Undaria Pinnatifida. Int. J. Biol. Macromol. 2019, 122, 255–264. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Tao, H.-Y.; Liu, S.-Q. Neuroprotective Effects of Carboxymethylated Chitosan on Hydrogen Peroxide Induced Apoptosis in Schwann Cells. Eur. J. Pharmacol. 2014, 740, 127–134. [Google Scholar] [CrossRef]
- Souza, R.B.; Frota, A.F.; Silva, J.; Alves, C.; Neugebauer, A.Z.; Pinteus, S.; Rodrigues, J.A.G.; Cordeiro, E.M.S.; de Almeida, R.R.; Pedrosa, R.; et al. In Vitro Activities of Kappa-Carrageenan Isolated from Red Marine Alga Hypnea Musciformis: Antimicrobial, Anticancer and Neuroprotective Potential. Int. J. Biol. Macromol. 2018, 112, 1248–1256. [Google Scholar] [CrossRef]
- Olasehinde, T.A.; Olaniran, A.O.; Okoh, A.I. Sulfated Polysaccharides of Some Seaweeds Exhibit Neuroprotection via Mitigation of Oxidative Stress, Cholinergic Dysfunction and Inhibition of Zn – Induced Neuronal Damage in HT-22 Cells. BMC Complement. Med. Ther. 2020, 20, 251. [Google Scholar] [CrossRef]
- Nelson, T.J.; Sun, M.-K.; Lim, C.; Sen, A.; Khan, T.; Chirila, F.V.; Alkon, D.L. Bryostatin Effects on Cognitive Function and PKCɛ in Alzheimer’s Disease Phase IIa and Expanded Access Trials. J. Alzheimers Dis. 2017, 58, 521–535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.-F.; Chakraborty, C.; Sung, C.-S.; Feng, C.-W.; Jean, Y.-H.; Lin, Y.-Y.; Hung, H.-C.; Huang, T.-Y.; Huang, S.-Y.; Su, T.-M.; et al. Neuroprotection by Marine-Derived Compound, 11-Dehydrosinulariolide, in an In Vitro Parkinson’s Model: A Promising Candidate for the Treatment of Parkinson’s Disease. Naunyn.-Schmiedeberg’s Arch. Pharmacol. 2012, 385, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.-W.; Hung, H.-C.; Huang, S.-Y.; Chen, C.-H.; Chen, Y.-R.; Chen, C.-Y.; Yang, S.-N.; Wang, H.-M.D.; Sung, P.-J.; Sheu, J.-H.; et al. Neuroprotective Effect of the Marine-Derived Compound 11-Dehydrosinulariolide through DJ-1-Related Pathway in In Vitro and In Vivo Models of Parkinson’s Disease. Mar. Drugs. 2016, 14, 187. [Google Scholar] [CrossRef] [PubMed]
- Alonso, E.; Fuwa, H.; Vale, C.; Suga, Y.; Goto, T.; Konno, Y.; Sasaki, M.; LaFerla, F.M.; Vieytes, M.R.; Giménez-Llort, L.; et al. Design and Synthesis of Skeletal Analogues of Gambierol: Attenuation of Amyloid-β and Tau Pathology with Voltage-Gated Potassium Channel and N-Methyl-d-Aspartate Receptor Implications. J. Am. Chem. Soc. 2012, 134, 7467–7479. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Strong, J.A.; Meij, J.T.A.; Zhang, J.-M.; Yu, L. Neuropathic Pain: Early Spontaneous Afferent Activity Is the Trigger. Pain 2005, 116, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Marcil, J.; Walczak, J.-S.; Guindon, J.; Ngoc, A.H.; Lu, S.; Beaulieu, P. Antinociceptive Effects of Tetrodotoxin (TTX) in Rodents. Br. J. Anaesth. 2006, 96, 761–768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kayser, V.; Viguier, F.; Ioannidi, M.; Bernard, J.-F.; Latrémolière, A.; Michot, B.; Vela, J.-M.; Buschmann, H.; Hamon, M.; Bourgoin, S. Differential Anti-Neuropathic Pain Effects of Tetrodotoxin in Sciatic Nerve- versus Infraorbital Nerve-Ligated Rats--Behavioral, Pharmacological and Immunohistochemical Investigations. Neuropharmacology 2010, 58, 474–487. [Google Scholar] [CrossRef] [PubMed]
- Pandya, A.; Yakel, J.L. Allosteric Modulator Desformylflustrabromine Relieves the Inhibition of Α2β2 and Α4β2 Nicotinic Acetylcholine Receptors by β-Amyloid1–42 Peptide. J. Mol. Neurosci. 2011, 45, 42–47. [Google Scholar] [CrossRef] [Green Version]
- Borris, R.P. Natural Products Research: Perspectives from a Major Pharmaceutical Company. J. Ethnopharmacol. 1996, 51, 29–38. [Google Scholar] [CrossRef]
- Sosa-Hernández, J.E.; Escobedo-Avellaneda, Z.; Iqbal, H.M.N.; Welti-Chanes, J. State-of-the-Art Extraction Methodologies for Bioactive Compounds from Algal Biome to Meet Bio-Economy Challenges and Opportunities. Molecules 2018, 23, 2953. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.-W.; Lin, L.-G.; Ye, W.-C. Techniques for Extraction and Isolation of Natural Products: A Comprehensive Review. Chin. Med. 2018, 13, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roh, M.-K.; Uddin, M.S.; Chun, B.-S. Extraction of Fucoxanthin and Polyphenol from Undaria Pinnatifida Using Supercritical Carbon Dioxide with Co-Solvent. Biotechnol. Bioproc. E 2008, 13, 724–729. [Google Scholar] [CrossRef]
- Rodriguez-Jasso, R.M.; Mussatto, S.I.; Pastrana, L.; Aguilar, C.N.; Teixeira, J.A. Microwave-Assisted Extraction of Sulfated Polysaccharides (Fucoidan) from Brown Seaweed. Carbohydr. Polym. 2011, 86, 1137–1144. [Google Scholar] [CrossRef] [Green Version]
- Molino, A.; Rimauro, J.; Casella, P.; Cerbone, A.; Larocca, V.; Chianese, S.; Karatza, D.; Mehariya, S.; Ferraro, A.; Hristoforou, E.; et al. Extraction of Astaxanthin from Microalga Haematococcus Pluvialis in Red Phase by Using Generally Recognized as Safe Solvents and Accelerated Extraction. J. Biotechnol. 2018, 283, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Kumari, M.; Taritla, S.; Sharma, A.; Jayabaskaran, C. Antiproliferative and Antioxidative Bioactive Compounds in Extracts of Marine-Derived Endophytic Fungus Talaromyces Purpureogenus. Front. Microbiol. 2018, 9. [Google Scholar] [CrossRef] [Green Version]
- Hwang, P.-A.; Yan, M.-D.; Lin, H.-T.V.; Li, K.-L.; Lin, Y.-C. Toxicological Evaluation of Low Molecular Weight Fucoidan In Vitro and In Vivo. Mar. Drugs 2016, 14, 121. [Google Scholar] [CrossRef] [Green Version]
- Feng, Y.; Wang, X. Antioxidant Therapies for Alzheimer’s Disease. Available online: https://www.hindawi.com/journals/omcl/2012/472932/ (accessed on 22 December 2020).
- Fadaka, A.O.; Ajiboye, B.O.; Adewale, I.; Ojo, O.A.; Oyinloye, B.E.; Okesola, M.A. Significance of Antioxidants in the Treatment and Prevention of Neurodegenerative Diseases. J. Phytopharmacol. 2019, 8, 75–83. [Google Scholar] [CrossRef]
- Kiokias, S.; Gordon, M.H. Dietary Supplementation with a Natural Carotenoid Mixture Decreases Oxidative Stress. European. J. Clin. Nutr. 2003, 57, 1135–1140. [Google Scholar] [CrossRef] [Green Version]
- Eggersdorfer, M.; Wyss, A. Carotenoids in Human Nutrition and Health. Arch. Biochem. Biophys. 2018, 652, 18–26. [Google Scholar] [CrossRef]
- Guedes, A.C.; Amaro, H.M.; Malcata, F.X. Microalgae as Sources of Carotenoids. Mar. Drugs 2011, 9, 625–644. [Google Scholar] [CrossRef]
- Maoka, T. Carotenoids in Marine Animals. Mar. Drugs 2011, 9, 278–293. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, C.-R.; Yang, H.; Liu, J.; Zhang, T.; Jiao, S.-S.; Wang, Y.-J.; Xu, Z.-Q. ProBDNF Attenuates Hippocampal Neurogenesis and Induces Learning and Memory Deficits in Aged Mice. Neurotox. Res. 2016, 29, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Wang, X.; Xiang, Q.; Meng, X.; Peng, Y.; Du, N.; Liu, Z.; Sun, Q.; Wang, C.; Liu, X. Astaxanthin Alleviates Brain Aging in Rats by Attenuating Oxidative Stress and Increasing BDNF Levels. Food. Funct. 2013, 5, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Galasso, C.; Orefice, I.; Pellone, P.; Cirino, P.; Miele, R.; Ianora, A.; Brunet, C.; Sansone, C. On the Neuroprotective Role of Astaxanthin: New Perspectives? Mar. Drugs 2018, 16, 247. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.-P.; Liu, S.-Y.; Sun, H.; Wu, X.-M.; Li, J.-J.; Zhu, L. Neuroprotective Effect of Astaxanthin on H2O2-Induced Neurotoxicity In Vitro and on Focal Cerebral Ischemia In Vivo. Brain Res 2010, 1360, 40–48. [Google Scholar] [CrossRef]
- Sekikawa, T.; Kizawa, Y.; Li, Y.; Takara, T. Cognitive Function Improvement with Astaxanthin and Tocotrienol Intake: A Randomized, Double-Blind, Placebo-Controlled Study. J. Clin. Biochemy. Nutr. 2020, 67, 307–316. [Google Scholar] [CrossRef]
- Sachindra, N.M.; Sato, E.; Maeda, H.; Hosokawa, M.; Niwano, Y.; Kohno, M.; Miyashita, K. Radical Scavenging and Singlet Oxygen Quenching Activity of Marine Carotenoid Fucoxanthin and Its Metabolites. J. Agric. Food Chem. 2007, 55, 8516–8522. [Google Scholar] [CrossRef]
- Heo, S.-J.; Ko, S.-C.; Kang, S.-M.; Kang, H.-S.; Kim, J.-P.; Kim, S.-H.; Lee, K.-W.; Cho, M.-G.; Jeon, Y.-J. Cytoprotective Effect of Fucoxanthin Isolated from Brown Algae Sargassum Siliquastrum against H2O2-Induced Cell Damage. Eur. Food Res. Technol. 2008, 228, 145–151. [Google Scholar] [CrossRef]
- Ha, A.W.; Na, S.J.; Kim, W.K. Antioxidant Effects of Fucoxanthin Rich Powder in Rats Fed with High Fat Diet. Nutr. Res. Pract. 2013, 7, 475–480. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.-Y.; Chen, N.-F.; Chen, W.-F.; Hung, H.-C.; Lee, H.-P.; Lin, Y.-Y.; Wang, H.-M.; Sung, P.-J.; Sheu, J.-H.; Wen, Z.-H. Sinularin from Indigenous Soft Coral Attenuates Nociceptive Responses and Spinal Neuroinflammation in Carrageenan-Induced Inflammatory Rat Model. Mar. Drugs 2012, 10, 1899–1919. [Google Scholar] [CrossRef] [Green Version]
- Pangestuti, R.; Kim, S.-K. Peptide-Derived from Seahorse Exerts a Protective Effect against Cholinergic Neuronal Death in In Vitro Model of Alzheimer’s Disease. Procedia Chem. 2015, 14, 343–352. [Google Scholar] [CrossRef] [Green Version]
- Wei, H.; Gao, Z.; Zheng, L.; Zhang, C.; Liu, Z.; Yang, Y.; Teng, H.; Hou, L.; Yin, Y.; Zou, X. Protective Effects of Fucoidan on Aβ25–35 and d-Gal-Induced Neurotoxicity in PC12 Cells and d-Gal-Induced Cognitive Dysfunction in Mice. Mar. Drugs 2017, 15, 77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, H.-A.; Hayden, M.M.; Bannerman, S.; Jansen, J.; Crowe-White, K.M. Anti-Apoptotic Effects of Carotenoids in Neurodegeneration. Molecules 2020, 25, 3453. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-Y.; Yang, D.; Fu, Z.J.; Woo, T.; Wong, D.; Lo, A.C.Y. Lutein Enhances Survival and Reduces Neuronal Damage in a Mouse Model of Ischemic Stroke. Neurobiol. Dis. 2012, 45, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Xiao, A.-J.; Chen, W.; Xu, B.; Liu, R.; Turlova, E.; Barszczyk, A.; Sun, C.L.; Liu, L.; Deurloo, M.; Wang, G.-L.; et al. Marine Compound Xyloketal B Reduces Neonatal Hypoxic-Ischemic Brain Injury. Mar. Drugs. 2015, 13, 29–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kijjoa, A.; Sawangwong, P. Drugs and Cosmetics from the Sea. Mar. Drugs. 2004, 2, 73–82. [Google Scholar] [CrossRef] [Green Version]
- Cragg, G.M.; Newman, D.J. Natural Products: A Continuing Source of Novel Drug Leads. Biochim. Biophys. Acta 2013, 1830, 3670–3695. [Google Scholar] [CrossRef] [Green Version]
- McGivern, J.G. Ziconotide: A Review of Its Pharmacology and Use in the Treatment of Pain. Neuropsychiatr. Dis. Treat. 2007, 3, 69–85. [Google Scholar] [CrossRef] [Green Version]
- Brookes, M.E.; Eldabe, S.; Batterham, A. Ziconotide Monotherapy: A Systematic Review of Randomised Controlled Trials. Curr. Neuropharmacol. 2017, 15, 217–231. [Google Scholar] [CrossRef]
- Moodie, L.W.K.; Sepčić, K.; Turk, T.; FrangeŽ, R.; Svenson, J. Natural Cholinesterase Inhibitors from Marine Organisms. Nat. Prod. Rep. 2019, 36, 1053–1092. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Sorribas, A.; Yoshida, W.Y.; Kelly, M.; Williams, P.G. Xestosaprols from the Indonesian Marine Sponge Xestospongia Sp. J. Nat. Prod. 2010, 73, 1188–1191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Zhang, W.; Li, L.; Salvador, L.A.; Chen, T.; Chen, W.; Felsenstein, K.M.; Ladd, T.B.; Price, A.R.; Golde, T.E.; et al. Cyanobacterial Peptides as a Prototype for the Design of Potent β-Secretase Inhibitors and the Development of Selective Chemical Probes for Other Aspartic Proteases. J. Med. Chem. 2012, 55, 10749–10765. [Google Scholar] [CrossRef]
- McCulloch, M.W.B.; Bugni, T.S.; Concepcion, G.P.; Coombs, G.S.; Harper, M.K.; Kaur, S.; Mangalindan, G.C.; Mutizwa, M.M.; Veltri, C.A.; Virshup, D.M.; et al. Carteriosulfonic Acids A−C, GSK-3β Inhibitors from a Carteriospongia Sp. J. Nat. Prod. 2009, 72, 1651–1656. [Google Scholar] [CrossRef] [Green Version]
- Tahtouh, T.; Elkins, J.M.; Filippakopoulos, P.; Soundararajan, M.; Burgy, G.; Durieu, E.; Cochet, C.; Schmid, R.S.; Lo, D.C.; Delhommel, F.; et al. Selectivity, Cocrystal Structures, and Neuroprotective Properties of Leucettines, a Family of Protein Kinase Inhibitors Derived from the Marine Sponge Alkaloid Leucettamine B. J. Med. Chem. 2012, 55, 9312–9330. [Google Scholar] [CrossRef] [PubMed]
- Gompel, M.; Leost, M.; De Kier Joffe, E.B.; Puricelli, L.; Franco, L.H.; Palermo, J.; Meijer, L. Meridianins, a New Family of Protein Kinase Inhibitors Isolated from the Ascidian Aplidium Meridianum. Bioorg. Med. Chem. Lett. 2004, 14, 1703–1707. [Google Scholar] [CrossRef] [PubMed]
- Meijer, L.; Thunnissen, A.M.; White, A.W.; Garnier, M.; Nikolic, M.; Tsai, L.H.; Walter, J.; Cleverley, K.E.; Salinas, P.C.; Wu, Y.Z.; et al. Inhibition of Cyclin-Dependent Kinases, GSK-3beta and CK1 by Hymenialdisine, a Marine Sponge Constituent. Chem. Biol. 2000, 7, 51–63. [Google Scholar] [CrossRef]
- Pettit, G.R.; McNulty, J.; Herald, D.L.; Doubek, D.L.; Chapuis, J.-C.; Schmidt, J.M.; Tackett, L.P.; Boyd, M.R. Antineoplastic Agents. 362. Isolation and X-ray Crystal Structure of Dibromophakellstatin from the Indian Ocean Sponge Phakellia Mauritiana. J. Nat. Prod. 1997, 60, 180–183. [Google Scholar] [CrossRef]
- Langjae, R.; Bussarawit, S.; Yuenyongsawad, S.; Ingkaninan, K.; Plubrukarn, A. Acetylcholinesterase-Inhibiting Steroidal Alkaloid from the Sponge Corticium Sp. Steroids 2007, 72, 682–685. [Google Scholar] [CrossRef]
- Nukoolkarn, V.S.; Saen-oon, S.; Rungrotmongkol, T.; Hannongbua, S.; Ingkaninan, K.; Suwanborirux, K. Petrosamine, a Potent Anticholinesterase Pyridoacridine Alkaloid from a Thai Marine Sponge Petrosia n. Sp. Bioorg. Med. Chem. 2008, 16, 6560–6567. [Google Scholar] [CrossRef]
- Sepcić, K.; Marcel, V.; Klaebe, A.; Turk, T.; Suput, D.; Fournier, D. Inhibition of Acetylcholinesterase by an Alkylpyridinium Polymer from the Marine Sponge, Reniera Sarai. Biochim. Biophys. Acta 1998, 1387, 217–225. [Google Scholar] [CrossRef]
- Ellison, M.; McIntosh, J.M.; Olivera, B.M. Alpha-Conotoxins ImI and ImII. Similar Alpha 7 Nicotinic Receptor Antagonists Act at Different Sites. J. Biol. Chem. 2003, 278, 757–764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, C.; Chen, W.; Han, Y.; Sanders, T.; Chew, G.; Liu, J.; Hawrot, E.; Chi, C.; Wang, C. Characterization of a Novel Alpha4/4-Conotoxin, Qc1.2, from Vermivorous Conus Quercinus. Acta Biochim. Biophys. Sin. (Shanghai) 2009, 41, 858–864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakai, R.; Swanson, G.T. Recent Progress in Neuroactive Marine Natural Products. Nat. Prod. Rep. 2014, 31, 273–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balansa, W.; Islam, R.; Fontaine, F.; Piggott, A.M.; Zhang, H.; Webb, T.I.; Gilbert, D.F.; Lynch, J.W.; Capon, R.J. Ircinialactams: Subunit-Selective Glycine Receptor Modulators from Australian Sponges of the Family Irciniidae. Bioorg. Med. Chem. 2010, 18, 2912–2919. [Google Scholar] [CrossRef] [PubMed]
- Suna, H.; Arai, M.; Tsubotani, Y.; Hayashi, A.; Setiawan, A.; Kobayashi, M. Dysideamine, a New Sesquiterpene Aminoquinone, Protects Hippocampal Neuronal Cells against Iodoacetic Acid-Induced Cell Death. Bioorg. Med. Chem. 2009, 17, 3968–3972. [Google Scholar] [CrossRef]
- Qi, J.; Ojika, M.; Sakagami, Y. Linckosides A and B, Two New Neuritogenic Steroid Glycosides from the Okinawan Starfish Linckia Laevigata. Bioorg. Med. Chem. 2002, 10, 1961–1966. [Google Scholar] [CrossRef]
- Mancini, F.; De Simone, A.; Andrisano, V. Beta-Secretase as a Target for Alzheimer’s Disease Drug Discovery: An Overview of In Vitro Methods for Characterization of Inhibitors. Anal. Bioanal. Chem. 2011, 400, 1979–1996. [Google Scholar] [CrossRef]
- Hooper, C.; Killick, R.; Lovestone, S. The GSK3 Hypothesis of Alzheimer’s Disease. J. Neurochem. 2008, 104, 1433–1439. [Google Scholar] [CrossRef] [Green Version]
- Sharma, K. Cholinesterase Inhibitors as Alzheimer’s Therapeutics (Review). Mol. Med. Rep. 2019, 20, 1479–1487. [Google Scholar] [CrossRef] [Green Version]
- Dineley, K.T.; Pandya, A.A.; Yakel, J.L. Nicotinic ACh Receptors as Therapeutic Targets in CNS Disorders. Trends Pharmacol. Sci. 2015, 36, 96–108. [Google Scholar] [CrossRef] [Green Version]
- Castelli, V.; d’Angelo, M.; Lombardi, F.; Alfonsetti, M.; Antonosante, A.; Catanesi, M.; Benedetti, E.; Palumbo, P.; Cifone, M.G.; Giordano, A.; et al. Effects of the Probiotic Formulation SLAB51 in In Vitro and In Vivo Parkinson’s Disease Models. Aging (Albany N. Y.) 2020, 12, 4641–4659. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Zhang, Q.; Wang, H.; Cui, Y.; Sun, Z.; Yang, J.; Zheng, Y.; Jia, J.; Yu, F.; Wang, X.; et al. Fucoidan Protects against Dopaminergic Neuron Death In Vivo and In Vitro. Eur. J. Pharmacol. 2009, 617, 33–40. [Google Scholar] [CrossRef]
- Lin, Y.; Wu, X.; Feng, S.; Jiang, G.; Luo, J.; Zhou, S.; Vrijmoed, L.L.P.; Jones, E.B.G.; Krohn, K.; Steingröver, K.; et al. Five Unique Compounds: Xyloketals from Mangrove Fungus Xylaria Sp. from the South China Sea Coast. J. Org. Chem. 2001, 66, 6252–6256. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, L.; Ling, C.; Li, J.; Pang, J.-Y.; Lin, Y.-C.; Liu, J.; Huang, R.; Wang, G.-L.; Pei, Z.; et al. Marine Compound Xyloketal B Protects PC12 Cells against OGD-Induced Cell Damage. Brain Res. 2009, 1302, 240–247. [Google Scholar] [CrossRef]
- Chen, W.-L.; Qian, Y.; Meng, W.-F.; Pang, J.-Y.; Lin, Y.-C.; Guan, Y.-Y.; Chen, S.-P.; Liu, J.; Pei, Z.; Wang, G.-L. A Novel Marine Compound Xyloketal B Protects against Oxidized LDL-Induced Cell Injury In Vitro. Biochem. Pharmacol. 2009, 78, 941–950. [Google Scholar] [CrossRef]
- Rueckschloss Uwe; Galle Jan; Holtz Juergen; Zerkowski Hans-Reinhard; Morawietz Henning Induction of NAD(P)H Oxidase by Oxidized Low-Density Lipoprotein in Human Endothelial Cells. Circulation 2001, 104, 1767–1772. [CrossRef] [Green Version]
- Hannan, M.A.; Dash, R.; Haque, M.N.; Mohibbullah, M.; Sohag, A.A.M.; Rahman, M.A.; Uddin, M.J.; Alam, M.; Moon, I.S. Neuroprotective Potentials of Marine Algae and Their Bioactive Metabolites: Pharmacological Insights and Therapeutic Advances. Mar. Drugs 2020, 18, 347. [Google Scholar] [CrossRef]
- Celikler, S.; Vatan, O.; Yildiz, G.; Bilaloglu, R. Evaluation of Anti-Oxidative, Genotoxic and Antigenotoxic Potency of Codium Tomentosum Stackhouse Ethanolic Extract in Human Lymphocytes In Vitro. Food. Chem. Toxicol. 2009, 47, 796–801. [Google Scholar] [CrossRef] [PubMed]
- Valentão, P.; Trindade, P.; Gomes, D.; Guedes de Pinho, P.; Mouga, T.; Andrade, P.B. Codium Tomentosum and Plocamium Cartilagineum: Chemistry and Antioxidant Potential. Food. Chem. 2010, 119, 1359–1368. [Google Scholar] [CrossRef]
- Genç, Y.; Bardakci, H.; Yücel, Ç.; Karatoprak, G.Ş.; Küpeli Akkol, E.; Hakan Barak, T.; Sobarzo-Sánchez, E. Oxidative Stress and Marine Carotenoids: Application by Using Nanoformulations. Mar. Drugs 2020, 18, 423. [Google Scholar] [CrossRef] [PubMed]
- Ambati, R.R.; Phang, S.-M.; Ravi, S.; Aswathanarayana, R.G. Astaxanthin: Sources, Extraction, Stability, Biological Activities and Its Commercial Applications—A Review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef] [PubMed]
- Grimmig, B.; Daly, L.; Hudson, C.; Nash, K.R.; Bickford, P.C. Astaxanthin Attenuates Neurotoxicity in a Mouse Model of Parkinson’s Disease. Funct. Foods Health Dis. 2017, 7, 562–576. [Google Scholar] [CrossRef]
- Catanesi, M.; d’Angelo, M.; Antonosante, A.; Castelli, V.; Alfonsetti, M.; Benedetti, E.; Desideri, G.; Ferri, C.; Cimini, A. Neuroprotective Potential of Choline Alfoscerate against β-Amyloid Injury: Involvement of Neurotrophic Signals. Cell Biol. Int. 2020, 44, 1734–1744. [Google Scholar] [CrossRef] [PubMed]
- Ulep, M.G.; Saraon, S.K.; McLea, S. Alzheimer Disease. J. Nurse Pract. 2018, 14, 129–135. [Google Scholar] [CrossRef]
- Lin, J.; Huang, L.; Yu, J.; Xiang, S.; Wang, J.; Zhang, J.; Yan, X.; Cui, W.; He, S.; Wang, Q. Fucoxanthin, a Marine Carotenoid, Reverses Scopolamine-Induced Cognitive Impairments in Mice and Inhibits Acetylcholinesterase In Vitro. Mar. Drugs 2016, 14, 67. [Google Scholar] [CrossRef] [Green Version]
- Pangestuti, R.; Vo, T.-S.; Ngo, D.-H.; Kim, S.-K. Fucoxanthin Ameliorates Inflammation and Oxidative Reponses in Microglia. J. Agric. Food Chem. 2013, 61, 3876–3883. [Google Scholar] [CrossRef]
- de Chaves, E.P.; Sipione, S. Sphingolipids and Gangliosides of the Nervous System in Membrane Function and Dysfunction. FEBS. Lett. 2010, 584, 1748–1759. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi, R.; Kanie, Y.; Kanie, O.; Shimizu, Y. A Unique Structural Distribution Pattern Discovered for the Cerebrosides from Starfish Asterias Amurensis. Carbohydr. Res. 2019, 473, 115–122. [Google Scholar] [CrossRef]
- Sun, Q.; Liu, F.; Sang, J.; Lin, M.; Ma, J.; Xiao, X.; Yan, S.; Naman, C.B.; Wang, N.; He, S.; et al. 9-Methylfascaplysin Is a More Potent Aβ Aggregation Inhibitor than the Marine-Derived Alkaloid, Fascaplysin, and Produces Nanomolar Neuroprotective Effects in SH-SY5Y Cells. Mar. Drugs. 2019, 17, 121. [Google Scholar] [CrossRef] [Green Version]
- Naveen kumar, S.; Rajivgandhi, G.; Ramachandran, G.; Manoharan, N. A Marine Sponge Fascaplysinopsis Sp. Derived Alkaloid Fascaplysin Inhibits the HepG2 Hepatocellular Carcinoma Cell. Front. Lab. Med. 2018, 2, 41–48. [Google Scholar] [CrossRef]
- Wernicke, C.; Hellmann, J.; Zięba, B.; Kuter, K.; Ossowska, K.; Frenzel, M.; Dencher, N.A.; Rommelspacher, H. 9-Methyl-β-Carboline Has Restorative Effects in an Animal Model of Parkinson’s Disease. Pharmacol Rep. 2010, 62, 35–53. [Google Scholar] [CrossRef]
- Horton, W.; Sood, A.; Peerannawar, S.; Kugyela, N.; Kulkarni, A.; Tulsan, R.; Tran, C.D.; Soule, J.; LeVine, H.; Török, B.; et al. Synthesis and Application of β-Carbolines as Novel Multi-Functional Anti-Alzheimer’s Disease Agents. Bioorg. Med. Chem. Lett. 2017, 27, 232–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manda, S.; Sharma, S.; Wani, A.; Joshi, P.; Kumar, V.; Guru, S.K.; Bharate, S.S.; Bhushan, S.; Vishwakarma, R.A.; Kumar, A.; et al. Discovery of a Marine-Derived Bis-Indole Alkaloid Fascaplysin, as a New Class of Potent P-Glycoprotein Inducer and Establishment of Its Structure-Activity Relationship. Eur. J. Med. Chem. 2016, 107, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.-F.; Liu, Z.; Bai, S.; Dong, X.-Y.; Sun, Y. Exploring the Inter-Molecular Interactions in Amyloid-β Protofibril with Molecular Dynamics Simulations and Molecular Mechanics Poisson-Boltzmann Surface Area Free Energy Calculations. J. Chem. Phys. 2012, 136, 145101. [Google Scholar] [CrossRef] [PubMed]
- Seo, D.; Boros, B.D.; Holtzman, D.M. The Microbiome: A Target for Alzheimer Disease? Cell Res. 2019, 29, 779–780. [Google Scholar] [CrossRef]
- Wang, X.; Sun, G.; Feng, T.; Zhang, J.; Huang, X.; Wang, T.; Xie, Z.; Chu, X.; Yang, J.; Wang, H.; et al. Sodium Oligomannate Therapeutically Remodels Gut Microbiota and Suppresses Gut Bacterial Amino Acids-Shaped Neuroinflammation to Inhibit Alzheimer’s Disease Progression. Cell. Res. 2019, 29, 787–803. [Google Scholar] [CrossRef]
- Shanghai Greenvalley Pharmaceutical Co., Ltd. A Phase 3, Multi-Center, Randomized, Double-Blind, Parallel-Group, Placebo-Controlled Clinical Trial to Evaluate the Efficacy and Safety of Sodium Oligomannate (GV-971) in Treatment of Mild to Moderate Alzheimer’s Disease (GREEN MEMORY: GREen Valley 971 EvaluatioN Memory); ClinicalTrials.gov: Bethesda, MD, USA, 2020. [Google Scholar]








| Class of Compounds | Identified Molecules of Interest | Marine Sources | Main Effects |
|---|---|---|---|
| Peptides | BPH (protein hydrolysate, consisting of active peptides Phe-Tyr-Tyr and Asp-Trp) | Lantern Fish (Benthosema pterotum) | Free radical scavenger, reduction of reactive species generation, and prevention of H2O2 mediated apoptosis [30] |
| CFH (protein hydrolysate, consisting of 40 oligopeptides | Sea cucumber (Cucumaria frondosa) | In vitro: oxidative stress attenuation | |
| In vivo: amelioration of learning and memory deficits in D-galactose-induced aging mice [12,31] | |||
| Conotoxins | Cone snails (genus Conus) | Anti-nociceptive activity and alleviation of neuropathic pain (ziconotide) [32]; | |
| functional recovery of damaged neurons (ACV1) [33]; anti-convulsant activity (conantokin-L) [34] | |||
| HTP-1 (H. trimaculatus-derived neuroprotective peptides Gly-Thr-Glu-Asp-Glu-Leu-AspLys) | Seahorse (Hippocampus trimaculatus) | In vitro: neuroprotective activity against Aβ42-induced apoptosis in PC12 cells [16,35] | |
| Glycoproteins | Lectins | Green algae (Caulerpa Cupressoides) | In vivo: anti-nociceptive and anti-inflammatory activity in Swiss mice [36] |
| Pigments | AXT | Microalgae (Haematococcus pluvialis) Shrimp, lobster, crustacean, krill, trout, salmon | In vitro: protection from 6-OHDA-induced apoptosis and inhibition of mitochondrial impairment in SH-SY5Y [37] |
| In vivo: anti-depressant effects, anti-oxidant activity (mediated by an increasing in GSH and superoxide dismutase) [38,39] | |||
| FX | Brown seaweed (Undaria pinnatifida) | Reduction of oxidative stress in rat hippocampal neurons [40]; | |
| Increase in neuron survivals in traumatic brain injury models [41] | |||
| Mytiloxanthin (metabolite of fucoxanthin) | Tunicates and shellfish | Scavenger of singlet oxygen [42] | |
| Lipids | Polyunsaturated fatty acids | fish oils (cod liver oil), algae, sea cucumber, microalgae | Reduction of Aβ-amyloid toxicity, anti-aggregation properties, inhibition of Aβ40 and Aβ42 fibrillogenesis [43] |
| Glycolipids | Glycosphingolipids (cerebrosides) | Echinoderms (sea cucumber), porifera and mollusks | Improvement of cognitive deficiency in AD rat model [44] |
| Glycosaminoglycans | Heparin and Heparan sulfate | Mollusks, shrimp heads (Litopenaeus vannamei and Penaeus brasiliensis), | Reduction of neuronal cell apoptosis and pro-inflammatory cytokines, neuroprotective effect in cerebral ischemia in gerbils [45]; amelioration of brain condition after stroke [46] |
| crabs (Goniopsis cruentata and Ucides cordatus), sea cucumber, ascidian (Styela plicata), scallop, cockle (Cerastoderma edule), sand dollar (Mellita quinquiesperforata) | |||
| Hyaluronic acid | Shark fins, tuna eyeballs, bivalves, mussels and codfish bones | Hyaluronic acid scaffolds with neuroprotective effects in spinal cord injury [28,47] | |
| Chondroitin sulfate | Shark and fish cartilage, blackmouth catshark | In vitro: protection of SH-SY5Y cells against oxidative stress [29,48] | |
| Polysaccharides | SV2-1 | Ommastrephes bartrami | In vitro: protection of PC12 cells from 6-OHDA-induced death; anti-oxidant activity [49] |
| Fucoidan | Brown algae (Undaria pinnatifida) | In vitro: reduction of Aβ1–42- and hydrogen peroxide-mediated cytotoxicity in PC12 cells [50] | |
| Chitosan and its derivatives | Crustaceans (shrimps and crabs) | Neuroprotective effects on peripheral nerves and Schwann cells [51] | |
| Carrageenan | Red algae (Hypnea musciformis) | In vitro: anti-oxidant and cytoprotective effects against 6-OHDA-induced neurotoxicity in SH-5YSY models [52] | |
| Sulfated polysaccharides | Sea weeds (Ecklonia maxima, Gelidium pristoides, Ulva lactuca, Ulva rigida and Gracilaria gracilis) | In vitro: stimulation of anti-oxidant activities (increase in anti-oxidant enzymes and glutathione content) in hippocampal cell line with Zn-induce damage [53] | |
| Macrolides | Bryostatin | Brown bryozoa (Bugula neritina) | Potent modulation of protein kinase C; induction of synaptogenesis and amelioration of deficits in rats and mice models of neurodegenerative diseases [54] |
| 11-dehydrosinulariolide | Soft coral (Sinularia flexibilis) | In vitro: anti-apoptotic and anti-inflammatory activity on SH-SY5Y cells treated with 6-OHDA [55]. | |
| In vivo: amelioration of PD symptoms in rat and zebrafish models [56] | |||
| Polycyclic ethers | Gambierol | Gambierdiscus toxicus | In vitro: decrease in intra- and extra-cellular levels of Aβ deposits and in tau hyperphosphorylation in triple transgenic (3xTg-AD) mice model [57] |
| Guanidine neurotoxins | Tetrodotoxin | Tetraodontiformes. (pufferfish) | Beneficial effects on acute [58], inflammatory [59] and neuropathic [60] pain |
| Indole alkaloids | Bromotriptamines | Bryozoa | In vitro: in Xenopus. oocytes, they act as positive allosteric modulator for two subtypes of nicotinic acetylcholine receptors (α4β2 and α2β2). They can attenuate the inhibition of Aβ1–42 on these receptors [61] |
| Pharmacological Activity | Compounds | Main Source |
|---|---|---|
| Beta-secretase 1 inhibitors | Xestosaprols | Indonesian marine sponges, genus Xestospongia. [95] |
| Tasiamide B | Cyanobacteria [96] | |
| Glycogen synthase kinase-3 inhibitors | Carteriosulfonic acids | Sponges, genus Carteriospongia [97] |
| Leucettamines | Sponge Leucetta microraphis [98] | |
| Merdidianins | Ascidian Aplidium meridianum [99] | |
| Hymenialdisine | Sponges (various species) [100,101] | |
| Cholinesterase inhibitors | 4-acetoxy-plakinamine B | Sponges, genus Corticium [102] |
| Petrosamine | Sponges, genus Petrosia n. [103] | |
| Alkylpyridine | Sponges, Reniera sarai [104] | |
| (and alkylpyridinium derivatives) | ||
| Nicotinic acetylcholine receptor antagonists | Α-conotoxins | Sea snail, genus Conus species: geographus, imperialis, vexillum, quercinum [105,106] Octocorals [107] |
| Cembranoids | ||
| (lophotoxin) | ||
| Glycine receptors modulators | Ircinialactams | Australian sponges, family Irciinidae [108] |
| Neuronal growth inducers | Dysideamine | Indonesian marine sponge, genus Dysidea [109] |
| Neurotrophic-like agents | Linckosides | Okinawan starfish Linckia laevigata [110] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Catanesi, M.; Caioni, G.; Castelli, V.; Benedetti, E.; d’Angelo, M.; Cimini, A. Benefits under the Sea: The Role of Marine Compounds in Neurodegenerative Disorders. Mar. Drugs 2021, 19, 24. https://doi.org/10.3390/md19010024
Catanesi M, Caioni G, Castelli V, Benedetti E, d’Angelo M, Cimini A. Benefits under the Sea: The Role of Marine Compounds in Neurodegenerative Disorders. Marine Drugs. 2021; 19(1):24. https://doi.org/10.3390/md19010024
Chicago/Turabian StyleCatanesi, Mariano, Giulia Caioni, Vanessa Castelli, Elisabetta Benedetti, Michele d’Angelo, and Annamaria Cimini. 2021. "Benefits under the Sea: The Role of Marine Compounds in Neurodegenerative Disorders" Marine Drugs 19, no. 1: 24. https://doi.org/10.3390/md19010024
APA StyleCatanesi, M., Caioni, G., Castelli, V., Benedetti, E., d’Angelo, M., & Cimini, A. (2021). Benefits under the Sea: The Role of Marine Compounds in Neurodegenerative Disorders. Marine Drugs, 19(1), 24. https://doi.org/10.3390/md19010024