New Haloterpenes from the Marine Red Alga Laurencia papillosa: Structure Elucidation and Biological Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Working up and Structure Identification
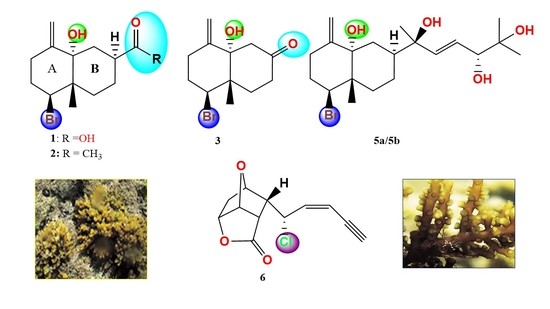
2.1.1. Aplysiolic Acid
2.1.2. 7-Acetyl-aplysiol
2.1.3. Aplysiol-7-one
2.1.4. 11,14-Dihydroaplysia-5,11,14,15-tetrols
2.1.5. 5-epi-Maneolactone
2.2. Biological Activities
3. Materials and Methods
3.1. General Procedures
3.2. Collection and Taxonomy of the Marine Alga
3.3. Extraction and Isolation of the Bioactive Constituents
3.4. Antimicrobial Assay
3.5. Ab Initio Calculations
3.6. Crystal Structure Determination of 7-acetylaplysiol (2) and 5-epi-maneolactone (6)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Author Statement
Abbreviations
References
- Pereira, R.C.; Dagama, B.A.P.; Teixeira, V.L.; Yoneshigue-Valentin, Y. Ecological roles of natural products of the Brazilian red seaweed Laurencia obtusa. Braz. J. Biol. 2003, 63, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Vairappan, C.S.; Daitoh, M.; Suzuki, M.; Abe, T.; Masuda, M. Antibacterial halogenated metabolites from the Malaysian Laurencia species. Phytochemistry 2001, 58, 291–297. [Google Scholar] [CrossRef]
- Vairappan, C.S.; Kawamoto, T.; Miwa, H.; Suzuki, M. Potent antibacterial activity of halogenated compounds against antibiotic-resistant bacteria. Planta Med. 2004, 70, 1087–1090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manilal, A.; Sujith, S.; Selvin, J.; Kiran, G.S.; Shakir, C.; Gandhimathi, R.; Panikkar, M.V.N. Biopotentials of seaweeds collected from southwest coast of India. J. Mar. Sci. Technol. 2009, 17, 67–73. [Google Scholar]
- Shanmughapriya, S.; Manilal, A.; Sujith, S.; Selvin, J.; Kiran, G.S.; Natarajaseenivasan, K. Antimicrobial activity of seaweeds extracts against multi-resistant pathogens. Ann. Microbiol. 2008, 58, 535–541. [Google Scholar] [CrossRef]
- El Sayed, K.A.; Dunbar, D.C.; Perry, T.L.; Wilkins, S.P.; Hamann, M.T.; Greenplate, J.T.; Wideman, M.A. Marine natural products as prototype insecticidal agents. J. Agric. Food Chem. 1997, 45, 2735–2739. [Google Scholar] [CrossRef]
- Koenig, G.M.; Wright, A.D. Sesquiterpene content of the antibacterial dichloromethane extract of the marine red alga Laurencia obtusa. Planta Med. 1997, 63, 186–187. [Google Scholar] [CrossRef]
- Sakemi, S.; Higa, T.; Jefford, C.W.; Bernardinelli, G.; Venustatriol, G. A new anti-viral triterpene tetracyclic ether from Laurencia venusta. Tetrahedron Lett. 1986, 27, 4287–4290. [Google Scholar] [CrossRef]
- De Oliveira, A.L.L.; de Felício, R.; Debonsi, H.M. Marine natural products: Chemical and biological potential of seaweeds and their endophytic fungi. Rev. Bras. Farmacogn. 2012, 22, 906–920. [Google Scholar] [CrossRef] [Green Version]
- Davyt, D.; Fernandez, R.; Suescun, L.; Mombrú, A.W.; Saldanña, J.; Dominguez, L.; Fujii, M.T.; Manta, E. Bisabolanes from the red alga Laurencia scoparia. J. Nat. Prod. 2006, 69, 1113–1116. [Google Scholar] [CrossRef]
- Ji, N.Y.; Li, X.M.; Cui, X.M.; Wang, B.G. Two new brominated diterpenes from Laurencia decumbens. Chin. Chem. Lett. 2007, 18, 957–959. [Google Scholar] [CrossRef]
- Iliopoulou, D.; Mihopoulos, N.; Vagias, C.; Papazafiri, P.; Roussis, V. Novel cytotoxic brominated diterpenes from the red alga Laurencia obtusa. J. Org. Chem. 2003, 68, 7667–7674. [Google Scholar] [CrossRef] [PubMed]
- Lyakhova, E.G.; Kalinovsky, A.I.; Dmitrenok, A.S.; Kolesnikova, S.A.; Fedorov, S.N.; Vaskovsky, V.E.; Stonik, V.A. Structures and absolute stereochemistry of nipponallene and neonipponallene, new brominated allenes from the red alga Laurencia nipponica. Tetrahedron Lett. 2006, 47, 6549–6552. [Google Scholar] [CrossRef]
- Kladi, M.; Vagias, C.; Stavri, M.; Rahman, M.M.; Gibbons, S.; Roussis, V. C15 acetogenins with antistaphylococcal activity from the red alga Laurencia glandulifera. Phytochem. Lett. 2008, 1, 31–36. [Google Scholar] [CrossRef]
- Ojika, M.; Yoshida, Y.; Okumura, M.; Ieda, S.; Yamada, K. Aplysiadiol, a new brominated diterpene from the marine mollusk Aplysia kurodai. J. Nat. Prod. 1990, 53, 1619–1622. [Google Scholar] [CrossRef]
- Vairappan, C.S.; Ishii, T.; Lee, T.K.; Suzuki, M.; Zhaoqi, Z. Antibacterial Activities of a New Brominated Diterpene from Borneon Laurencia spp. Mar. Drugs 2010, 8, 1743–1749. [Google Scholar] [CrossRef]
- Waraszkiewicz, S.M.; Sun, H.H.; Erickson, K.L.; Finer, J.; Clardy, J. C15 halogenated compounds from the Hawaiian marine alga Laurencia nidifica. Maneonenes and isomaneonenes. J. Org. Chem. 1978, 43, 3194–3204. [Google Scholar] [CrossRef]
- Ayyad, S.-E.N.; Al-Footy, K.O.; Alarif, W.M.; Sobahi, T.R.; Bassaif, S.A.; Makki, M.S.; Asiri, A.M.; Halwani, A.A.Y.; Badria, A.F.; Badria, F.A.-R. Bioactive C15 acetogenins from the red alga Laurencia obtusa. Chem. Pharm. Bull. 2011, 59, 1294–1298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, N.-Y.; Li, X.-M.; Xie, H.; Ding, J.; Lia, K.; Ding, L.-P.; Wang, B.-G. Highly Oxygenated Triterpenoids from the Marine Red Alga Laurencia mariannensis (Rhodomelaceae). Helv. Chim. Acta 2008, 91, 1940–1946. [Google Scholar] [CrossRef]
- Ji, N.-Y.; Li, X.-M.; Li, K.; Ding, L.-P.; Gloer, J.B.; Wang, B.-G. Diterpenes, Sesquiterpenes, and a C15-Acetogenin from the Marine Red Alga Laurencia Mariannensis. J. Nat. Prod. 2007, 70, 1901–1905. [Google Scholar] [CrossRef]
- Takahashi, Y.; Suzuki, M.; Abe, T.; Masuda, M. Anhydroaplysiadiol from Laurencia japonensis. Phytochemistry 1998, 48, 987–990. [Google Scholar] [CrossRef]
- Zhao, Y.; Yue, J.-M.; He, Y.-N.; Lin, Z.-W.; Sun, H.-D. Eleven New Eudesmane Derivatives from Laggera pterodonta. J. Nat. Prod. 1997, 60, 545–549. [Google Scholar] [CrossRef]
- Sun, H.H.; Waraszkiewicz, S.M.; Erickson, K.L.; Finer, K.L.; Clardy, J. Dictyoxepin and Dictyolene, Two New Diterpenes from the Marine Alga Dictyota acutiloba (Phaeophyta). J. Am. Chem. Soc. 1977, 99, 3516–3517. [Google Scholar] [CrossRef] [PubMed]
- Bowden, B.F.; Coll, J.C.; Liyanage, N.; Mitcell, S.J.; Stokie, G.J.; Blount, J.F. Studies of Australian soft corals. VIII. A chemical and crystallographic study of a novel bicyclic diterpene alcohol with a rearranged skeleton from an unknown species of soft coral. Aust. J. Chem. 1978, 31, 2039–2047. [Google Scholar] [CrossRef]
- Poet, S.E.; Ravi, B.N. Three new diterpenes from a soft coral Nephthea species. Aust. J. Chem. 1982, 35, 77–83. [Google Scholar] [CrossRef]
- Goll, J.C.; Bowden, B.F.; Konig, G.M.; Braslau, R.; Price, I.R. Studies of Australian Soft Corals. The Natural Products Chemistry of Alcyonacean Soft Corals with Special Reference to the Genus Lobophytum. Bull. Soc. Chim. Belg. 1986, 95, 815. [Google Scholar]
- Laatsch, H. AntiBase: The Natural Compound Identifier; Wiley-VCH Verlag: Weinheim, Germany, 2017. [Google Scholar]
- Vairappan, C.S.; Suzuki, M.; Abe, T.; Masuda, M. Halogenated metabolites with antibacterial activity from the Okinawan Laurencia species. Phytochemistry 2001, 58, 517–523. [Google Scholar] [CrossRef]
- Lindel, T.; Junker, J.; Koeck, M. 2D-NMR-guided constitutional analysis of organic compounds employing the computer program COCON. Eur. J. Org. Chem. 1999, 1999, 573–577. [Google Scholar] [CrossRef]
- Nasr, A.H. The Marine Algae of Alexandria. 1—A Report on Some Marine Algae Collected from the Vicinity of Alexandria; Notes and Memoirs No. 36; Government Press: Bulaq, Cairo, 1940; p. 33. [Google Scholar]
- Abou-El Wafa, G.S.E.; El-Naggar, M.E.E. Studies on the Biological Activities of Some Species of Egyptian Marine Algae, Private Communication; Mansoura University: El-Mansoura, Egypt, 2005. [Google Scholar]
- Burkholder, P.R.; Burkholder, L.M.; Almodovar, L.R. Antibiotic activity of some marine algae of Puerto Rico. Bot. Mar. 1960, 2, 149–156. [Google Scholar] [CrossRef]
- Sajid, I.; Fondja, Y.C.B.; Shaaban, K.A.; Hasnain, S.; Laatsch, H. Antifungal and antibacterial activities of indigenous Streptomyces isolates from saline farmlands: Prescreening, ribotyping and metabolic diversity. World J. Microbiol. Biotechnol. 2009, 25, 601–610. [Google Scholar] [CrossRef]
- Shaaban, K.A.; Shaaban, M.; Rahman, H.; Grün-Wollny, I.; Laatsch, H. Karamomycins, A.-C: Novel 2-Naphthalen-2-yl-thiazoles from Nonomuraea endophytica sp. J. Nat. Prod. 2019, 82, 870–877. [Google Scholar] [CrossRef] [PubMed]

 , 4J
, 4J  ) and HMBC (
) and HMBC (  ,
,  ) correlations of 1–3, 5a.
) correlations of 1–3, 5a.
 = front side, β-orientation,
= front side, β-orientation,  = weak);
= weak);  = backside, α-orientation;) of all-(S)-aplysiolic acid (1); proton shifts = values at the atoms. For atom distances, see Table S1.
= backside, α-orientation;) of all-(S)-aplysiolic acid (1); proton shifts = values at the atoms. For atom distances, see Table S1.
 = front side, β-orientation,
= front side, β-orientation,  = weak);
= weak);  = backside, α-orientation;) of all-(S)-aplysiolic acid (1); proton shifts = values at the atoms. For atom distances, see Table S1.
= backside, α-orientation;) of all-(S)-aplysiolic acid (1); proton shifts = values at the atoms. For atom distances, see Table S1.
 = front side, β-orientation
= front side, β-orientation  = weak;
= weak;  = backside, α-orientation;
= backside, α-orientation;  geminal correlations) of all-(S)-7-acetyl-aplysiol (2) and all-(S)-aplysiol-7-one (3).
geminal correlations) of all-(S)-7-acetyl-aplysiol (2) and all-(S)-aplysiol-7-one (3).
 = front side, β-orientation
= front side, β-orientation  = weak;
= weak;  = backside, α-orientation;
= backside, α-orientation;  geminal correlations) of all-(S)-7-acetyl-aplysiol (2) and all-(S)-aplysiol-7-one (3).
geminal correlations) of all-(S)-7-acetyl-aplysiol (2) and all-(S)-aplysiol-7-one (3).

 ) and selected HMBC (
) and selected HMBC (  ) correlations of 5-epi-maneolactone (6, left), (b) NOESY connectivities (
) correlations of 5-epi-maneolactone (6, left), (b) NOESY connectivities (  ) of 5-epi-maneolactone (6, right).
) of 5-epi-maneolactone (6, right).

 ) and selected HMBC (
) and selected HMBC (  ) correlations of 5-epi-maneolactone (6, left), (b) NOESY connectivities (
) correlations of 5-epi-maneolactone (6, left), (b) NOESY connectivities (  ) of 5-epi-maneolactone (6, right).
) of 5-epi-maneolactone (6, right).

| Aplysiolic Acid (1) | 7-Acetyl-aplysiol (2) | Aplysiol-7-one (3) | Dihydroaplysiatetrol (5a) | 5-epi-Maneolactone (6) | |
|---|---|---|---|---|---|
| Appearance | colorless solid | colorless solid | colorless solid | colorless oil | colorless solid |
| Rf | 0.26 a | 0.28 b | 0.39 b | 0.30 a | 0.50 b |
| Anisaldehyde/ sulfuric acid | pink, turning later to violet | pink, turning later to violet | pink, turning later to violet | pink, turning later to violet | brownish gray |
| Molecular formula | C13H19BrO3 | C14H21BrO2 | C12H17BrO2 | C20H33BrO4 | C12H11ClO3 |
| (+)-ESIMS: m/z (%) | 323/325 [M + Na]+ (100:95.4), 623/625/627 [2M + Na]+, (10:31:10) | 296/298 [M + Na]+ (88:100), 569 [2M + Na]+ | 439/441 [M + Na]+ (100:97), 855/857/859 [2M + Na]+, (20:57:19) | 261/263 [M + Na]+ (100:31), 499 [2M + Na]+ (4) | |
| (−)-ESIMS: m/z (%) | 301/303 [M − H]– (100:90.5), 603/605/607 [2M − H]–(37:57:21) | 335/337/339 [M + Cl]− (69:100:25) | 461/463 [M + 2Na − 3H]− (100:97), 831/833/855 [2M − H]–, (25:52:27) | ||
| (+)-ESIHRMS: (m/z) | 323.0615 [M + Na]+ (calc. 323.0617 for C14H21BrNaO2), 625.1326 [2M + Na]+ (calc. 625.1327 for C28H42Br2NaO4) | 439.1454 [M + Na]+ (calc. 439.1454 for C20H33BrO4Na) | 261.0299 [M + Na]+ (calc. 261.0289 for C12H11ClO3Na) | ||
| (−)-ESIHRMS (m/z) | 301.0441 [M − H]- (calc. 301.0444 for C13H18BrO3) | 335.0418 [M + H]– (calc. 335.0418 for C14H21BrClO2) | |||
| IR (KBr) ν cm−1 | 3259, 2361, 2182, 1782, 1592, 1358, 1160, 1018, 895, 841, 794, 665 | ||||
| [α]20D (MeOH) | −34.8 (c, 0.13) | −55.4 (c, 0.24) | − 31.9 (c, 0.12) | −155.8 (c, 0.26) |
| Nr. | 1 | 2 | 3 | |||
|---|---|---|---|---|---|---|
| δC | δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) | |
| 1 | 62.9 | 4.72 (dd, 12.4, 4.7) | 63.2 | 4.69 (dd, 12.3, 4.7) | 61.5 | 4.67 (dd, 12.7, 4.5) |
| 2 | 33.9 | 2.20 (m), 2.13 (m) | 33.9 | 2.15 (m), 2.08 (m) | 34.0 | 2.24 (m), 2.09 (m) |
| 3 | 32.5 | 2.70 (dddd, 13.5, 9.6, 5.7, 2.1)2.15 (m) | 32.5 | 2.11 (m), 2.67 (tdt, 13.0, 5.6, 2.0) | 32.1 | 2.64 (ddd, 13.6, 5.0, 2.8), 2.17 (ddd, 13.6, 5.0, 2.0) |
| 4 | 148.5 | 148.6 | 147.7 | |||
| 5 | 76.2 | 76.3 | 79.6 | |||
| 6 | 34.1 | 2.04 (dd, 13.8, 12.8), 1.83 (m) | 33.1 | 1.65 (ddd, 14.0, 3.9, 1.3), 1.91 (m) | 48.5 | 2.86 (d, 14.6), 2.38 (m) |
| 7 | 38.2 | 2.94 (tt, 12.9, 4.1) | 46.3 | 2.90 (tt, 12.7, 4.0) | 209.9 | |
| 8 | 23.5 | 1.90 (m), 1.66 (m) | 23.4 | 1.80 (m), 1.45 (m) | 37.5 | 2.41 (m) |
| 9 | 32.0 | 1.77 (m) | 32.2 | 1.74 (m) | 33.3 | 2.08 (m) |
| 10 | 43.0 | 43.0 | 43.4 | |||
| 11 | 181.0 | 211.8 | 110.9 | 4.94 (d, 2.0), 4.74 (d, 1.5) | ||
| 12 | 14.8 | 0.96 (s) | 14.7 | 0.89 (s) | 14.8 | 1.18 (s) |
| 13 | 110.2 | 4.90 (dd, 2.0, 1.0), 4.81 (d, 1.5) | 110.0 | 4.86 (m), 4.79 (t, 1.3) | ||
| 14 | - | 28.3 | 2.13 (s) | |||
| Nr. | 5a | Epimer 5b | ||
|---|---|---|---|---|
| δC | δH (J in Hz) | δC | δH (J in Hz) | |
| 1 | 65.23 | 4.72 (m) | 65.23 | 4.72 (m) |
| 2 | 35.61 | 2.14 (m), 2.10 (m) | 35.61 | 2.13 (m), 2.08 (m) |
| 3 | 33.69 | 2.77 (m), 2.12 (m) | 33.69 | 2.77 (m), 2.09 (m) |
| 4 | 151.64 | 151.61 | ||
| 5 | 77.28 | 77.27 | ||
| 6 | 32.75 | 1.66 (m), 1.75 (m) | 32.75 | 1.66 (m), 1.73 |
| 7 | 43.48 | 1.95 (m) | 43.65 | 1.95 (m) |
| 8 | 22.22 | 1.33, 1.67 (m) | 22.93 | 1.33, 1.60 (m) |
| 9 | 34.03 | 1.68 (m) | 33.96 | 1.67 (m) |
| 10 | 44.17 | 44.17 | ||
| 11 | 75.39 | 75.38 | ||
| 12 | 139.83 | 5.79 (d, 16.3) | 140.46 | 5.77 (d, 15.6) |
| 13 | 128.08 | 5.74 (dd, 15–16, 6–7) | 127.95 | 5.71 (dd, 15–16, 6–7) |
| 14 | 80.33 | 3.85 (m) | 80.39 | 3.86 (m) |
| 15 | 73.69 | 73.66 | ||
| 16 | 25.97 | 1.15 (s) | 26.07 | 1.15 (s) |
| 17 | 25.11 | 1.15 (s) | 25.16 | 1.16 (s) |
| 18 | 26.30 | 1.27 (s) | 26.22 | 1.27 (s) |
| 19 | 15.21 | 0.89 (s) | 15.21 | 0.891 (s) |
| 20 | 109.15 | 4.84 (s), 4.74 (s) | 109.17 | 4.83 (s), 4.75 (s) |
| Nr. | δC | δH (J in Hz) |
|---|---|---|
| 1 | 86.5 | 3.36 (dd, 2.4, 1.0) |
| 2 | 78.2 | |
| 3 | 113.8 | 5.71 (ddd, 10.5, 2.5, 0.5) |
| 4 | 139.2 | 6.01 (ddd, 10.5, 9.5, 1.1) |
| 5 | 55.3 | 4.81 (m) |
| 6 | 52.9 | 2.83 (dddd, 12.1, 10.4, 4.1, 1.7) |
| 7 | 80.0 | 4.83 (m) |
| 8 | 33.9 | 2.11 (d,18.0), 2.02 (m) |
| 9 | 78.9 | 4.85 (m) |
| 10 | 84.1 | 5.36 (td, 5.0, 0.9) |
| 11 | 42.9 | 2.71 (ddd, 10.2, 5.1, 0.8) |
| 12 | 173.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaaban, M.; Abou-El-Wafa, G.S.E.; Golz, C.; Laatsch, H. New Haloterpenes from the Marine Red Alga Laurencia papillosa: Structure Elucidation and Biological Activity. Mar. Drugs 2021, 19, 35. https://doi.org/10.3390/md19010035
Shaaban M, Abou-El-Wafa GSE, Golz C, Laatsch H. New Haloterpenes from the Marine Red Alga Laurencia papillosa: Structure Elucidation and Biological Activity. Marine Drugs. 2021; 19(1):35. https://doi.org/10.3390/md19010035
Chicago/Turabian StyleShaaban, Mohamed, Ghada S. E. Abou-El-Wafa, Christopher Golz, and Hartmut Laatsch. 2021. "New Haloterpenes from the Marine Red Alga Laurencia papillosa: Structure Elucidation and Biological Activity" Marine Drugs 19, no. 1: 35. https://doi.org/10.3390/md19010035
APA StyleShaaban, M., Abou-El-Wafa, G. S. E., Golz, C., & Laatsch, H. (2021). New Haloterpenes from the Marine Red Alga Laurencia papillosa: Structure Elucidation and Biological Activity. Marine Drugs, 19(1), 35. https://doi.org/10.3390/md19010035