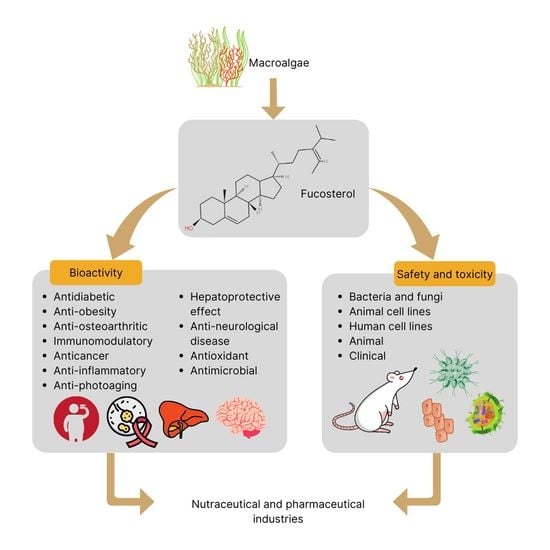
Fucosterol of Marine Macroalgae: Bioactivity, Safety and Toxicity on Organism
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characteristics and Structure of Fucosterol
2.2. Bioactivity of Fucosterol
2.2.1. Antidiabetic Activity
2.2.2. Anti-Obesity Activity
2.2.3. Anti-Osteoarthritis Activity
2.2.4. Immunomodulatory Activity
2.2.5. Anticancer Properties
2.2.6. Anti-Inflammatory Activity
2.2.7. Anti-Photoaging Effect
2.2.8. Hepatoprotective Effect
2.2.9. Anti-Neurological Disease
2.2.10. Antioxidant Activity
2.2.11. Antimicrobial Activity
2.3. Safety and Toxicity of Fucosterol in Bacteria and Fungi
2.4. Safety and Toxicity of Fucosterol in Cell Lines
2.5. Safety and Toxicity of Fucosterol in Animals
3. Materials and Methods
3.1. Literature Search
3.2. Selection Criteria
3.3. Data Extraction
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Hannan, M.A.; Sohag, A.A.M.; Dash, R.; Haque, M.N.; Mohibbullah, M.; Oktaviani, D.F.; Hossain, M.T.; Choi, H.J.; Moon, I.S. Phytosterols of marine algae: Insights into the potential health benefits and molecular pharmacology. Phytomedicine 2020, 69, 153201. [Google Scholar] [CrossRef]
- Heilbron, I.; Phipers, R.F.; Wright, H.R. The chemistry of the algae. Part I. The algal sterol fucosterol. J. Chem. Soc. 1934, 1572–1576. [Google Scholar] [CrossRef]
- Lopes, G.; Sousa, C.; Bernardo, J.; Andrade, P.B.; Valentão, P.; Ferreres, F.; Mouga, T. Sterol profiles in 18 macroalgae of the portuguese coast. J. Phycol. 2011, 47, 1210–1218. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Mahomoodally, M.F.; Sadeer, N.B.; Keum, Y.S.; Rengasamy, K.R. Characterization of nutritionally important lipophilic constituents from brown kelp Ecklonia radiata (C. Ag.) J. Agardh. Food Chem. 2021, 340, 127897. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Machado, D.I.; López-Hernández, J.; Paseiro-Losada, P.; López-Cervantes, J. An HPLC method for the quantification of sterols in edible seaweeds. Biomed. Chromatogr. 2004, 18, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Terasaki, M.; Hirose, A.; Narayan, B.; Baba, Y.; Kawagoe, C.; Yasui, H.; Saga, N.; Hosokawa, M.; Miyashita, K. Evaluation of recoverable functional lipid components of several brown seaweeds (phaeophyta) from Japan with special reference to fucoxanthin and fucosterol contents1. J. Phycol. 2009, 45, 974–980. [Google Scholar] [CrossRef]
- Kılınç, B.; Cirik, S.; Turan, G.; Tekogul, H.; Koru, E. Seaweed for Food and Industrial Application. In Food Industry; IntechOpen: London, UK, 2013; pp. 735–748. [Google Scholar]
- McHugh, D.J. Seaweeds uses as Human Foods; Food and Agriculture Organization of United Nations: Rome, Italy, 2003; ISBN 92-5-104958-0. [Google Scholar]
- Choi, J.S.; Seo, H.J.; Lee, Y.R.; Kwon, S.J.; Moon, S.H.; Park, S.M.; Sohn, J.H. Characteristics and in vitro anti-diabetic properties of the Korean rice wine, Makgeolli fermented with Laminaria japonica. Prev. Nutr. Food Sci. 2014, 19, 98–107. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.S.; Lee, K.; Lee, B.B.; Kim, Y.C.; Cho, K.K.; Choi, I.S. Safety, functionality and stability tests of Ecklonia cava extract for dermal application. Toxicol. Environ. Health Sci. 2014, 6, 61–66. [Google Scholar] [CrossRef]
- Negara, B.F.S.P.; Sohn, J.H.; Kim, J.S.; Choi, J.S. Antifungal and larvicidal activities of phlorotannins from brown seaweeds. Mar. Drugs 2021, 19, 223. [Google Scholar] [CrossRef]
- Khan, M.N.A.; Yoon, S.J.; Choi, J.S.; Park, N.G.; Lee, H.H.; Cho, J.Y.; Hong, Y.K. Anti-edema effects of brown seaweed (Undaria pinnatifida) extract on phorbol 12-myristate 13-Acetate-Induced mouse ear inflammation. Am. J. Chin. Med. 2009, 37, 373–381. [Google Scholar] [CrossRef]
- Lee, S.; Yeon, S.L.; Sang, H.J.; Sam, S.K.; Kuk, H.S. Anti-oxidant activities of fucosterol from the marine algae Pelvetia siliquosa. Arch. Pharm. Res. 2003, 26, 719–722. [Google Scholar] [CrossRef]
- Jung, H.A.; Jin, S.E.; Ahn, B.R.; Lee, C.M.; Choi, J.S. Anti-inflammatory activity of edible brown alga Eisenia bicyclis and its constituents fucosterol and phlorotannins in LPS-stimulated RAW264.7 macrophages. Food Chem. Toxicol. 2013, 59, 199–206. [Google Scholar] [CrossRef]
- Lee, J.H.; Jung, H.A.; Kang, M.J.; Choi, J.S.; Kim, G. Do Fucosterol, isolated from Ecklonia stolonifera, inhibits adipogenesis through modulation of FoxO1 pathway in 3T3-L1 adipocytes. J. Pharm. Pharmacol. 2017, 69, 325–333. [Google Scholar] [CrossRef]
- Gan, S.Y.; Wong, L.Z.; Wong, J.W.; Tan, E.L. Fucosterol exerts protection against amyloid β-induced neurotoxicity, reduces intracellular levels of amyloid β and enhances the mRNA expression of neuroglobin in amyloid β-induced SH-SY5Y cells. Int. J. Biol. Macromol. 2019, 121, 207–213. [Google Scholar] [CrossRef]
- Mao, Z.; Shen, X.; Dong, P.; Liu, G.; Pan, S.; Sun, X.; Hu, H.; Pan, L.; Huang, J. Fucosterol exerts antiproliferative effects on human lung cancer cells by inducing apoptosis, cell cycle arrest and targeting of Raf/MEK/ERK signalling pathway. Phytomedicine 2019, 61, 152809. [Google Scholar] [CrossRef] [PubMed]
- Meinita, M.D.N.; Harwanto, D.; Sohn, J.H.; Kim, J.S.; Choi, J.S. Hizikia fusiformis: Pharmacological and Nutritional Properties. Foods 2021, 10, 1660. [Google Scholar] [CrossRef] [PubMed]
- Negara, B.F.P.S.; Sohn, J.H.; Kim, J.S.; Choi, J.S. Effects of Phlorotannins on Organisms: Focus on the Safety, Toxicity, and Availability of Phlorotannins. Foods. 2021, 10, 452. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.A.; Islam, M.N.; Lee, C.M.; Oh, S.H.; Lee, S.; Jung, J.H.; Choi, J.S. Kinetics and molecular docking studies of an anti-diabetic complication inhibitor fucosterol from edible brown algae Eisenia bicyclis and Ecklonia stolonifera. Chem. Biol. Interact. 2013, 206, 55–62. [Google Scholar] [CrossRef]
- Huh, G.W.; Lee, D.Y.; In, S.J.; Lee, D.G.; Park, S.Y.; Yi, T.H.; Kang, H.C.; Seo, W.D.; Baek, N.I. Fucosterols from Hizikia fusiformis and their proliferation activities on osteosarcoma-derived cell MG63. J. Korean Soc. Appl. Biol. Chem. 2012, 55, 551–555. [Google Scholar] [CrossRef]
- Tang, H.F.; Yi, Y.H.; Yao, X.S.; Xu, Q.Z.; Zhang, S.Y.; Lin, H.W. Bioactive steroids from the brown alga Sargassum carpophyllum. J. Asian Nat. Prod. Res. 2002, 4, 95–101. [Google Scholar] [CrossRef]
- Hwang, E.; Park, S.Y.; Sun, Z.W.; Shin, H.S.; Lee, D.G.; Yi, T.H. The Protective Effects of Fucosterol Against Skin Damage in UVB-Irradiated Human Dermal Fibroblasts. Mar. Biotechnol. 2014, 16, 361–370. [Google Scholar] [CrossRef]
- Yoon, N.Y.; Chung, H.Y.; Kim, H.R.; Choi, J.S. Acetyl- and butyrylcholinesterase inhibitory activities of sterols and phlorotannins from Ecklonia stolonifera. Fish. Sci. 2008, 74, 200–207. [Google Scholar] [CrossRef]
- Jung, H.A.; Jung, H.J.; Jeong, H.Y.; Kwon, H.J.; Kim, M.S.; Choi, J.S. Anti-adipogenic activity of the edible brown alga Ecklonia stolonifera and its constituent fucosterol in 3T3-L1 adipocytes. Arch. Pharm. Res. 2014, 37, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Bouzidi, N.; Viano, Y.; Ortalo-Magné, A.; Seridi, H.; Alliche, Z.; Daghbouche, Y.; Culioli, G.; El Hattab, M. Sterols from the brown alga Cystoseira foeniculacea: Degradation of fucosterol into saringosterol epimers. Arab. J. Chem. 2019, 12, 1474–1478. [Google Scholar] [CrossRef] [Green Version]
- Jung, H.A.; Bhakta, H.K.; Min, B.S.; Choi, J.S. Fucosterol activates the insulin signaling pathway in insulin resistant HepG2 cells via inhibiting PTP1B. Arch. Pharm. Res. 2016, 39, 1454–1464. [Google Scholar] [CrossRef] [PubMed]
- Seong, S.H.; Nguyen, D.H.; Wagle, A.; Woo, M.H.; Jung, H.A.; Choi, J.S. Experimental and Computational Study to Reveal the Potential of Non-Polar Constituents from Hizikia fusiformis as Dual Protein Tyrosine Phosphatase 1B and α-Glucosidase Inhibitors. Mar. Drugs 2019, 17, 302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.S.; Shin, K.H.; Kim, B.K.; Lee, S. Anti-diabetic activities of fucosterol from Pelvetia siliquosa. Arch. Pharm. Res. 2004, 27, 1120–1122. [Google Scholar] [CrossRef]
- Noureddin, M.; Mato, J.M.; Lu, S.C. Nonalcoholic fatty liver disease: Update on pathogenesis, diagnosis, treatment and the role of S-adenosylmethionine. Exp. Biol. Med. 2015, 240, 809–820. [Google Scholar] [CrossRef]
- Lee, D.G.; Park, S.Y.; Chung, W.S.; Park, J.H.; Shin, H.S.; Hwang, E.; Kim, I.H.; Yi, T.H. The bone regenerative effects of fucosterol in in vitro and in vivo models of postmenopausal osteoporosis. Mol. Nutr. Food Res. 2014, 58, 1249–1257. [Google Scholar] [CrossRef]
- Bang, M.H.; Kim, H.H.; Lee, D.Y.; Han, M.W.; Baek, Y.S.; Chung, D.K.; Baek, N.I. Anti-osteoporotic activities of fucosterol from sea mustard (Undaria pinnatifida). Food Sci. Biotechnol. 2011, 20, 343–347. [Google Scholar] [CrossRef]
- Mayer, A.M.S.; Rodríguez, A.D.; Taglialatela-Scafati, O.; Fusetani, N. Marine pharmacology in 2009–2011: Marine compounds with antibacterial, antidiabetic, antifungal, anti-inflammatory, antiprotozoal, antituberculosis, and antiviral activities; affecting the immune and nervous systems, and other miscellaneous mechanisms of action. Mar. Drugs 2013, 11, 2510–2573. [Google Scholar] [CrossRef]
- Park, S.; Hwang, E.; Shin, Y.; Lee, D.; Yang, J.; Park, J.; Yi, T. Immunostimulatory Effect of Enzyme-Modified Hizikia fusiforme in a Mouse Model In Vitro and Ex Vivo. Mar. Biotechnol. 2017, 19, 65–75. [Google Scholar] [CrossRef]
- Bae, H.; Lee, J.Y.; Song, G.; Lim, W. Fucosterol suppresses the progression of human ovarian cancer by inducing mitochondrial dysfunction and endoplasmic reticulum stress. Mar. Drugs 2020, 18, 261. [Google Scholar] [CrossRef]
- Kala, K.J.; Prashob Peter, K.J.; Chandramohanakumar, N. Cyto-toxic potential of fucosterol isolated from Turbinaria conoides against dalton’s lymphoma ascites. Int. J. Pharmacogn. Phytochem. Res. 2015, 7, 1217–1221. [Google Scholar]
- Caamal-Fuentes, E.; Moo-Puc, R.; Freile-Pelegrín, Y.; Robledo, D. Cytotoxic and antiproliferative constituents from Dictyota ciliolata, Padina sanctae-crucis and Turbinaria tricostata. Pharm. Biol. 2014, 52, 1244–1248. [Google Scholar] [CrossRef] [Green Version]
- Khanavi, M.; Gheidarloo, R.; Sadati, N.; Shams Ardekani, M.R.; Bagher Nabavi, S.M.; Tavajohi, S.; Ostad, S.N. Cytotoxicity of fucosterol containing fraction of marine algae against breast and colon carcinoma cell line. Pharmacogn. Mag. 2012, 8, 60–64. [Google Scholar] [CrossRef] [Green Version]
- Jiang, H.; Li, J.; Chen, A.; Li, Y.; Xia, M.; Guo, P.; Yao, S.; Chen, S. Fucosterol exhibits selective antitumor anticancer activity against hela human cervical cell line by inducing mitochondrial mediated apoptosis, cell cycle migration inhibition and downregulation of m-TOR/PI3K/Akt signalling pathway. Oncol. Lett. 2018, 15, 3458–3463. [Google Scholar] [CrossRef] [PubMed]
- Prayong, P.; Barusrux, S.; Weerapreeyakul, N. Cytotoxic activity screening of some indigenous Thai plants. Fitoterapia 2008, 79, 598–601. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.J.; Yoon, W.J.; Kim, K.N.; Ahn, G.N.; Kang, S.M.; Kang, D.H.; Oh, C.; Jung, W.K.; Jeon, Y.J. Evaluation of anti-inflammatory effect of fucoxanthin isolated from brown algae in lipopolysaccharide-stimulated RAW 264.7 macrophages. Food Chem. Toxicol. 2010, 48, 2045–2051. [Google Scholar] [CrossRef] [PubMed]
- Yoo, M.S.; Shin, J.S.; Choi, H.E.; Cho, Y.W.; Bang, M.H.; Baek, N.I.; Lee, K.T. Fucosterol isolated from Undaria pinnatifida inhibits lipopolysaccharide- induced production of nitric oxide and pro-inflammatory cytokines via the inactivation of nuclear factor-κB and p38 mitogen-activated protein kinase in RAW264.7 macrophages. Food Chem. 2012, 135, 967–975. [Google Scholar] [CrossRef] [PubMed]
- Jayawardena, T.U.; Sanjeewa, K.K.A.; Lee, H.G.; Nagahawatta, D.P.; Yang, H.W.; Kang, M.C.; Jeon, Y.J. Particulate Matter-Induced Inflammation/Oxidative Stress in Macrophages: Fucosterol from Padina boryana as a Potent Protector, Activated via NF-κB/MAPK Pathways and Nrf2/HO-1 Involvement. Mar. Drugs 2020, 18, 628. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Jayawardena, T.U.; Kim, H.S.; Lee, W.W.; Vaas, A.P.J.P.; De Silva, H.I.C.; Abayaweera, G.S.; Nanayakkara, C.M.; Abeytunga, D.T.U.; Lee, D.S.; et al. Beijing urban particulate matter-induced injury and inflammation in human lung epithelial cells and the protective effects of fucosterol from Sargassum binderi (Sonder ex J. Agardh). Environ. Res. 2019, 172, 150–158. [Google Scholar] [CrossRef]
- Kim, M.S.; Oh, G.H.; Kim, M.J.; Hwang, J.K. Fucosterol inhibits matrix metalloproteinase expression and promotes type-1 procollagen production in UVB-induced HaCaT cells. Photochem. Photobiol. 2013, 89, 911–918. [Google Scholar] [CrossRef]
- Gossard, A.A.; Lindor, K.D. Autoimmune hepatitis: A review. J. Gastroenterol. 2012, 47, 498–503. [Google Scholar] [CrossRef]
- Choi, J.S.; Han, Y.R.; Byeon, J.S.; Choung, S.Y.; Sohn, H.S.; Jung, H.A. Protective effect of fucosterol isolated from the edible brown algae, Ecklonia stolonifera and Eisenia bicyclis, on tert-butyl hydroperoxide- and tacrine-induced HepG2 cell injury. J. Pharm. Pharmacol. 2015, 67, 1170–1178. [Google Scholar] [CrossRef]
- Hoang, M.H.; Jia, Y.; Jun, H.J.; Lee, J.H.; Lee, B.Y.; Lee, S.J. Fucosterol is a selective liver X receptor modulator that regulates the expression of key genes in cholesterol homeostasis in macrophages, hepatocytes, and intestinal cells. J. Agric. Food Chem. 2012, 60, 11567–11575. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, J.; Fu, Z.; Ye, C.; Zhang, R.; Song, Y.; Zhang, Y.; Li, H.; Ying, H.; Liu, H. 24(S)-saringosterol from edible marine seaweed Sargassum fusiforme is a novel selective LXRβ agonist. J. Agric. Food Chem. 2014, 62, 6130–6137. [Google Scholar] [CrossRef] [PubMed]
- Mo, W.; Wang, C.; Li, J.; Chen, K.; Xia, Y.; Li, S.; Xu, L.; Lu, X.; Wang, W.; Guo, C. Fucosterol protects against concanavalin a-induced acute liver injury: Focus on P38 MAPK/NF-κ B pathway activity. Gastroenterol. Res. Pract. 2018, 2018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishwarya, M.; Narendhirakannan, R.T. The advances in neurobiology. Adv. Neurobiol. 2016, 12, 293–306. [Google Scholar] [CrossRef]
- Oh, J.H.; Choi, J.S.; Nam, T.J. Fucosterol from an edible brown alga Ecklonia stolonifera prevents soluble amyloid beta-induced cognitive dysfunction in aging rats. Mar. Drugs 2018, 16, 368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, H.A.; Ali, M.Y.; Choi, R.J.; Jeong, H.O.; Chung, H.Y.; Choi, J.S. Kinetics and molecular docking studies of fucosterol and fucoxanthin, BACE1 inhibitors from brown algae Undaria pinnatifida and Ecklonia stolonifera. Food Chem. Toxicol. 2016, 89, 104–111. [Google Scholar] [CrossRef]
- Wong, C.H.; Gan, S.Y.; Tan, S.C.; Gany, S.A.; Ying, T.; Gray, A.I.; Igoli, J.; Chan, E.W.L.; Phang, S.M. Fucosterol inhibits the cholinesterase activities and reduces the release of pro-inflammatory mediators in lipopolysaccharide and amyloid-induced microglial cells. J. Appl. Phycol. 2018, 30, 3261–3270. [Google Scholar] [CrossRef]
- Paudel, P.; Seong, S.H.; Jung, H.A.; Choi, J.S. Characterizing fucoxanthin as a selective dopamine D3/D4 receptor agonist: Relevance to Parkinson’s disease. Chem. Biol. Interact. 2019, 310, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Oktaviani, D.F.; Bae, Y.; Dyah, M.; Meinita, N.; Moon, I.S. An Ethanol Extract of the Brown Seaweed Hizikia fusiformis and Its Active Constituent, Fucosterol, Extend the Lifespan of the Nematode Caenorhabditis elegans. J. Life Sci. 2019, 29, 1120–1125. [Google Scholar] [CrossRef]
- Tyskiewicz, K.; Tyskiewicz, R.; Konkol, M.; Rój, E.; Jaroszuk-Sciseł, J.; Skalicka-Wozniak, K. Antifungal Properties of Fucus vesiculosus L. Supercritical Fluid Extract against Fusarium culmorum and Fusarium oxysporum. Molecules 2019, 24, 3518. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.S.; Kumar, Y.; Khan, M.S.Y.; Gupta, V. New antifungal steroids from Turbinaria conoides (J. Agardh) Kutzing. Nat. Prod. Res. 2010, 24, 1481–1487. [Google Scholar] [CrossRef]
- Rajendran, I.; Chakraborty, K.; Pananghat, V. Bioactive sterols from the brown alga Anthophycus longifolius (Turner) Kützing, 1849 (= Sargassum longifolium). Indian J. Fish. 2013, 60, 83–86. [Google Scholar]
- Fernando, I.P.S.; Jayawardena, T.U.; Kim, H.S.; Vaas, A.P.J.P.; De Silva, H.I.C.; Nanayakkara, C.M.; Abeytunga, D.T.U.; Lee, W.W.; Ahn, G.; Lee, D.S.; et al. A keratinocyte and integrated fibroblast culture model for studying particulate matter-induced skin lesions and therapeutic intervention of fucosterol. Life Sci. 2019, 233, 116714. [Google Scholar] [CrossRef]
- Sun, Z.; Mohamed, M.A.A.; Park, S.Y.; Yi, T.H. Fucosterol protects cobalt chloride induced inflammation by the inhibition of hypoxia-inducible factor through PI3K/Akt pathway. Int. Immunopharmacol. 2015, 29, 642–647. [Google Scholar] [CrossRef]
- Ramos, A.A.; Almeida, T.; Lima, B.; Rocha, E. Cytotoxic activity of the seaweed compound fucosterol, alone and in combination with 5-fluorouracil, in colon cells using 2D and 3D culturing. J. Toxicol. Environ. Heal.-Part A Curr. Issues 2019, 82, 537–549. [Google Scholar] [CrossRef]
- Ji, Y.B.; Ji, C.F.; Yue, L. Study on human promyelocytic leukemia HL-60 cells apoptosis induced by fucosterol. Biomed. Mater. Eng. 2014, 24, 845–851. [Google Scholar] [CrossRef]
- Malhão, F.; Ramos, A.A.; Rocha, E. Cytotoxic and Anti-Proliferative Effects of Fucosterol, Alone and in Combination with Doxorubicin, in 2D and 3D Cultures of Triple-Negative Breast Cancer Cells. Med. Sci. Forum 2021, 2, 8600. [Google Scholar] [CrossRef]
- Pacheco, B.S.; dos Santos, M.A.Z.; Schultze, E.; Martins, R.M.; Lund, R.G.; Seixas, F.K.; Colepicolo, P.; Collares, T.; Paula, F.R.; De Pereira, C.M.P. Cytotoxic activity of fatty acids from Antarctic macroalgae on the growth of human breast cancer cells. Front. Bioeng. Biotechnol. 2018, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhen, X.H.; Quan, Y.C.; Jiang, H.Y.; Wen, Z.S.; Qu, Y.L.; Guan, L.P. Fucosterol, a sterol extracted from Sargassum fusiforme, shows antidepressant and anticonvulsant effects. Eur. J. Pharmacol. 2015, 768, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Altman, D.; Antes, G.; Atkins, D.; Barbour, V.; Barrowman, N.; Berlin, J.A.; et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guiry, M.D.; Guiry, G.M. AlgaeBase. World-Wide Electronic Publication; National University of Ireland: Galway, UK, 2021; Available online: http://www.algaebase.org (accessed on 10 September 2021).






| Bacteria/ Fungi | Extract or Chemical | Sources | Method | Concentration | Toxicity | Ref. |
|---|---|---|---|---|---|---|
| Pyricularia oryzae | Fucosterol extract | Sargassum carpophyllum | Screening | Nd | Affected the morphology | [22] |
| Staphylococcus epidermidis | Fucosterol extract | Turbinaria conoides | Broth dilution susceptibility assay | 2–256 µg/mL | Inhibited bacteria growth | [58] |
| Aspergillus niger | Fucosterol extract | Turbinaria conoides | Broth dilution susceptibility assay | 2–256 µg/mL | Inhibited fungal growth | [58] |
| Candida albicans | Fucosterol extract | Turbinaria conoides | Broth dilution susceptibility assay | 2–256 µg/mL | Inhibited fungal growth | [58] |
| Escherichia coli | Fucosterol extract | Turbinaria conoides | Broth dilution susceptibility assay | 2–256 µg/mL | Inhibited bacteria growth | [58] |
| Staphylococcus aureus | Fucosterol extract | Turbinaria conoides | Broth dilution susceptibility assay | 2–256 µg/mL | Inhibited bacteria growth | [58] |
| Pseudomonas aeruginosa | Fucosterol extract | Turbinaria conoides | Broth dilution susceptibility assay | 2–256 µg/mL | Inhibited bacteria growth | [58] |
| Fusarium culmorum | Commercial fucosterol | Nd | Determined on a liquid RB medium | 0.05–1.0% | Inhibited fungal growth and caused total degradation | [57] |
| Cell Lines | Extract or Chemical | Sources | Method | Concentration | Toxicity | Ref. |
|---|---|---|---|---|---|---|
| RAW 264.7 macrophage cells | Fucosterol extract | Padina boryana | MMT assay and incubated for 23 h | 12.5, 25, and 50 µg/mL | Increased cell viability | [43] |
| A549 human lung epithelial cells exposed to CPM | Fucosterol extract | Sargassum aquifolium (formerly Sargassum binderi) | MMT assay and incubated for 24 h | 3.125, 6.25, 12.5, 25, 50, and 100 µg/mL | Low toxicity and increased cell viability | [44] |
| Chinese hamster ovary (CHO) cells, rat basophil leukemia (RBL) cells, U373 cells, and BA/F3 cells | Fucosterol extract | Undaria pinnatifida and Eisenia bicyclis | Human monoamine oxidase (hMAO) inhibition and functional assay | 500 μM | Had no effects on the viability of the cells | [55] |
| Human dermal fibroblasts (HDF) and HaCaT cells | Fucosterol extract | Sargassum aquifolium (formerly Sargassum binderi) | MMT assay and incubated for 3 h | 3.125, 6.25, 12.5, 25, 50, and 100 µg/mL | Not toxic and increased cell viability | [60] |
| Human recombinant PTP1B | Fucosterol extract | Sargassum fusiforme | Docking simulation | 0 to 2 mM | Inhibited PTP1B and α-glucosidase | [28] |
| RAW 264.7 macrophages | Fucosterol extract | Undaria pinnatifida | Western blot analysis | 10, 25, or 50 µM | Had no effects on the viability of the cells | [42] |
| Murine 3T3-L1 preadipocytes | Fucosterol extract | Ecklonia cava subsp. stolonifera (formerly Ecklonia stolonifera) | Western blot analysis | 25 and 50 µM | No significant effects up to 50 µM | [15] |
| β-Site amyloid precursor protein cleaving enzyme 1 (BACE1) | Fucosterol extract | Undaria pinnatifida and Ecklonia cava subsp. stolonifera (formerly Ecklonia stolonifera) | Kinetics and molecular docking simulation | 0, 5.0, 20, and 100 µM | Inhibited BACE1 and not toxic | [53] |
| Insulin-resistant HepG2 (human hepatocarcinoma) cells | Fucosterol extract | Ecklonia cava subsp. stolonifera (formerly Ecklonia stolonifera) | MMT assay and incubated for 2 h | 12.5, 25, 50, 100, and 200 µM | No significant effect up to 100 µM | [27] |
| HepG2 cells induced t-BHP and tacrine | Fucosterol extract | Ecklonia cava subsp. stolonifera (formerly Ecklonia stolonifera)and Eisenia bicyclis | MMT assay and incubated for 2 h | 0, 25, 50 and 100 μM | Low toxicity and increased cell viability | [47] |
| HaCaT cells induced cobalt chloride (CoCl2) | Fucosterol extract | Sargassum fusiforme (formerly Hizikia fusiformis) | MMT assay and incubated for 2 h | 1, 2, 5, and 10 μM | Low toxicity and increased cell viability | [61] |
| C8-B4 microglial cells | Fucosterol extract | Padina australis | MMT assay and incubated for 4 h | 12, 24, 48, 96, and 192 μM | Had no effects on the viability of the cells | [54] |
| Colon carcinoma (HT-29), colorectal adenocarcinoma (Caco-2), and breast ductal carcinoma (T47D) cell lines | Fucosterol extract | Sargassum angustifolium | MMT assay and incubated for 4 h | 4.5, 18, 36, and 72 μg/mL | Low toxicity | [38] |
| Oral carcinoma (KB), epithelial carcinoma of the larynx (Hep-2), MCF-7, cervix adenocarcinoma (SiHa), and a human cell embryonic kidney cell line (HEK-293) | Fucosterol extract | Dictyota ciliolataPadina sanctae-crucis, and Turbinaria tricostata | MMT assay and incubated for 2 h | Nd | Only inactive on HEK-293 | [37] |
| Dalton’s Lymphoma Ascites (DLA) cells | Fucosterol extract | Turbinaria conoides | Trypan blue viability assay | 100 and 200 μg/mL | Not toxic | [36] |
| Lung cancer cell and human normal cell | Commercial fucosterol | Nd | MMT assay and incubated for 24 h | 1.55, 3.12, 6.25, 12.5, 25, 50, and 100 µg/mL | Decreased cell viability in cancer cell and low toxicity in normal cell | [17] |
| Human cancer cell lines (HT29 and HCT116) and CCD-18Co fibroblasts | Commercial fucosterol | Nd | MMT assay and incubated for 24 h | 5 and 10 µM | Decreased cell viability in HT29 cells, but no effect in HCT116 and CCD-18Co | [62] |
| Human promyelocytic leukemia cells (HL-60) | Commercial fucosterol | Nd | MMT assay and incubated for 4 h | 7.55, 15.1, 30.2, 60.4, and 120.8 µM | Not toxic | [63] |
| Human ovarian cancer (ES2 and OV90) cells | Commercial fucosterol | Nd | 2′,7′-dichlorofluorescin diacetate assay | 0, 20, 40, 60, 80, and 100 µM | Not toxic | [35] |
| HaCaT cells and monkey kidney COS-7 cells | Commercial fucosterol | Nd | MMT assay and incubated for 3 h | 0.5, 1, and 5 μM | Had no effects on the viability of the cells | [45] |
| Animal | Extract or Chemical | Sources | Method | Concentration | Toxicity | Ref. |
|---|---|---|---|---|---|---|
| E18 aging rats | Fucosterol extract | Ecklonia cava subsp. stolonifera | Dorsal hippocampus injected by fucosterol for 4 weeks | 10 µmol/h | Increased the latency to reach the platform | [52] |
| C57BL/6 mice | Fucosterol extract | Sargassum fusiforme | Oral administration | 50, 100, and 200 mg/kg | Increased splenocyte proliferation and increased NO production with no cytotoxicity | [34] |
| Balb/e mice | Fucosterol extract | Sargassum fusiforme | Administered by via gastric intubation route | 0.1 mL/20 g of mouse | Not neurotoxic | [66] |
| Ovariectomized (OVX) rats | Fucosterol extract | Sargassum fusiforme | Oral, for 7 weeks beginning 12 weeks post-operation | 25, 50, and 100 mg/kg | Had no toxic effects | [31] |
| Caenorhabditis elegans | Fucosterol extract | Sargassum fusiforme | Measured on both NGM agar and broth containing fucosterol | Up to 0.1 mg/mL in 2% dimethyl sulfoxide (DMSO) | Low toxicity | [56] |
| Institute of Cancer Research (ICR) mice | Fucosterol extract | Ecklonia cava subsp. stolonifera and Eisenia bicyclis | Oral, for 3 consecutive days | 200 μL fucosterol (25, 50, and 100 mg/kg) | No mortality | [47] |
| BALB/c mice weighing | Commercial fucosterol | Nd | Oral, administered daily for 3 days | 25, 50, or 100 mg/kg | Inhibited ConA-induced acute liver injury significantly | [50] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meinita, M.D.N.; Harwanto, D.; Tirtawijaya, G.; Negara, B.F.S.P.; Sohn, J.-H.; Kim, J.-S.; Choi, J.-S. Fucosterol of Marine Macroalgae: Bioactivity, Safety and Toxicity on Organism. Mar. Drugs 2021, 19, 545. https://doi.org/10.3390/md19100545
Meinita MDN, Harwanto D, Tirtawijaya G, Negara BFSP, Sohn J-H, Kim J-S, Choi J-S. Fucosterol of Marine Macroalgae: Bioactivity, Safety and Toxicity on Organism. Marine Drugs. 2021; 19(10):545. https://doi.org/10.3390/md19100545
Chicago/Turabian StyleMeinita, Maria Dyah Nur, Dicky Harwanto, Gabriel Tirtawijaya, Bertoka Fajar Surya Perwira Negara, Jae-Hak Sohn, Jin-Soo Kim, and Jae-Suk Choi. 2021. "Fucosterol of Marine Macroalgae: Bioactivity, Safety and Toxicity on Organism" Marine Drugs 19, no. 10: 545. https://doi.org/10.3390/md19100545
APA StyleMeinita, M. D. N., Harwanto, D., Tirtawijaya, G., Negara, B. F. S. P., Sohn, J. -H., Kim, J. -S., & Choi, J. -S. (2021). Fucosterol of Marine Macroalgae: Bioactivity, Safety and Toxicity on Organism. Marine Drugs, 19(10), 545. https://doi.org/10.3390/md19100545