Applying Seaweed Compounds in Cosmetics, Cosmeceuticals and Nutricosmetics
Abstract
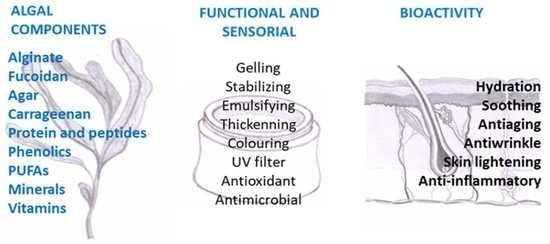
:1. Introduction
2. Seaweed Components and Bioactivity
2.1. Polysaccharides
2.2. Proteins, Peptides and Aminoacids
2.3. Phenolics and Terpenoids
2.4. Lipids
2.5. Vitamins
2.6. Minerals
2.7. Pigments
3. Technological Functions
3.1. Antimicrobial Agents
3.2. Antioxidants
3.3. Sensorial Properties
3.4. Texturizing
4. Bioactive Functions
4.1. Moisturization
4.2. Skin Whitening
4.3. UV Protection, Antioxidant and Antiaging
5. Patents
6. Conclusions and Future Trends
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Soulioti, I.; Diomidous, M.; Theodosopoulou, H.; Violaki, N.; Plessa, H.; Charalambidou, M.; Pistolis, J.; Plessas, S.T. Cosmetics: History, products, industry, legislation, regulations and implications in public health. Rev. Clin. Pharmacol. Pharmacokinet. 2013, 27, 5–15. [Google Scholar]
- Pangestuti, R.; Shin, K.-H.; Kim, S.-K. Anti-photoaging and potential skin health benefits of seaweeds. Mar. Drugs 2021, 19, 172. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, F.B.; Alves, R.C.; Rodrigues, F.; Oliveira, M.B.P.P. Macroalgae-Derived Ingredients for Cosmetic Industry—An Update. Cosmetics 2018, 5, 2. [Google Scholar] [CrossRef] [Green Version]
- Resende, D.I.S.P.; Ferreira, M.; Magalhães, C. Trends in the use of marine ingredients in anti-aging cosmetics. Algal Res. 2021, 55, 102273. [Google Scholar] [CrossRef]
- Aslam, A.; Bahadar, A.; Liaquat, R.; Saleem, M.; Waqas, A.; Zwawi, M. Algae as an attractive source for cosmetics to counter environmental stress. Sci. Total Environ. 2021, 772, 144905. [Google Scholar] [CrossRef]
- Preetha, J.P.; Karthika, K. Cosmeceuticals—An evolution. Int. J. Chemtech Res. 2009, 1, 1217–1223. [Google Scholar]
- Chaudhari, P.M.; Kawade, P.V.; Funne, S.M. Cosmeceuticals-a review. Int. J. Pharm. Technol. 2011, 3, 774–798. [Google Scholar]
- Draelos, Z.D. Cosmeceuticals: Efficacy and influence on skin tone. Dermatol. Clin. 2014, 32, 137–143. [Google Scholar] [CrossRef]
- Querellou, J.; Børresen, T.; Boyen, C.; Dobson, A.; Höfle, M.G.; Ianora, A.; Jaspars, M.; Kijjoa, A.; Olafsen, J.; Rigos, G.; et al. Marine Biotechnology: Realising the Full Potential of Europe. In EurOCEAN—Challenges for Marine Research in the Next Decade; McDonough, N., Ed.; VLIZ Special Publication: Oostende, Belgium, 2010; Volume 47, p. 21. [Google Scholar]
- Brandt, F.S.; Cazzaniga, A.; Hann, M. Cosmeceuticals: Current trends and market analysis. Semin. Cutan. Med. Surg. 2011, 30, 141–143. [Google Scholar] [CrossRef]
- Pham, A.K.; Dinulos, J.G. Cosmeceuticals for children: Should you care? Curr. Opin. Pediatr. 2014, 26, 446–451. [Google Scholar] [CrossRef]
- De Lacerda, D.; Thioly-Bensoussan, D.; Burke, K. Cosmeceuticals for Men. Available online: https://pubmed.ncbi.nlm.nih.gov/24308151/ (accessed on 28 September 2021).
- Draelos, Z.D. Cosmeceuticals for Male Skin. Dermatol. Clin. 2018, 36, 17–20. [Google Scholar] [CrossRef]
- Lin, T.J. Evolution of cosmetics: Increased need for experimental clinical medicine. J. Exp. Clin. Med. 2010, 2, 49–52. [Google Scholar] [CrossRef]
- Faria-Silva, C.; Ascenso, A.; Costa, A.M.; Marto, J.; Carvalheiro, M.; Ribeiro, H.M.; Simões, S. Feeding the skin: A new trend in food and cosmetics convergence. Trends Food Sci. Technol. 2020, 95, 21–32. [Google Scholar] [CrossRef]
- Pereira, L. Seaweeds as Source of Bioactive Substances and Skin Care Therapy—Cosmeceuticals, Algotheraphy, and Thalassotherapy. Cosmetics 2018, 5, 68. [Google Scholar] [CrossRef] [Green Version]
- Sotelo, C.G.; Blanco, M.; Ramos, P.; Vazquez, J.A.; Perez-Martin, R.I. Sustainable sources from aquatic organisms for cosmeceuticals ingredients. Cosmetics 2021, 8, 48. [Google Scholar] [CrossRef]
- Zárate, R.; Portillo, E.; Teixidó, S.; de Carvalho, M.A.A.P.; Nunes, N.; Ferraz, S.; Seca, A.M.L.; Rosa, G.P.; Barreto, M.C. Pharmacological and cosmeceutical potential of Seaweed Beach-casts of Macaronesia. Appl. Sci. 2020, 10, 5831. [Google Scholar] [CrossRef]
- Félix, R.; Carmona, A.M.; Félix, C.; Novais, S.C.; Lemos, M.F.L. Industry-friendly hydroethanolic extraction protocols for Grateloupia turuturu UV-shielding and antioxidant compounds. Appl. Sci. 2020, 10, 5304. [Google Scholar] [CrossRef]
- Balboa, E.M.; Soto, M.L.; Nogueira, D.R.; González-López, N.; Conde, E.; Moure, A.; Vinardell, M.P.; Mitjans, M.; Domínguez, H. Potential of antioxidant extracts produced by aqueous processing of renewable resources for the formulation of cosmetics. Ind. Crop. Prod. 2014, 58, 104–110. [Google Scholar] [CrossRef] [Green Version]
- Dolorosa, M.T.; Nurjanah; Purwaningsih, S.; Anwar, E. Utilization of Kappaphycus alvarezii and Sargassum plagyophyllum from Banten as cosmetic creams. IOP Conf. Ser. Earth Environ. Sci. 2019, 404, 012008. [Google Scholar] [CrossRef]
- Wahyuni, T. The Potential and Application of Eucheuma sp. For Solid Soap: A Review. IOP Conf. Ser. Earth Environ. Sci. 2021, 750, 012048. [Google Scholar] [CrossRef]
- Jesumani, V.; Du, H.; Aslam, M.; Pei, P.; Huang, N. Potential use of seaweed bioactive compounds in skincare—A review. Mar. Drugs 2019, 17, 688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jesumani, V.; Du, H.; Pei, P.; Zheng, C.; Cheong, K.-L.; Huang, N. Unravelling property of polysaccharides from Sargassum sp. as an anti-wrinkle and skin whitening property. Int. J. Biol. Macromol. 2019, 140, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Lordan, S.; Ross, R.P.; Stanton, C. Marine bioactives as functional food ingredients: Potential to reduce the incidence of chronic diseases. Mar. Drugs 2011, 9, 1056–1100. [Google Scholar] [CrossRef] [Green Version]
- Gellenbeck, K.W. Utilization of algal materials for nutraceutical and cosmeceutical applications—What do manufacturers need to know? J. Appl. Phycol. 2012, 24, 309–313. [Google Scholar] [CrossRef]
- Thomas, N.V.; Kim, S. Beneficial effects of marine algal compounds in cosmeceuticals. Mar. Drugs 2013, 11, 146–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.M.D.; Chen, C.C.; Huynh, P.; Chang, J.S. Exploring the potential of using algae in cosmetics. Biores. Technol. 2015, 184, 355–362. [Google Scholar] [CrossRef]
- Couteau, C.; Coiffard, L. Phycocosmetics and other marine cosmetics, specific cosmetics formulated using marine resources. Mar. Drugs 2020, 18, 322. [Google Scholar] [CrossRef]
- Kharkwal, H.; Joshi, D.; Panthari, P.; Pant, M.K.; Kharkwal, A.C. Algae as future drugs. Asian J. Pharm. Clin. Res. 2012, 5, 1–4. [Google Scholar]
- Senevirathne, W.S.M.; Kim, S.K. Cosmeceuticals from Algae. In Functional Ingredients from Algae for Foods and Nutraceuticals, 1st ed.; Domínguez, H., Ed.; Woodhead Publishing: Cambridge, UK, 2013; pp. 694–713. [Google Scholar]
- Vo, T.; Ngo, D.; Kang, K.; Jung, W.; Kim, S. The beneficial properties of marine polysaccharides in alleviation of allergic responses. Mol. Nutr. Food Res. 2015, 59, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Sharifi-Rad, J.; Seca, A.M.L.; Pinto, D.C.G.A.; Michalak, I.; Trincone, A.; Mishra, A.P.; Nigam, M.; Zam, W.; Martins, N. Current Trends on Seaweeds: Looking at Chemical Composition, Phytopharmacology, and Cosmetic Applications. Molecules 2019, 24, 4182. [Google Scholar] [CrossRef] [Green Version]
- Morais, T.; Cotas, J.; Pacheco, D.; Pereira, L. Seaweeds compounds: An ecosustainable source of cosmetic ingredients? Cosmetics 2021, 8, 8. [Google Scholar] [CrossRef]
- Bedoux, G.; Hardouin, K.; Burlot, A.S.; Bourgougnon, N. Bioactive components from seaweeds: Cosmetic applications and future development. Adv. Bot. Res. 2014, 71, 345–378. [Google Scholar]
- Agatonovic-Kustrin, S.; Morton, D.W. Cosmeceuticals derived from bioactive substances found in marine algae. J. Oceanogr. Mar. Res. 2013, 1, 106. [Google Scholar]
- Couteau, C.; Coiffard, L. Seaweed Application in Cosmetics in Seaweed. In Health and Disease Prevention; Fleurence., J., Levine, I., Eds.; Nikki Levy. Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 423–441. [Google Scholar]
- Kim, S.K.; Ravichandran, Y.D.; Khan, S.B.; Kim, Y.T. Prospective of the cosmeceuticals derived from marine organisms. Biotechnol. Bioproc. Engineer. 2008, 13, 511–523. [Google Scholar] [CrossRef]
- Kim, S.K. Review: Marine cosmeceuticals. J. Cosmet. Dermatol. 2014, 13, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Thiyagarasaiyar, K.; Goh, B.-H.; Jeon, Y.-J.; Yow, Y.-Y. Algae Metabolites in Cosmeceutical: An Overview of Current Applications and Challenges. Mar. Drugs 2020, 18, 323. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.B.A.; Adel, M.; Karimi, P.; Peidayesh, M. Pharmaceutical, cosmeceutical, and traditional applications of marine carbohydrates. Adv. Food Nutr. Res. 2014, 73, 197–220. [Google Scholar]
- Kim, J.H.; Lee, J.-E.; Kim, K.H.; Kang, N.J. Beneficial effects of marine algae-derived carbohydrates for skin health. Mar. Drugs 2018, 16, 459. [Google Scholar] [CrossRef] [Green Version]
- Shanura Fernando, I.P.; Asanka Sanjeewa, K.K.; Samarakoon, K.W.; Kim, H.S.; Gunasekara, U.K.D.S.S.; Park, Y.J.; Abeytungaa, D.T.U.; Lee, W.W.; Jeon, Y.-J. The potential of fucoidans from Chnoospora minima and Sargassum polycystum in cosmetics: Antioxidant, anti-inflammatory, skin-whitening, and antiwrinkle activities. J. Appl. Phycol. 2018, 30, 3223–3232. [Google Scholar] [CrossRef]
- Morya, V.; Kim, J.; Kim, E. Algal fucoidan: Structural and size-dependent bioactivities and their perspectives. Appl. Microbiol. Biotechnol. 2012, 93, 71–82. [Google Scholar] [CrossRef]
- Casas, M.P.; Rodríguez-Hermida, V.; Pérez-Larrán, P.; Conde, E.; Liveri, M.T.; Ribeiro, D.; Fernandes, E.; Domínguez, H. In vitro bioactive properties of phlorotannins recovered from hydrothermal treatment of Sargassum muticum. Sep. Purif. Technol. 2016, 167, 117–126. [Google Scholar] [CrossRef]
- Ruocco, N.; Costantini, S.; Guariniello, S.; Costantini, M. Polysaccharides from the marine environment with pharmacological, cosmeceutical and nutraceutical potential. Molecules 2016, 21, 551. [Google Scholar] [CrossRef]
- Lee, Y.-E.; Kim, H.; Seo, C.; Park, T.; Lee, K.B.; Yoo, S.-Y.; Hong, S.-C.; Kim, J.T.; Lee, J. Marine polysaccharides: Therapeutic efficacy and biomedical applications. Arch. Pharm. Res. 2017, 40, 1006–1020. [Google Scholar] [CrossRef]
- Sanjeewa, K.K.A.; Kang, N.; Ahn, G.; Jee, Y.; Kim, Y.T.; Jeon, Y.J. Bioactive potentials of sulfated polysaccharides isolated from brown seaweed Sargassum spp in related to human health applications: A review. Food Hydrocoll. 2018, 81, 200–208. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Kim, K.N.; Kim, D.; Jeon, Y.J. Algal polysaccharides: Potential bioactive substances for cosmeceutical applications. Crit. Rev. Biotechnol. 2019, 39, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Cikoš, A.M.; Jerković, I.; Molnar, M.; Šubarić, D.; Jokić, S. New trends for macroalgal natural products applications. Nat. Prod. Res. 2021, 35, 1180–1191. [Google Scholar] [CrossRef] [PubMed]
- Fournière, M.; Bedoux, G.; Lebonvallet, N.; Lescchiera, R.; Goff-Pain, C.L.; Bourgougnon, N.; Latire, T. Poly-and oligosaccharide ulva sp. Fractions from enzyme-assisted extraction modulate the metabolism of extracellular matrix in human skin fibroblasts: Potential in anti-aging dermo-cosmetic applications. Mar. Drugs 2021, 19, 156. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.; Brault, L.; Gasparotto, E.; Vallée, R.; Morvan, P.Y.; Ferrières, V.; Nugier-Chauvin, C. Formation of Amphiphilic Molecules from the Most Common Marine Polysaccharides, toward a Sustainable Alternative? Molecules 2021, 26, 4445. [Google Scholar] [CrossRef]
- Xu, S.-Y.; Kan, J.; Hu, Z.; Liu, Y.; Du, H.; Pang, G.-C.; Cheong, K.-L. Quantification of Neoagaro-Oligosaccharide Production through Enzymatic Hydrolysis and Its Anti-Oxidant Activities. Molecules 2018, 23, 1354. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Jin, W.; Duan, D.; Zhang, Q. Structural analysis and anti-complement activity of polysaccharides extracted from Grateloupia livida (Harv.) Yamada. J. Oceanol. Limnol. 2019, 37, 806–814. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Song, X.N.; Lin, Y.; Xiao, Q.; Du, X.P.; Chen, Y.H.; Xiao, A.F. Antioxidant capacity and prebiotic effects of Gracilaria neoagaro oligosaccharides prepared by agarase hydrolysis. Int. J. Biol. Macromol. 2019, 137, 177–186. [Google Scholar] [CrossRef]
- Sachan, N.K.; Pushkar, S.; Jha, A.; Bhattcharya, A. Sodium alginate: The wonder polymer for controlled drug delivery. J. Pharm. Res. 2009, 2, 1191–1199. [Google Scholar]
- Priyadarshani, I.; Rath, B. Commercial and industrial applications of micro algae—A review. J. Algal Biomass Util. 2012, 3, 89–100. [Google Scholar]
- Xue, C.; Yu, G.; Hirata, T.; Terao, J.; Lin, H. Antioxidative activities of several marine polysaccharides evaluated in a phosphatidylcholine-liposomal suspension and organic solvents. Biosci. Biotechnol. Biochem. 1998, 62, 206–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thevanayagam, H.; Mohamed, S.M.; Chu, W.-L. Assessment of UVB-photoprotective and antioxidative activities of carrageenan in keratinocytes. J. Appl. Phycol. 2014, 26, 1813–1821. [Google Scholar] [CrossRef]
- Sun, Z.; Mohamed, M.A.A.; Park, S.Y.; Yi, T.H. Fucosterol protects cobalt chloride induced inflammation by the inhibition of hypoxia-inducible factor through PI3K/Akt pathway. Int. Immunopharmacol. 2015, 29, 642–647. [Google Scholar] [CrossRef] [PubMed]
- Fernando, I.P.S.; Jayawardena, T.U.; Kim, H.S.; Vaas, A.; De Silva, H.I.C.; Nanayakkara, C.M.; Abeytunga, D.T.U.; Lee, W.; Ahn, G.; Lee, D.S.; et al. A keratinocyte and integrated fibroblast culture model for studying particulate matter-induced skin lesions and therapeutic intervention of fucosterol. Life Sci. 2019, 233, 116714. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.-T.; Zhang, X.; Liu, Y.; Chen, X.-Q.; Cheong, K.-L. Quantification of 3,6-anhydro-galactose in red seaweed polysaccharides and their potential skin-whitening activity. 3 Biotech 2020, 10, 189. [Google Scholar] [CrossRef]
- Senni, K.; Gueniche, F.; Foucault-Bertaud, A.; Igondjo-Tchen, S.; Fioretti, F.; Colliec-Jouault, S.; Durand, P.; Guezennec, J.; Godeau, G.; Letourneur, D. Fucoidan a sulfated polysaccharide from brown algae is a potent modulator of connective tissue proteolysis. Arch. Biochem. Biophys. 2006, 445, 56–64. [Google Scholar] [CrossRef] [Green Version]
- Moon, H.J.; Lee, S.H.; Ku, M.J.; Yu, B.C.; Jeon, M.J.; Jeong, S.H.; Stonik, V.A.; Zvyagintseca, T.N.; Ermakova, S.P.; Lee, Y.P. Fucoidan inhibits UVB-induced MMP-1 promoter expression and down regulation of type I procollagen synthesis in human skin fibroblasts. Eur. J. Dermatol. 2009, 19, 129–134. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Oh, J.-Y.; Lee, W.; Jeon, Y.-J. Fucoidan isolated from Hizikia fusiforme suppresses ultraviolet B-induced photodamage by down-regulating the expressions of matrix metalloproteinases and pro-inflammatory cytokines via inhibiting NF-κB, AP-1, and MAPK signaling pathways. Int. J. Biol. Macromol. 2021, 166, 751–759. [Google Scholar] [CrossRef]
- Su, W.; Wang, L.; Fu, X.; Ni, L.; Duan, D.; Xu, J.; Gao, X. Protective effect of a fucose-rich fucoidan isolated from Saccharina japonica against ultraviolet B-induced photodamage in vitro in human keratinocytes and in vivo in Zebrafish. Mar. Drugs 2020, 18, 316. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Jayawardena, T.U.; Yang, H.-W.; Lee, H.-G.; Jeon, Y.-J. The potential of sulfated polysaccharides isolated from the brown seaweed Ecklonia maxima in cosmetics: Antioxidant, anti-melanogenesis, and photoprotective activities. Antioxidants 2020, 9, 724. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, A.; Pereira, J.M.; Rioux, L.; Turgeon, S.L.; Beaulieu, M.; Moulin, V.J. Role of seaweed Laminaran from Saccharina longicruris on matrix deposition during dermal tissue-engineered production. Int. J. Biol. Macromol. 2015, 75, 13–20. [Google Scholar] [CrossRef]
- Ozanne, H.; Toumi, H.; Roubinet, B.; Landemarre, L.; Lespessailles, E.; Daniellou, R.; Cesaro, A. Laminarin Effects, a β-(1,3)-Glucan, on Skin Cell Inflammation and Oxidation. Cosmetics 2020, 7, 66. [Google Scholar] [CrossRef]
- Jiang, N.; Li, B.; Wang, X.; Xu, X.; Liu, X.; Li, W.; Chang, X.; Li, H.; Qi, H. The antioxidant and antihyperlipidemic activities of phosphorylated polysaccharide from Ulva pertusa. Int. J. Biol. Macromol. 2020, 145, 1059–1065. [Google Scholar] [CrossRef]
- Yu, B.; Bi, D.; Yao, L.; Li, T.; Gu, L.; Xu, H.; Li, X.; Li, H.; Hu, Z.; Xu, X. The inhibitory activity of alginate against allergic reactions in an ovalbumin-induced mouse model. Food Funct. 2020, 11, 2704–2713. [Google Scholar] [CrossRef]
- Szekalska, M.; Sosnowska, K.; Tomczykowa, M.; Winnicka, K.; Kasacka, I.; Tomczyk, M. In vivo anti-inflammatory and anti-allergic activities of cynaroside evaluated by using hydrogel formulations. Biomed. Pharmacother. 2020, 121, 109681. [Google Scholar] [CrossRef]
- Holdt, S.L.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Xing, M.; Cao, Q.; Wang, Y.; Xiao, H.; Zhao, J.; Zhang, Q.; Ji, A.; Song, S. Advances in Research on the Bioactivity of Alginate Oligosaccharides. Mar. Drugs 2020, 18, 144. [Google Scholar] [CrossRef] [Green Version]
- Deville, C.; Gharbi, M.; Dandrifosse, G.; Peulen, O. Study on the effects of laminarin, a polysaccharide from seaweed, on gut characteristics. J. Sci. Food Agric. 2007, 87, 1717–1725. [Google Scholar] [CrossRef]
- Shannon, E.; Conlon, M.; Hayes, M. Seaweed Components as Potential Modulators of the Gut Microbiota. Mar. Drugs 2021, 19, 358. [Google Scholar] [CrossRef]
- Choi, J.; Ha, Y.; Joo, C.; Cho, K.K.; Kim, S.; Choi, I.S. Inhibition of oral pathogens and collagenase activity by seaweed extracts. J. Environ. Biol. 2012, 33, 115–121. [Google Scholar]
- Gómez-Mascaraque, L.G.; Martínez-Sanz, M.; Martínez-López, R.; Martínez-Abad, A.; Panikuttira, B.; López-Rubio, A.; Tuohy, M.G.; Hogan, S.A.; Brodkorb, A. Characterization and gelling properties of a bioactive extract from Ascophyllum nodosum obtained using a chemical-free approach. Curr. Res. Food Sci. 2021, 4, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Jiang, H.; Mao, X.; Ci, F. Laminarin and Laminarin Oligosaccharides Originating from Brown Algae: Preparation, Biological Activities, and Potential Applications. J. Ocean Univ. China 2021, 20, 641–653. [Google Scholar] [CrossRef]
- Kadam, S.U.; Tiwari, B.K.; O’Donnell, C.P. Extraction, structure and biofunctional activities of laminarin from brown algae. Int. J. Food Sci. Technol. 2015, 50, 24–31. [Google Scholar] [CrossRef]
- Costa, A.M.S.; Rodrigues, J.M.M.; Pérez-Madrigal, M.M.; Dove, A.P.; Mano, J.F. Modular Functionalization of Laminarin to Create Value-Added Naturally Derived Macromolecules. J. Am. Chem. Soc. 2020, 142, 19689–19697. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cai, C.; Yang, C.; Li, J.; Sun, T.; Yu, G. Recent advances in pharmaceutical potential of brown algal polysaccharides and their derivatives. Curr. Pharm. Des. 2019, 25, 1290–1311. [Google Scholar] [CrossRef]
- Tümen Erden, S.; Ekentok Atici, C.; Cömez, B.; Sezer, A.D. Preparation and in vitro characterization of laminarin based hydrogels. J. Res. Pharm. 2021, 25, 164–172. [Google Scholar]
- Zargarzadeh, M.; Amaral, A.J.R.; Custódio, C.A.; Mano, J.F. Biomedical applications of laminarin. Carbohydr. Polym. 2020, 232, 115774. [Google Scholar] [CrossRef]
- Narayanaswamy, R.; Jo, B.W.; Choi, S.K.; Ismail, I.S. Fucoidan: Versatile cosmetic ingredient. An overview. J. Appl. Cosmetol. 2013, 31, 131–138. [Google Scholar]
- Baweja, P.; Kumar, S.; Sahoo, D.; Levine, I. Biology of Seaweeds in Seaweed. In Health and Disease Prevention; Fleurence, J., Levine, I., Eds.; Nikki Levy Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 41–106. [Google Scholar]
- Li, N.; Zhang, Q.; Song, J. Toxicological evaluation of fucoidan extracted from Laminaria japonica in Wistar rats. Food Chem. Toxicol. 2005, 43, 421–426. [Google Scholar] [CrossRef]
- Citkowska, A.; Szekalska, M.; Winnicka, K. Possibilities of fucoidan utilization in the development of pharmaceutical dosage forms. Mar. Drugs 2019, 17, 458. [Google Scholar] [CrossRef] [Green Version]
- Jiao, G.L.; Yu, G.L.; Zhang, J.Z.; Ewart, H.S. Chemical structures and bioactivities of sulfated polysaccharides from marine algae. Mar. Drugs 2011, 9, 196–223. [Google Scholar] [CrossRef] [Green Version]
- Wijesinghe, W.; Jeon, Y. Biological activities and potential industrial applications of fucose rich sulfated polysaccharides and fucoidans isolated from brown seaweeds: A review. Carbohydr. Polym. 2012, 88, 13–20. [Google Scholar] [CrossRef]
- Kartik, A.; Akhil, D.; Lakshmi, D.; Panchamoorthy Gopinath, K.; Arun, J.; Sivaramakrishnan, R.; Pugazhendhi, A. A critical review on production of biopolymers from algae biomass and their applications. Bioresour. Technol. 2021, 329, 124868. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.-H.; Fang, Y.; Lin, H.; Chen, L.; Li, Z.-J.; Deng, D.; Lu, C.-X. Chemical characters and antioxidative properties of sulfated polysaccharides from Laminaria japonica. J. Appl. Phycol. 2001, 13, 67–70. [Google Scholar] [CrossRef]
- Kim, S.M.; Kang, K.; Jeon, J.S.; Jho, E.H.; Kim, C.Y.; Nho, C.W.; Um, B.H. Isolation of phlorotannins from Eisenia bicyclis and their hepatoprotective effect against oxidative stress induced by tert-butyl hyperoxide. Appl. Biochem. Biotechnol. 2011, 165, 1296–1307. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, X.; Jiang, Y.; Wang, J.; Hwang, H.; Yang, X.; Wang, P. Structure characterization of low molecular weight sulfate Ulva polysaccharide and the effect of its derivative on iron deficiency anemia. Int. J. Biol. Macromol. 2019, 126, 747–754. [Google Scholar] [CrossRef]
- Senni, K.; Pereira, J.; Gueniche, F.; Delbarre-Ladrat, C.; Sinquin, C.; Ratiskol, J.; Godeau, G.; Fischer, A.-M.; Helley, D.; Colliec-Jouault, S. Marine polysaccharides: A source of bioactive molecules for cell therapy and tissue engineering. Mar. Drugs 2011, 9, 1664–1681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali Karami, M.; Sharif Makhmalzadeh, B.; Pooranian, M.; Rezai, A. Preparation and optimization of silibinin-loaded chitosan–fucoidan hydrogel: An in vivo evaluation of skin protection against UVB. Pharm. Dev. Technol. 2021, 26, 209–219. [Google Scholar] [CrossRef]
- Costa, L.S.; Fidelis, G.P.; Telles, C.B.S.; Dantas-Santos, N.; Camara, R.B.G.; Cordeiro, S.L.; Costa, M.S.; Almetida-Lima, J.; Oliveira, R.M.; Alburquerque, I.R.; et al. Antioxidant and antiproliferative activities of heterofucans from the seaweed Sargassum filipendula. Mar. Drugs 2011, 9, 952–966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiao, L.; Li, Y.; Chi, Y.; Ji, Y.; Gao, Y.; Hwang, H.; Aker, W.G.; Wang, P. Rheological properties, gelling behavior and texture characteristics of polysaccharide from Enteromorpha prolifera. Carbohydr. Polym. 2016, 136, 1307–1314. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.-P.; Wu, D.-S.; Xiao, X.-W.; Huang, Q.-Y.; Chen, H.-B.; Liu, D.; Fu, H.; Chen, X.-H.; Zhao, C. Structural characterization and antioxidant effect of green alga Enteromorpha prolifera polysaccharide in Caenorhabditis elegans via modulation of microRNAs. Int. J. Biol. Macromol. 2020, 150, 1084–1092. [Google Scholar] [CrossRef]
- Adrien, A.; Bonnet, A.; Dufour, D.; Badouin, S.; Maugard, T.; Bridiau, N. Pilot production of ulvans from Ulva sp. and their effects on hyaluronan and collagen production in cultured dermal fibroblasts. Carbohydr. Polym. 2017, 157, 1306–1314. [Google Scholar] [CrossRef]
- Lahaye, M.; Robic, A. Structure and function properties of Ulvan, a polysaccharide from green seaweeds. Biomacromol 2007, 8, 1765–1774. [Google Scholar] [CrossRef]
- Mo’o, F.R.C.; Wilar, G.; Devkota, H.P.; Wathoni, N. Ulvan, a polysaccharide from Macroalga Ulva sp.: A review of chemistry, biological activities and potential for food and biomedical applications. Appl. Sci. 2020, 10, 5488. [Google Scholar] [CrossRef]
- Kidgell, J.T.; Carnachan, S.M.; Magnusson, M.; Lawton, R.J.; Sims, I.M.; Hinkley, S.F.R.; de Nys, R.; Glasson, C.R.K. Are all ulvans equal? A comparative assessment of the chemical and gelling properties of ulvan from blade and filamentous Ulva. Carbohyd. Polym. 2021, 264, 118010. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Xu, H.; Wang, X.; Wang, Y.; Jiang, N.; Qi, H.; Liu, X. Antioxidant and antihyperlipidemic activities of high sulfate content purified polysaccharide from Ulva pertusa. Int. J. Biol. Macromol. 2020, 146, 756–762. [Google Scholar] [CrossRef]
- Chen, X.; Fu, X.; Huang, L.; Xu, J.; Gao, X. Agar oligosaccharides: A review of preparation, structures, bioactivities and application. Carbohydr. Polym. 2021, 265, 118076. [Google Scholar]
- Aziz, E.; Batool, R.; Khan, M.U.; Rauf, A.; Akhtar, W.; Heydary, M.; Rehman, S.; Shahzad, T.; Malik, A.; Mosavat, S.-H.; et al. An overview on red algae bioactive compounds and their pharmaceutical applications. J. Altern. Complement. Med. 2021, 17, 20190203. [Google Scholar]
- Laurienzo, P. Marine polysaccharides in pharmaceutical applications: An overview. Mar. Drugs 2010, 8, 2435–2465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghanbarzadeh, M.; Golmoradizadeh, A.; Homaei, A. Carrageenans and carrageenases: Versatile polysaccharides and promising marine enzymes. Phytochem. Rev. 2018, 17, 535–571. [Google Scholar] [CrossRef]
- Isaka, S.; Cho, K.; Nakazono, S.; Abu, R.; Ueno, M.; Kim, D.; Oda, T. Antioxidant and anti-inflammatory activities of porphyrin isolated from discolored nori (Porphyra yezoensis). Int. J. Biol. Macromol. 2015, 74, 68–75. [Google Scholar] [CrossRef]
- Beaumont, M.; Tran, R.; Vera, G.; Niedrist, D.; Rousset, A.; Pierre, R.; Shastri, V.P.; Forget, A. Hydrogel-Forming Algae Polysaccharides: From Seaweed to Biomedical Applications. Biomacromolecules 2021, 22, 1027–1052. [Google Scholar] [CrossRef]
- Kwon, M.; Nam, T. Porphyran induces apoptosis related signal pathway in AGS gastric cancer cell lines. Life Sci. 2006, 79, 1956–1962. [Google Scholar] [CrossRef]
- Pacheco-Quito, E.-M.; Ruiz-Caro, R.; Veiga, M.-D. Carrageenan: Drug Delivery Systems and Other Biomedical Applications. Mar. Drugs 2020, 18, 583. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Jiang, H.; Fu, L.; Ci, F.; Mao, X. Porphyran and oligo-porphyran originating from red algae Porphyra: Preparation, biological activities, and potential applications. Food Chem. 2021, 349, 129209. [Google Scholar] [CrossRef]
- Araujo, I.W.F.; Vanderlei, E.S.O.; Rodrigues, J.A.G.; Coura, C.O.; Quindere, A.L.G.; Fontes, B.P.; Queiroz, I.N.L.; Jorge, R.J.B.; Bezerra, M.M.; Silva, A.A.R.; et al. Effects of a sulfated polysaccharide isolated from the red seaweed Solieria filiformis on models of nociception and inflammation. Carbohydr. Polym. 2011, 86, 1207–1215. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Tian, R.; Wu, H.; Li, X.; Li, S.; Bian, L. Evaluation of acute and sub-chronic toxicity of Lithothamnion sp. in mice and rats. Toxicol. Rep. 2020, 7, 852–858. [Google Scholar] [CrossRef]
- Cheong, K.-L.; Qiu, H.-M.; Du, H.; Liu, Y.; Khan, B.M. Oligosaccharides derived from red seaweed: Production, properties, and potential health and cosmetic application. Molecules 2018, 23, 245. [Google Scholar] [CrossRef] [Green Version]
- Bleakley, S.; Hayes, M. Algal proteins: Extraction, application, and challenges concerning production. Foods 2017, 6, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fleurence, J. Seaweed Proteins. In Proteins in Food Processing; Yada, R.Y., Ed.; Woodhead Publishing Limited: Cambridge, UK, 2004; pp. 197–213. [Google Scholar]
- Gressler, V.; Fujii, M.T.; Martins, A.P.; Colepicolo, P.; Mancini-Filho, J.; Pinto, E. Biochemical composition of two red seaweed species grown on the Brazilian coast. J. Sci. Food Agric. 2011, 91, 1687–1692. [Google Scholar] [CrossRef]
- Cheung, R.C.F.; Ng, T.B.; Wong, J.H. Marine peptides: Bioactivities and applications. Mar. Drugs 2015, 13, 4006–4043. [Google Scholar] [CrossRef] [PubMed]
- Qasim, R. Amino acids composition of some common seaweeds. Pak. J. Pharmac. Sci. 1991, 4, 49–54. [Google Scholar]
- Wong, K.H.; Cheung, P.C.K. Nutritional evaluation of some subtropical red and green seaweeds. Part II. In vitro protein digestibility and amino acid profiles of protein concentrates. Food Chem. 2001, 72, 11–17. [Google Scholar] [CrossRef]
- Matanjun, P.; Mohamed, S.; Mustapha, N.M.; Muhammad, K. Nutrient content of tropical edible seaweeds, Eucheuma cottoni, Caulerpa lentillifera and Sargassum polycysum. J. Appl. Phycol. 2009, 21, 75–80. [Google Scholar] [CrossRef]
- Admassu, H.; Abdalbasit, M.; Gasmalla, A.; Yang, R.; Zhao, W. Bioactive peptides derived from seaweed protein and their health benefits: Antihypertensive, antioxidant, and antidiabetic properties. J. Food Sci. 2018, 83, 6–16. [Google Scholar] [CrossRef] [Green Version]
- Meisel, H. Multifunctional peptides encrypted in milk proteins. Biofactors 2004, 21, 55–61. [Google Scholar] [CrossRef]
- Murray, B.A.; FitzGerald, R.J. Angiotensin converting enzyme inhibitory peptides derived from food proteins: Biochemistry, bioactivity and production. Curr. Pharmac. Des. 2007, 13, 773–791. [Google Scholar] [CrossRef]
- Harnedy, P.A.; FitzGerald, R.J. Bioactive proteins, peptides, and amino acids from macroalgae. J. Phycol. 2011, 47, 218–232. [Google Scholar] [CrossRef]
- Indumathi, P.; Mehta, A. A novel anticoagulant peptide from the nori hydrolysate. J. Funct. Foods 2016, 20, 606–617. [Google Scholar] [CrossRef]
- Jo, C.; Khan, F.F.; Khan, M.I.; Iqbal, J. Marine bioactive peptides: Types, structures, and physiological functions. Food Rev. Int. 2016, 33, 44–61. [Google Scholar] [CrossRef]
- Lafarga, T.; Acién-Fernández, F.G.; Garcia-Vaquero, M. Bioactive peptides and carbohydrates from seaweed for food applications: Natural occurrence, isolation, purification, and identification. Algal Res. 2020, 48, 101909. [Google Scholar] [CrossRef]
- Sridhar, K.; Inbaraj, B.S.; Chen, B.-H. Recent developments on production, purification and biological activity of marine peptides. Food Res. Int. 2021, 47, 110468. [Google Scholar] [CrossRef]
- Pangestuti, R.; Kim, S.K. Seaweed Proteins, Peptides, and Amino Acids. In Seaweed Sustainability: Food and Non-Food Applications; Tiwari, B.K., Toy, D.J., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 125–140. [Google Scholar]
- Yanshin, N.; Kushnareva, A.; Lemesheva, V.; Birkemeyer, C.; Tarakhovskaya, E. Chemical composition and potential practical application of 15 red algal species from the White Sea Coast (the Arctic Ocean). Molecules 2021, 26, 2489. [Google Scholar] [CrossRef]
- Lee, H.-A.; Kim, I.-H.; Nam, T.-J. Bioactive peptide from Pyropia yezoensis and its anti-inflammatory activities. Int. J. Molec. Med. 2015, 36, 1701–1706. [Google Scholar] [CrossRef] [Green Version]
- Ryu, J.; Park, S.J.; Kim, I.H.; Choi, Y.H.; Nam, T.J. Protective effect of porphyra-334 on UVA-induced photoaging in human skin fibroblasts. Int. J. Mol. Medic. 2014, 34, 796–803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romarís-Hortas, V.; Bermejo-Barrera, P.; Moreda-Piñeiro, A. Ultrasound-assisted enzymatic hydrolysis for iodinated amino acid extraction from edible seaweed before reversed-phase high performance liquid chromatography-inductively coupled plasma-mass spectrometry. J. Chromatogr. A 2013, 1309, 33–40. [Google Scholar] [CrossRef]
- Shick, J.M.; Dunlap, W.C.; Buettner, G.R. Ultraviolet (UV) Protection in Marine Organisms II, Biosynthesis, Accumulation, and Sunscreening Function of Mycosporine-Like Amino Acids. In Free Radicals in Chemistry, Biology and Medicine; Yoshikawa, S., Toyokuni, S., Yamamoto, Y., Naito, Y., Eds.; OICA International: London, UK, 2000; pp. 215–228. [Google Scholar]
- Bedoux, G.; Hardouin, K.; Marty, C.; Taupin, L.; Vandanjon, L.; Bourgougnon, N. Chemical characterization and photoprotective activity measurement of extracts from the red macroalga Solieria chordalis. Bot. Mar. 2014, 57, 291–301. [Google Scholar] [CrossRef] [Green Version]
- Dunlap, W.C.; Yamamoto, Y. Small-molecule antioxidants in marine organisms: Antioxidant activity of mycosporine-glycine. Compar. Biochem. Physiol. 1995, 112, 105–114. [Google Scholar] [CrossRef]
- Bandaranayake, W.M. Mycosporines: Are they nature’s sunscreens? Nat. Prod. Rep. 1998, 15, 159–172. [Google Scholar] [CrossRef]
- Conde, F.R.; Churio, M.S.; Previtali, C.M. The photoprotector mechanism of mycosporine-like amino acids. Excited-state properties and photostability of porphyra-334 in aqueous solution. J. Photochem. Photobiol. B Biol. 2000, 56, 139–144. [Google Scholar] [CrossRef]
- Gröniger, A.; Sinha, R.P.; Klisch, M.; Häder, D.P. Photoprotective compounds in cyanobacteria, phytoplankton and macroalgae—A database. J. Photochem. Photobiol. B Biol. 2000, 58, 115–122. [Google Scholar] [CrossRef]
- Adams, N.L.; Shick, J.M. Mycosporine-like amino acids prevent UVB-induced abnormalities durin early development of the green sea urchin Strongylocentrotus droebachiensis. Mar. Biol. 2001, 138, 267–280. [Google Scholar] [CrossRef]
- Singh, S.P.; Kumari, S.; Rastogi, R.P.; Singh, K.L.; Sinha, R.P. Mycosporine-like amino acids (MAAs): Chemical structure, biosynthesis and significance as UV-absorbing/screening compounds. Indian J. Experim. Biol. 2008, 46, 7–17. [Google Scholar]
- Karsten, U.; Sawall, T.; Wiencke, C. A survey of the distribution of UV-absorbing substances in tropical macroalgae. Phycol. Res. 2006, 46, 271–279. [Google Scholar]
- Rastogi, R.P.; Sonani, R.R.; Madamwar, D. UV Photoprotectants from Algae—Synthesis and Bio-Functionalities. In Algal Green Chemistry; Rastogi, R.P., Madamwar, D., Pandey, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 17–38. [Google Scholar]
- Orfanoudaki, M.; Hartmann, A. Chemical profiling of mycosporine-like amino acids in twenty-three red algal species. J. Phycol. 2019, 55, 393–403. [Google Scholar] [CrossRef]
- Gianeti, M.D.; Maia Campos, P.M.B.G. Efficacy evaluation of a multifunctional cosmetic formulation: The benefits of a combination of active antioxidant substances. Molecules 2014, 19, 18268–18282. [Google Scholar] [CrossRef]
- Leandro, A.; Pereira, L.; Gonçalves, A.M.M. Diverse applications of marine macroalgae. Mar. Drugs 2020, 18, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chrapusta, E.; Kaminski, A.; Duchnik, K.; Bober, B.; Adamski, M.; Bialczyk, J. Mycosporine-like amino acids: Potential health and beauty ingredients. Mar. Drugs 2017, 15, 326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmid, D.; Schürch, C.; Zülli, F. UV-A sunscreen from red algae for protection against premature skin aging. Cosmetics 2004, 2004, 139–143. [Google Scholar]
- Rangel, K.C.; Villela, L.Z.; Pereira, K.D.C.; Debonsi, H.M.; Gaspar, L.R. Assessment of the photoprotective potential and toxicity of Antarctic red macroalgae extracts from Curdiea racovitzae and Iridaea cordata for cosmetic use. Algal Res. 2020, 50, 101984. [Google Scholar] [CrossRef]
- Barceló-Villalobos, M.; Figueroa, F.L.; Korbee, N.; Álvarez-Gómez, F.; Abreu, M.H. Production of Mycosporine-Like amino acids from Gracilaria vermiculophylla (Rhodophyta) cultured through one year in an integrated multi-trophic aquaculture (IMTA) system. Mar. Biotechnol. 2017, 19, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Athukorala, Y.; Trang, S.; Kwok, C.; Yuan, Y.V. Antiproliferative and antioxidant activities and mycosporine-Like amino acid profiles of wild-Harvested and cultivated edible Canadian marine red macroalgae. Molecules 2016, 21, 119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pliego-Cortés, H.; Bedoux, G.; Boulho, R.; Taupin, L.; Freile-Pelegrin, Y.; Bourgougnon, N.; Robledo, D. Stress tolerance and photoadaptation to solar radiation in Rhodymenia pseudopalmata (Rhodophyta) through mycosporine-like amino acids, phenolic compounds, and pigments in an Integrated Multi-Trophic Aquaculture System. Algal Res. 2019, 41, 101542. [Google Scholar] [CrossRef]
- Leelapornpisid, P.; Mungmai, L.; Sirithunyalug, B.; Jiranusornkul, S.; Peerapornpisal, Y. A novel moisturizer extracted from freshwater macroalga [Rhizoclonium hieroglyphicum (C. Agardh) Kützing] for skin care cosmetic. Chiang Mai J. Sci. 2014, 41, 1195–1207. [Google Scholar]
- Francavilla, M.; Franchi, M.; Monteleone, M.; Caroppo, C. The red seaweed Gracilaria gracilis as a multi products source. Mar. Drugs 2013, 11, 3754–3776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, X.; Bai, L.; Mao, X.; Zhang, X. Novel peptides with anti-proliferation activity from the Porphyra haitanesis hydrolysate. Process Biochem. 2017, 60, 98–107. [Google Scholar] [CrossRef]
- Verdy, C.; Branka, J.E.; Mekideche, N. Quantitative assessment of lactate and progerin production in normal human cutaneous cells during normal ageing: Effect of an Alaria esculenta extract. Int. J. Cosmet. Sci. 2011, 33, 462–466. [Google Scholar] [CrossRef]
- Arad, S.; Yaron, A. Natural pigments from red microalgae for use in foods and cosmetics. Trends Food Sci. Technol. 1992, 3, 92–97. [Google Scholar] [CrossRef]
- Bermejo, R.; Talavera, E.M.; del Valle, C.; Alvarez-Pez, J.M. C-phycocyanin incorporated into reverse micelles: A fluorescence study. Colloids Surf. B Biointerfaces 2000, 18, 51–59. [Google Scholar] [CrossRef]
- Chronakis, I.S. Biosolar proteins from aquatic algae. Dev. Food Sci. 2000, 41, 39–75. [Google Scholar] [CrossRef]
- Rossano, R.; Ungaro, N.; D’Ambrosio, A.; Liuzzi, G.M.; Riccio, P. Extracting and purifying R-phycoeythrin from Mediterranean red algae Corallina elongata Ellis and Solander. J. Biotecnol. 2003, 101, 289–296. [Google Scholar] [CrossRef]
- Sekar, S.; Chandramohan, M. Phycobiliproteins as a commodity: Trends in applied research, patents and commercialization. J. Appl. Phycol. 2008, 20, 113–136. [Google Scholar] [CrossRef]
- Viskari, P.J.; Colyer, C.L. Rapid extraction of phycobiliproteins from cultures cyanobacteria samples. Anal. Biochem. 2003, 319, 263–271. [Google Scholar] [CrossRef]
- Martins, M.; Vieira, F.A.; Correia, I.; Ferreira, R.A.S.; Abreu, H.; Coutinho, J.A.P.; Ventura, S.P.M. Recovery of phycobiliproteins from the red macroalga Gracilaria sp. using ionic liquid aqueous solutions. Green Chem. 2016, 18, 4287–4296. [Google Scholar] [CrossRef]
- Saluri, M.; Kaldmäe, M.; Rospu, M.; Sirkel, H.; Paalme, T.; Landreh, M.; Tuvikene, R. Spatial variation and structural characteristics of phycobiliproteins from the red algae Furcellaria lumbricalis and Coccotylus truncatus. Algal Res. 2020, 52, 102058. [Google Scholar] [CrossRef]
- Osório, C.; Machado, S.; Peixoto, J.; Bessada, S.; Pimentel, F.B.; Alves, R.C.; Oliveira, M.B.P.P. Pigments content (chlorophylls, fucoxanthin and phycobiliproteins) of different commercial dried algae. Separations 2020, 7, 33. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Lee, W.; Ahn, G. Marine algal flavonoids and phlorotannins; an intriguing frontier of biofunctional secondary metabolites. Crit. Rev. Biotechnol. 2021, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, A.; Pradhan, R.; Ghotekar, S.; Dahikar, S.; Marasini, B.P. Phytochemical Analysis and Anti-Microbial Activity of Desmostachya Bipinnata: A review. J. Med. Chem. Sci. 2021, 4, 36–41. [Google Scholar]
- Wijesinghe, W.; Jeon, Y. Biological activities and potential cosmeceutical applications of bioactive components from brown seaweeds: A review. Phytochem. Rev. 2011, 10, 431–443. [Google Scholar] [CrossRef]
- Ferreres, F.; Lopes, G.; Gil-Izquierdo, A.; Andrade, P.B.; Sousa, C.; Mouga, T.; Valentão, P. Phlorotannin extracts from fucales characterized by HPLC-DAD-ESI-MSn: Approaches to hyaluronidase inhibitory capacity and antioxidant properties. Mar. Drugs 2012, 10, 2766–2781. [Google Scholar] [CrossRef] [Green Version]
- Balboa, E.M.; Conde, E.; Moure, A.; Falqué, E.; Domínguez, H. In vitro antioxidant properties of crude extracts and compounds from brown algae. Food Chem. 2013, 138, 1764–1785. [Google Scholar] [CrossRef] [PubMed]
- Phasanasophon, K.; Kim, S. Antioxidant and Cosmeceutical Activities of Agarum cribrosum Phlorotannin Extracted by Ultrasound Treatment. Nat. Prod. Commun. 2018, 13, 565–570. [Google Scholar] [CrossRef]
- Gheda, S.; Naby, M.A.; Mohamed, T.; Pereira, L.; Khamis, A. Antidiabetic and antioxidant activity of phlorotannins extracted from the brown seaweed Cystoseira compressa in streptozotocin-induced diabetic rats. Environ. Sci. Pollut. Res. 2021, 28, 22886–22901. [Google Scholar] [CrossRef] [PubMed]
- Joe, M.; Kim, S.; Choi, H.; Shin, W.; Park, G.; Kang, D.; Kim, Y.K. The inhibitory effects of eckol and dieckol from Ecklonia stolonifera on the expression of matrix metalloproteinase-1 in human dermal fibroblasts. Biol. Pharm. Bull. 2006, 29, 1735–1739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le, Q.; Li, Y.; Qian, Z.; Kim, M.; Kim, S. Inhibitory effects of polyphenols isolated from marine alga Ecklonia cava on histamine release. Process Biochem. 2009, 44, 168–176. [Google Scholar] [CrossRef]
- Barbosa, M.; Lopes, G.; Valentão, P.; Ferreres, F.; Gil-Izquierdo, A.; Pereira, D.M.; Andrade, P.B. Edible seaweeds’ phlorotannins in allergy: A natural multi-target approach. Food Chem. 2018, 265, 233–241. [Google Scholar] [CrossRef]
- Sugiura, Y.; Kinoshita, Y.; Misumi, S.; Yamatani, H.; Katsuzaki, H.; Hayashi, Y.; Murase, N. Correlation between the seasonal variations in phlorotannin content and the antiallergic effects of the brown alga Ecklonia cava subsp. stolonifera. Algal Res. 2021, 58, 102398. [Google Scholar] [CrossRef]
- Barbosa, M.; Lopes, G.; Andrade, P.-B.; Valentão, P. Bioprospecting of brown seaweeds for biotechnological applications: Phlorotannin actions in inflammation and allergy network. Trends Food Sci. Technol. 2019, 86, 153–171. [Google Scholar] [CrossRef]
- Catarino, M.D.; Amarante, S.J.; Mateus, N.; Silva, A.M.S.; Cardoso, S.M. Brown algae phlorotannins: A marine alternative to break the oxidative stress, inflammation and cancer network. Foods 2021, 10, 1478. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.S.; Kim, H.R.; Byun, D.S.; Son, B.W.; Nam, T.J.; Choi, J.S. Tyrosinase inhibitors isolated from the edible brown alga Ecklonia stolonifera. Arch. Pharm. Res. 2004, 27, 1226–1232. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.J.; Ko, S.; Cha, S.H.; Kang, D.H.; Park, H.S.; Choi, Y.U.; Kim, D.; Jung, W.K.; Yeon, Y.J. Effect of phlorotannins isolated from Ecklonia cava on melanogenesis and their protective effect against photo-oxidative stress induced by UV-B radiation. Toxicol. Vitr. 2009, 23, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.M.; Heo, S.J.; Kim, K.N.; Lee, S.H.; Yang, H.M.; Kim, A.D.; Jeon, Y.J. Molecular docking studies of a phlorotannin, dieckol isolated from Ecklonia cava with tyrosinase inhibitory activity. Bioorg. Med. Chem. 2012, 20, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lee, S.; Park, S.; Park, J.S.; Kim, Y.H.; Yang, S.Y. Slow-Binding Inhibition of Tyrosinase by Ecklonia cava Phlorotannins. Mar. Drugs 2019, 17, 359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Susano, P.; Silva, J.; Alves, C.; Martins, A.; Gaspar, H.; Pinteus, S.; Mouga, T.; Goettert, M.I.; Petrovski, Ž.; Branco, L.B.; et al. Unravelling the Dermatological Potential of the Brown Seaweed Carpomitra costata. Mar. Drugs 2021, 19, 135. [Google Scholar] [CrossRef]
- Hwang, H.; Chen, T.; Nines, R.G.; Shin, H.; Stoner, G.D. Photochemoprevention of UVB-induced skin carcinogenesis in SKH-1 mice by brown algae polyphenols. Int. J. Cancer 2006, 119, 2742–2749. [Google Scholar] [CrossRef]
- Hwang, E.; Park, S.-Y.; Sun, Z.-W.; Shin, H.-S.; Lee, D.-G.; Yi, T.H. The protective effects of fucosterol against skin damage in UVB-Irradiated human dermal fibroblasts. Mar. Biotechnol. 2014, 16, 361–370. [Google Scholar] [CrossRef]
- Handajani, F.; Prabowo, S. Sargassum duplicatum extract reduced artritis severity score and periarticular tissue matrix metalloproteinase- 1 (MMP-1) expression in ajuvan artritis exposed to cold stressor. Sys. Rev. Pharm. 2020, 11, 302–307. [Google Scholar]
- Ryu, B.; Ahn, B.-N.; Kang, K.-H.; Kim, Y.-S.; Li, Y.-X.; Kong, C.-S.; Kim, S.-K.; Kim, D.G. Dioxinodehydroeckol protects human keratinocyte cells from UVB-induced apoptosis modulated by related genes Bax/Bcl-2 and caspase pathway. J. Photochem. Photobiol. B 2015, 153, 352–357. [Google Scholar] [CrossRef]
- Vo, T.S.; Kim, S.-K.; Ryu, B.; Ngo, D.H.; Yoon, N.-Y.; Bach, L.G.; Hang, N.T.N.; Ngo, D.N. The suppressive activity of fucofuroeckol-a derived from brown algal Ecklonia stolonifera okamura on UVB-induced mast cell degranulation. Mar. Drugs 2018, 16, 1. [Google Scholar] [CrossRef] [Green Version]
- Manandhar, B.; Paudel, P.; Seong, S.H.; Jung, H.A.; Choi, J.S. Characterizing eckol as a therapeutic aid: A systematic review. Mar. Drugs 2019, 17, 361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manandhar, B.; Wagle, A.; Seong, S.H.; Paudel, P.; Kim, H.-R.; Jung, H.A.; Choi, J.S. Phlorotannins with potential anti-tyrosinase and antioxidant activity isolated from the marine seaweed Ecklonia stolonifera. Antioxidants 2019, 8, 240. [Google Scholar] [CrossRef] [Green Version]
- Sanjeewa, K.K.A.; Kim, E.-A.; Son, K.-T.; Jeon, Y.-J. Bioactive properties and potentials cosmeceutical applications of phlorotannins isolated from brown seaweeds: A review. J. Photochem. Photobiol. B 2016, 162, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Freitas, R.; Martins, A.; Silva, J.; Alves, C.; Pinteus, S.; Alves, J.; Teodoro, F.; Ribeiro, H.M.; Gonçalves, L.; Petrovski, Ž.; et al. Highlighting the Biological Potential of the Brown Seaweed Fucus spiralis for Skin Applications. Antioxidants 2020, 9, 611. [Google Scholar]
- Arunkumar, K.; Raj, R.; Raja, R.; Carvalho, I.S. Brown seaweeds as a source of anti-hyaluronidase compounds. S. Afr. J. Bot. 2021, 139, 470–477. [Google Scholar] [CrossRef]
- Ko, S.; Lee, M.; Lee, J.; Lee, S.; Lim, Y.; Jeon, Y. Dieckol, a phlorotannin isolated from a brown seaweed, Ecklonia cava, inhibits adipogenesis through AMP-activated protein kinase (AMPK) activation in 3T3-L1 preadipocytes. Environ. Toxicol. Pharmacol. 2013, 36, 1253–1260. [Google Scholar] [CrossRef]
- Eom, S.H.; Lee, E.H.; Park, K.; Kwon, J.Y.; Kim, P.H.; Jung, W.K.; Kim, Y.M. Eckol from Eisenia bicyclis inhibits inflammation through the Akt/NF-κB signaling in Propionibacterium acnes-induced human keratinocyte Hacat cells. J. Food Biochem. 2017, 41, 12312. [Google Scholar] [CrossRef]
- Hermund, D.B.; Plaza, M.; Turner, C.; Jónsdóttir, R.; Kristinsson, H.G.; Jacobsen, C.; Nielsen, K.F. Structure dependent antioxidant capacity of phlorotannins from Icelandic Fucus vesiculosus by UHPLC-DAD-ECD-QTOFMS. Food Chem. 2018, 240, 904–909. [Google Scholar]
- Jang, J.; Ye, B.-R.; Heo, S.-J.; Oh, C.; Kang, D.-H.; Kim, J.H.; Affan, A.; Yoon, K.-T.; Choi, Y.-U.; Park, S.C.; et al. Photo-oxidative stress by ultraviolet-B radiation and antioxidative defense of eckstolonol in human keratinocytes. Environ. Toxicol. Pharmacol. 2012, 34, 926–934. [Google Scholar] [CrossRef]
- Lee, J.-H.; Eom, S.-H.; Lee, E.-H.; Jung, Y.-J.; Kim, H.-J.; Jo, M.-R.; Son, K.-T.; Lee, H.-J.; Kim, J.H.; Lee, M.-S.; et al. In vitro antibacterial and synergistic effect of phlorotannins isolated from edible brown seaweed Eisenia bicyclis against acne-related bacteria. Algae 2014, 29, 47–55. [Google Scholar] [CrossRef]
- Sugiura, Y.; Matsuda, K.; Yamada, Y.; Nishikawa, M.; Shioya, K.; Katsuzaki, H.; Imai, K.; Amano, H. Isolation of a new anti-allergic phlorotannin, phlorofucofuroeckol-B, from an edible brown alga, Eisenia arborea. Biosci. Biotechnol. Biochem. 2006, 70, 2807–2811. [Google Scholar] [CrossRef] [Green Version]
- Le Lann, K.; Surget, G.; Couteau, C.; Coiffard, L.; Cerantola, S.; Gaillard, F.; Larnicol, M.; Zubia, M.; Gerard, F.; Poupart, N.; et al. Sunscreen, antioxidant, and bactericide capacities of phlorotannins from the brown macroalga Halidrys siliquosa. J. Appl. Phycol. 2016, 28, 3547–3559. [Google Scholar] [CrossRef] [Green Version]
- Gager, L.; Connan, S.; Molla, M.; Couteau, C.; Arbona, J.F.; Coiffard, L.; Cérantola, S.; Stiger-Pouvreau, V. Active phlorotannins from seven brown seaweeds commercially harvested in Brittany (France) detected by 1H NMR and in vitro assays: Temporal variation and potential valorization in cosmetic applications. J. Appl. Phycol. 2020, 32, 2375–2386. [Google Scholar] [CrossRef]
- Lee, J.H.; Ko, J.-Y.; Samarakoon, K.; Oh, J.-Y.; Heo, S.-J.; Kim, C.-Y.; Nah, J.-W.; Jang, M.-K.; Lee, J.-S.; Jeon, Y.-J. Preparative isolation of sargachromanol E from Sargassum siliquastrum by centrifugal partition chromatography and its anti-inflammatory activity. Food Chem. Toxicol. 2013, 62, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Ahn, B.; Kong, C.; Kim, S. The chromenesargachromanol E inhibits ultraviolet A-induced ageing of skin in human dermal fibroblasts. Br. J. Dermatol. 2013, 168, 968–976. [Google Scholar] [CrossRef] [PubMed]
- Balboa, E.M.; Li, Y.; Ahn, B.; Eom, S.; Domínguez, H.; Jiménez, C.; Rodríguez, J. Photodamage attenuation effect by a tetraprenyltoluquinol chromane meroterpenoid isolated from Sargassum muticum. J. Photochem. Photobiol. B 2015, 148, 51–58. [Google Scholar] [CrossRef]
- Azam, M.S.; Choi, J.; Lee, M.-S.; Kim, H.-R. Hypopigmenting Effects of Brown Algae-Derived Phytochemicals: A Review on Molecular Mechanisms. Mar. Drugs 2017, 15, 297. [Google Scholar] [CrossRef] [Green Version]
- Hamid, N.; Ma, Q.; Boulom, S.; Liu, T.; Zheng, Z.; Balbas, J.; Robertson, J. Seaweed Minor Constituents. In Seaweed Sustainability: Food and Non-Food Applications; Tiwari, B.K., Troy, D., Eds.; Academic Press: London, UK, 2015; pp. 193–242. [Google Scholar]
- Stiger-Pouvreau, V.; Zubia, M. Macroalgal diversity for sustainable biotechnological development in French tropical overseas territories. Bot. Mar. 2020, 63, 17–41. [Google Scholar] [CrossRef] [Green Version]
- Neto, R.T.; Marçal, C.; Queirós, A.S.; Abreu, H.; Silva, A.M.S.; Cardoso, S.M. Screening of Ulva rigida, Gracilaria sp., Fucus vesiculosus and Saccharina latissima as Functional Ingredients. Int. J. Mol. Sci. 2018, 19, 2987. [Google Scholar] [CrossRef] [Green Version]
- Biris-Dorhoi, E.-S.; Michiu, D.; Pop, C.R.; Rotar, A.M.; Tofana, M.; Pop, O.L.; Socaci, S.A.; Farcas, A.C. Macroalgae—A Sustainable Source of Chemical Compounds with Biological Activities. Nutrients 2020, 12, 3085. [Google Scholar] [CrossRef] [PubMed]
- Kumari, P.; Kumar, M.; Reddy, C.R.K.; Jha, B. Algal Lipids, Fatty Acids and Sterols. In Functional Ingredients from Algae for Foods and Nutraceuticals; Domínguez, H., Ed.; Woodhead Publishing: Cambridge, UK, 2013; pp. 87–134. [Google Scholar]
- Lever, J.; Brkljača, R.; Kraft, G.; Urban, S. Natural Products of Marine Macroalgae from South Eastern Australia, with Emphasis on the Port Phillip Bay and Heads Regions of Victoria. Mar. Drugs 2020, 18, 142. [Google Scholar] [CrossRef] [Green Version]
- Saadaoui, I.; Rasheed, R.; Abdulrahman, N.; Bounnit, T.; Cherif, M.; Al Jabri, H.; Mraiche, F. Algae-Derived Bioactive Compounds with Anti-Lung Cancer Potential. Mar. Drugs 2020, 18, 197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plaza, M.; Cifuentes, A.; Ibáñez, E. In the search of new functional food ingredients from algae. Trends Food Sci. Technol. 2008, 19, 31–39. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Zorita, S.; González-Arceo, M.; Trepiana, J.; Eseberri, I.; Fernández-Quintela, A.; Milton-Laskibar, I.; Aguirre, L.; González, M.; Portillo, M.P. Anti-Obesity Effects of Macroalgae. Nutrients 2020, 12, 2378. [Google Scholar] [CrossRef] [PubMed]
- Lange, K.W.; Hauser, J.; Nakamura, Y.; Kanaya, S. Dietary seaweeds and obesity. Food Sci. Hum. Wellness 2015, 4, 87–96. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Lee, Y.S.; Jung, S.H.; Kang, S.S.; Shin, K.H. Anti-oxidant activities of fucosterol from the marine algae Pelvetia siliquosa. Arch. Pharm. Res. 2003, 26, 719–722. [Google Scholar] [CrossRef] [PubMed]
- Abdul, Q.A.; Choi, R.J.; Jung, H.A.; Choi, J.S. Health benefit of fucosterol from marine algae: A review. J. Sci. Food Agric. 2016, 96, 1856–1866. [Google Scholar] [CrossRef]
- Hannan, M.A.; Sohag, A.A.M.; Dash, R.; Haque, M.N.; Mohibbullah, M.; Oktaviani, D.F.; Hossain, M.T.; Choi, H.J.; Moon, I.S. Phytosterols of marine algae: Insights into the potential health benefits and molecular pharmacology. Phytomedicine 2020, 69, 153201. [Google Scholar] [CrossRef]
- Perumal, P.; Sowmiya, R.; Prasanna Kumar, S.; Ravikumar, S.; Deepak, P.; Balasubramani, G. Isolation, structural elucidation and antiplasmodial activity of fucosterol compound from brown seaweed, Sargassum linearifolium against malarial parasite Plasmodium falciparum. Nat. Prod. Res. 2017, 32, 1316–1319. [Google Scholar] [CrossRef]
- Sugawara, T.; Kushiro, M.; Zhang, H.; Nara, E.; Ono, H.; Nagao, A. Lysophosphatidylcholine enhances carotenoid uptake from mixed micelles by Caco-2 human intestinal cells. J. Nutr. 2001, 131, 2921–2927. [Google Scholar] [CrossRef] [Green Version]
- Okada, T.; Mizuno, Y.; Sibayama, S.; Hosokawa, M.; Miyashita, H. Antiobesity effects of Undaria lipid capsules prepared with scallop phospholipids. J. Food Sci. 2011, 76, 2–6. [Google Scholar] [CrossRef]
- Rexliene, J.; Sridhar, J. Extraction and characterization of essential oil from Portieria hornemannii with applications in antibacterial edible films. Res. J. Biotechnol. 2021, 16, 81–89. [Google Scholar]
- Lee, H.J.; Dang, H.T.; Kang, G.J.; Yang, E.J.; Park, S.S.; Yoon, W.J.; Jung, J.H.; Kang, H.K.; Yoo, E.S. Two enone fatty acids isolated from Gracilaria verrucosa suppress the production of inflammatory mediators by down-regulating NF-êB and STAT1 activity in lipopolysaccharide-stimulated raw 264.7 cells. Arch. Pharm. Res. 2009, 32, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.K.; Das, G.; Baek, K. Chemical composition and antioxidant and antibacterial activities of an essential oil extracted from an edible seaweed, Laminaria japonica L. Molecules 2015, 20, 12093–12113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.; Shin, K.; Jung, S.; Lee, S. Effects of the extracts from the marine algae Pelvetia siliquosa on hyperlipidemia in rats. Korean J. Pharmacogn. 2004, 35, 143–146. [Google Scholar]
- Zhangfan, M.; Xiaoling, S.; Ping, D.; Gaoli, L.; Shize, P.; Xiangran, S.; Haifeng, H.; Li, P.; Jie, H. Fucosterol exerts antiproliferative effects on human lung cancer cells by inducing apoptosis, cel cycle arrest and targeting of Raf/MEK/ERK signaling pathway. Phytomedicine 2019, 61, 152809. [Google Scholar]
- Richard, D.; Kefi, K.; Barbe, U.; Bausero, P.; Visioli, F. Polyunsaturated fatty acids as antioxidants. Pharmacol. Res. 2008, 57, 451–455. [Google Scholar] [CrossRef]
- Mohy El-Din, S.M. Fatty acid profiling as bioindicator of chemical stress in marine Pterocladia capillacea, Sargassum hornschuchii and Ulva lactuca. Int. J. Environ. Sci. Technol. 2018, 15, 791–800. [Google Scholar] [CrossRef]
- Calandra, I.P.; Simonetti, S. Nutritional supplements in dermocosmetology. Ann. Ital. Dermatol. Clin. Sper. 1995, 49, 49–56. [Google Scholar]
- Jacob, L.; Baker, C.; Farris, P. Vitamin-based cosmeceuticals. Cosmet. Dermatol. 2012, 25, 405. [Google Scholar]
- Peñalver, R.; Lorenzo, J.M.; Ros, G.; Amarowicz, R.; Pateiro, M.; Nieto, G. Seaweeds as a Functional Ingredient for a Healthy Diet. Mar. Drugs 2020, 18, 301. [Google Scholar] [CrossRef]
- Kafi, R.; Kwak, H.S.R.; Schumacher, W.E.; Cho, S.; Hanft, V.N.; Hamilton, T.A.; King, A.L.; Neal, J.D.; Varani, J.; Fisher, G.J.; et al. Improvement of naturally aged skin with vitamin a (retinol). Arch. Dermatol. 2007, 143, 606–612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Searle, T.; Al-Niaimi, F.; Ali, F.R. The top 10 cosmeceuticals for facial hyperpigmentation. Dermatol. Ther. 2020, 33, 14095. [Google Scholar] [CrossRef] [PubMed]
- Bissett, D.L.; Oblong, J.E.; Goodman, L.J. Topical Vitamins. In Cosmetic Dermatology: Products and Procedures; Draelos, Z.D., Ed.; Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2015; pp. 336–345. [Google Scholar]
- Kilinç, B.; Semra, C.; Gamze, T.; Hatice, T.; Koru, E. Seaweeds for Food and Industrial Applications. In Food Industry; Muzzalupo, I., Ed.; InTech: Rijeka, Croatia, 2013; pp. 735–748. [Google Scholar]
- Campiche, R.; Curpen, S.J.; Lutchmanen-Kolanthan, V.; Gougeon, S.; Cherel, M.; Laurent, G.; Gempeler, M.; Schuetz, R. Pigmentation effects of blue light irradiation on skin and how to protect against them. Int. J. Cosmet. Sci. 2020, 42, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Bhatnagar, I. Physical, chemical, and biological properties of wonder kelp-Laminaria. Adv. Food Nutr. Res. 2011, 64, 85–96. [Google Scholar] [PubMed]
- Watanabe, F.; Yabuta, Y.; Bito, T.; Teng, F. Vitamin B12-containing plant food sources for vegetarians. Nutrients 2014, 6, 1861–1873. [Google Scholar] [CrossRef] [Green Version]
- Bito, T.; Tanioka, Y.; Watanabe, F. Characterization of vitamin B12 compounds from marine foods. Fish. Sci. 2018, 84, 747–755. [Google Scholar] [CrossRef]
- Manela-Azulay, M.; Bagatin, E. Cosmeceuticals vitamins. Clin. Dermatol. 2009, 27, 469–474. [Google Scholar] [CrossRef]
- Lorencini, M.; Brohem, C.A.; Dieamant, G.C.; Zanchin, N.I.; Maibach, H.I. Active ingredients against human epidermal aging. Ageing Res. Rev. 2014, 15, 100–115. [Google Scholar] [CrossRef]
- Paiva, L.; Lima, E.; Neto, A.I.; Marcone, M.; Baptista, J. Health-promoting ingredients from four selected Azorean macroalgae. Food Res. Int. 2016, 89, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Mercurio, D.G.; Wagemaker, T.A.L.; Alves, V.M.; Benevenuto, C.G.; Gaspar, L.R.; Maia Campos, P.M.B.G. In vivo photoprotective effects of cosmetic formulations containing UV filters, vitamins, Ginkgo biloba and red algae extracts. J. Photochem. Photobiol. B 2015, 153, 121–126. [Google Scholar] [CrossRef]
- Sumi, H.; Osada, K.; Yatagai, C.; Naito, S.; Yanagisawa, Y. High concentration of vitamin K1 proved in the seaweeds and sweet potato leaves. J.-Jpn. Soc. Food Sci. Technol. 2003, 50, 63–66. [Google Scholar] [CrossRef] [Green Version]
- Kamao, M.; Suhara, Y.; Tsugawa, N.; Uwano, M.; Yamaguchi, N.; Uenishi, K.; Ishida, H.; Sasaki, S.; Okano, T. Vitamin K content of foods and dietary vitamin K intake in Japanese young women. J. Nutr. Sci. Vitaminol. 2007, 53, 464–470. [Google Scholar] [CrossRef] [Green Version]
- Del Mondo, A.; Smerilli, A.; Sané, E.; Sansone, C.; Brunet, C. Challenging microalgal vitamins for human health. Microb. Cell Factories 2020, 19, 201. [Google Scholar] [CrossRef] [PubMed]
- Rupérez, P. Mineral content of edible marine seaweeds. Food Chem. 2002, 79, 23–26. [Google Scholar] [CrossRef]
- Circuncisão, A.R.; Catarino, M.D.; Cardoso, S.M.; Silva, A.M.S. Minerals from Macroalgae Origin: Health Benefits and Risks for Consumers. Mar. Drugs 2018, 16, 400. [Google Scholar] [CrossRef] [Green Version]
- Lozano Muñoz, I.; Díaz, N.F. Minerals in edible seaweed: Health benefits and food safety issues. Crit. Rev. Food Sci. Nutr. 2020, 18, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Tarnowska, M.; Briancon, S.; Resende de Azevedo, J.; Chevalier, Y.; Bolzinger, M.-A. Inorganic ions in the skin: Allies or enemies? Int. J. Pharm. 2020, 591, 119991. [Google Scholar] [CrossRef]
- Kraan, S. Pigments and Minor Compounds in Algae. In Functional Ingredients from Algae for Foods and Nutraceuticals; Domínguez, H., Ed.; Woodhead Publishing: Cambridge, UK, 2013; pp. 205–251. [Google Scholar]
- Mohamed, S.; Hashim, S.N.; Rahman, H.A. Seaweeds: A sustainable functional food for complementary and alternative therapy. Trends Food Sci. Technol. 2012, 23, 83–96. [Google Scholar] [CrossRef]
- Polefka, T.G.; Bianchini, R.J.; Shapiro, S. Interaction of mineral salts with the skin: A literature survey. Int. J. Cosmet. Sci. 2012, 34, 416–423. [Google Scholar] [CrossRef]
- Martins, A.; Vieira, H.; Gaspar, H.; Santos, S. Review: Marketed marine natural products in the pharmaceutical and cosmeceutical industries: Tips for success. Mar. Drugs 2014, 12, 1066–1101. [Google Scholar] [CrossRef] [Green Version]
- Guillerme, J.-B.; Couteau, C.; Coiffard, L. Applications for Marine Resources in Cosmetics. Cosmetics 2017, 4, 35. [Google Scholar] [CrossRef] [Green Version]
- Alves, A.; Sousa, E.; Sousa, E.; Kijjoa, A.; Pinto, M. Marine-derived compounds with potential use as cosmeceuticals and nutricosmetics. Molecules 2020, 25, 2536. [Google Scholar] [CrossRef] [PubMed]
- Chakdar, H.; Pabbi, S. Algal Pigments for Human Health and Cosmeceuticals. In Algal Green Chemistry; Rastogi, R.P., Madamwar, D., Pandey, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 171–188. [Google Scholar]
- Christaki, E.; Bonos, E.; Giannenasa, I.; Florou-Paneria, P. Functional properties of carotenoids originating from algae. J. Sci. Food Agric. 2013, 93, 5–11. [Google Scholar] [CrossRef]
- Rajauria, G. In-Vitro Antioxidant Properties of Lipophilic Antioxidant Compounds from 3 Brown Seaweed. Antioxidants 2019, 8, 596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maeda, H.; Hosokawa, M.; Sashima, T.; Funayama, K.; Miyashita, K. Fucoxanthin from edible seaweed, Undaria pinnatifida, shows antiobesity effect through UCP1 expression in white adipose tissues. Biochem. Biophys. Res. Commun. 2005, 332, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Gammone, M.A.; D’Orazio, N. Anti-obesity activity of the marine carotenoid fucoxanthin. Mar. Drugs 2015, 13, 2196–2214. [Google Scholar] [CrossRef] [PubMed]
- Rajauria, G.; Foley, B.; Abu-Ghannam, N. Characterization of dietary fucoxanthin from Himanthalia elongata brown seaweed. Food Res. Int. 2017, 99, 995–1001. [Google Scholar] [CrossRef]
- Shimoda, H.; Tanaka, J.; Shan, S.; Maoka, T. Anti-pigmentary activity of fucoxanthin and its influence on skin mRNA expression of melanogenic molecules. J. Pharm. Pharmacol. 2010, 62, 1137–1145. [Google Scholar] [CrossRef]
- Peng, J.; Yuan, J.P.; Wu, C.F.; Wang, J.H. Fucoxanthin, a marine carotenoid present in brown seaweeds and diatoms: Metabolism and bioactivities relevant to human health. Mar. Drugs 2011, 9, 1806–1828. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.; Jeon, Y. Protective effect of fucoxanthin isolated from Sargassum siliquastrum on UV-B induced cell damage. J. Photochem. Photobiol. B 2009, 95, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.-J.; Yoon, W.-J.; Kim, K.-N.; Ahn, G.-N.; Kang, S.-M.; Kang, D.-H.; Affan, A.; Oh, C.; Jung, W.-K.; Jeon, Y.-J. Evaluation of anti-inflammatory effect of fucoxanthin isolated from brown algae in lipopolysaccharide-stimulated RAW 264.7 macrophages. Food Chem. Toxicol. 2010, 48, 2045–2051. [Google Scholar] [CrossRef]
- Matsui, M.; Tanaka, K.; Higashiguchi, N.; Okawa, H.; Yamada, Y.; Tanaka, K.; Taira, S.; Aoyama, T.; Takanishi, M.; Natsume, C.; et al. Protective and therapeutic effects of fucoxanthin against sunburn caused by UV irradiation. J. Pharmacol. Sci. 2016, 132, 55–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nie, J.; Chen, D.; Lu, Y.; Dai, Z. Effects of various blanching methods on fucoxanthin degradation kinetics, antioxidant activity, pigment composition, and sensory quality of Sargassum fusiforme. LWT-Food Sci. Tecnol. 2021, 143, 111179. [Google Scholar] [CrossRef]
- Jia, Y.P.; Sun, L.; Yu, H.S.; Liang, L.P.; Li, W.; Ding, H.; Song, X.B.; Zhang, L.J. The Pharmacological Effects of Lutein and Zeaxanthin on Visual Disorders and Cognition Diseases. Molecules 2017, 22, 610. [Google Scholar] [CrossRef] [PubMed]
- Naser, W. The cosmetic effects of various natural biofunctional ingredients against skin aging: A review. Int. J. Appl. Pharm. 2021, 13, 10–18. [Google Scholar] [CrossRef]
- Bagal-Kestwal, D.R.; Pan, M.H.; Chiang, B.-H. Properties and Applications of Gelatin, Pectin, and Carrageenan Gels. In Bio Monomers for Green Polymeric Composite Materials; Visakh, P.M., Bayraktar, O., Menon, G., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2019; pp. 117–140. [Google Scholar]
- Tarman, K.; Ain, N.H.; Sulistiawati, S.; Hardjito, L.; Sadi, U. Biological process to valorise marine algae. IOP Conf. Ser. Earth Environ. Sci. 2020, 414, 012026. [Google Scholar] [CrossRef]
- Li, D.; Wu, Z.; Martini, N.; Wen, J. Advanced carrier systems in cosmetics and cosmeceuticals: A review. J. Cosmet. Sci. 2011, 62, 549–563. [Google Scholar]
- Peng, J.; Xu, W.; Ni, D.; Zhang, W.; Zhang, T.; Guang, C.; Mu, W. Preparation of a novel water-soluble gel from Erwinia amylovora levan. Int. J. Biol. Macromol. 2019, 122, 469–478. [Google Scholar] [CrossRef]
- Prima, N.R.; Andriyono, S. Techniques of additional Kappaphycus alvarezii on seaweed face mask production. IOP Conf. Ser. Earth Environ. Sci. 2021, 679, 012021. [Google Scholar] [CrossRef]
- Eom, S.; Kim, Y.; Kim, S. Antimicrobial effect of phlorotannins from marine brown algae. Food Chem. Toxicol. 2012, 50, 3251–3255. [Google Scholar] [CrossRef]
- Abu-Ghannam, N.; Rajauria, G. Antimicrobial Activity of Compounds Isolated from Algae. In Functional Ingredients from Algae for Foods and Nutraceuticals; Domínguez, H., Ed.; Woodhead Publishing: Cambridge, UK, 2013; pp. 287–306. [Google Scholar]
- Pérez, M.J.; Falqué, E.; Domínguez, H. Antimicrobial action of compounds from marine seaweed. Mar. Drugs 2016, 14, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopes, G.; Sousa, C.; Silva, L.R.; Pinto, E.; Andrade, P.B.; Bernardo, J.; Mouga, T.; Valentão, P. Can phlorotannins purified extracts constitute a novel pharmacological alternative for microbial infections with associated inflammatory conditions? PLoS ONE 2012, 7, 31145. [Google Scholar] [CrossRef]
- Amiguet, V.T.; Jewell, L.E.; Mao, H.; Sharma, M.; Hudson, J.B.; Durst, T.; Allard, M.; Rochefort, G.; Arnason, J.T. Antibacterial properties of a glycolipid-rich extract and active principle from Nunavik collections of the macroalgae Fucus evanescens C. Agardh (Fucaceae). Can. J. Microbiol. 2011, 57, 745–749. [Google Scholar] [CrossRef] [PubMed]
- Arguelles, E.D.L.R.; Sapin, A.B. Bioprospecting of Turbinaria ornata (Fucales, phaeophyceae) for cosmetic application: Antioxidant, tyrosinase inhibition and antibacterial activities. J. Int. Soc. Southeast Asian Agric. Sci. 2020, 26, 30–41. [Google Scholar]
- Arguelles, E.R.; Sapin, A.B. Bioactive properties of Sargassum siliquosum J. Agardh (Fucales, Ochrophyta) and its potential as source of skin-lightening active ingredient for cosmetic application. J. Appl. Pharm. Sci. 2020, 10, 51–58. [Google Scholar]
- Arguelles, E.L.R. Evaluation of antioxidant capacity, tyrosinase inhibition, and antibacterial activities of brown seaweed, Sargassum ilicifolium (Turner) c. agardh 1820 for cosmeceutical application. J. Fish. Environ. 2021, 45, 64–77. [Google Scholar]
- Kim, I.H.; Lee, D.G.; Lee, S.H.; Ha, J.M.; Ha, B.J.; Kim, S.K.; Le, J.H. Antibacterial activity of Ulva lactuca against methicillin-resistant Staphylococcus aureus (MRSA). Biotechnol. Bioprocess Eng. 2007, 12, 579–582. [Google Scholar] [CrossRef]
- Pierre, G.; Sopena, V.; Juin, C.; Mastouri, A.; Graber, M.; Maugard, T. Antibacterial activity of a sulfated galactan extracted from the marine alga Chaetomorpha aerea against Staphylococcus aureus. Biotechnol. Bioprocess Eng. 2011, 16, 937–945. [Google Scholar] [CrossRef]
- Ha, Y.; Choi, J.; Lee, B.; Moon, H.E.; Cho, K.K.; Choi, I.S. Inhibitory effects of seaweed extracts on the growth of the vaginal bacterium Gardnerella vaginalis. J. Environ. Biol. 2014, 35, 537–542. [Google Scholar] [PubMed]
- Wei, Y.; Liu, Q.; Yu, J.; Feng, Q.; Zhao, L.; Song, H.; Wang, W. Antibacterial mode of action of 1, 8-dihydroxy-anthraquinone from porphyrahaitanensis against Staphylococcus aureus. Nat. Prod. Res. 2015, 29, 976–979. [Google Scholar] [CrossRef]
- Widowati, I.; Suprijanto, J.; Trianto, A.; Puspita, M.; Bedoux, G.; Bourgougnon, N. Antibacterial activity and proximate analysis of Sargassum extracts as cosmetic additives in a moisturizer cream. AACL Bioflux 2019, 12, 1961–1969. [Google Scholar]
- Poyato, C.; Thomsen, B.R.; Hermund, D.B.; Ansorena, D.; Astiasarán, I.; Jónsdóttir, R.; Kristinsson, H.G.; Jacobsen, C. Antioxidant effect of water and acetone extracts of Fucus vesiculosus on oxidative stability of skin care emulsions. Eur. J. Lipid Sci. Technol. 2017, 119, 1600072. [Google Scholar] [CrossRef] [Green Version]
- Paiva, A.A.D.O.; Castro, A.J.G.; Nascimento, M.S.; Will, L.S.E.P.; Santos, N.D.; Araújo, R.M.; Xavier, C.A.C.; Rocha, F.A.; Leite, E.L. Antioxidant and anti-inflammatory effect of polysaccharides from Lobophora variegata on zymosan-induced arthritis in rats. Int. Immunopharmacol. 2011, 11, 1241–1250. [Google Scholar] [CrossRef] [Green Version]
- Nursid, M.; Khatulistiani, T.S.; Noviendri, D.; Hapsari, F.; Hardiyati, T. Total phenolic content, antioxidant activity and tyrosinase inhibitor from marine red algae extract collected from Kupang, East Nusa Tenggara. IOP Conf. Ser. Earth Environ. Sci. 2020, 493, 012013. [Google Scholar] [CrossRef]
- Sari, D.S.P.; Saputra, E.; Alamsjah, M.A. Potential of fucoxanthin content in Sargassum sp. On sunscreen cream preparation. Int. J. Recent Technol. Eng. 2019, 7, 448–451. [Google Scholar]
- Fransiska, D.; Darmawan, M.; Sinurat, E.; Sedayu, B.B.; WArdana, Y.W.; Herdiana, Y.; Setiana, G.P. Characteristics of Oil in Water (o/w) Type Lotions Incorporated with Kappa/Iota Carrageenan. IOP Conf. Ser. Earth Environ. Sci. 2021, 715, 012050. [Google Scholar] [CrossRef]
- Cunha, S.C.; Fernandes, J.O.; Vallecillos, L.; Cano-Sancho, G.; Domingo, J.L.; Pocurull, E.; Borrull, F.; Mauvault, A.L.; Ferrari, E.; Fernández-Tejedor, M.; et al. Co-occurrence of musk fragrances and UV-filters in seafood and macroalgae collected in European hotspots. Environ. Res. 2015, 143, 65–71. [Google Scholar] [CrossRef]
- Tiwari, R.; Tiwari, G.; Lahiri, A.; Vadivelan, R.; Rai, A.K. Localized delivery of drugs through medical textiles for treatment of burns: A perspective approach. Adv. Pharm. Bull. 2021, 11, 248–260. [Google Scholar]
- Mohan, R.; Singh, S.; Kumar, G.; Srivastava, M. Evaluation of gelling behavior of natural gums and their formulation prospects. Indian J. Pharm. Educ. Res. 2020, 54, 1016–1023. [Google Scholar] [CrossRef]
- Dita, L.R.; Sudarno, S.; Triastuti, J. Utilization of agar Gracilaria sp. as a natural thickener on liquid bath soap formulation. IOP Conf. Ser. Earth Environ. Sci. 2020, 441, 012021. [Google Scholar] [CrossRef]
- Hu, B.; Han, L.; Ma, R.; Phillips, G.O.; Nishinari, K.; Fang, Y. All-natural food-grade hydrophilic–hydrophobic core–shell microparticles: Facile fabrication based on gel-network-restricted antisolvent method. ACS Appl. Mater. Interfaces 2019, 11, 11936–11946. [Google Scholar] [CrossRef] [PubMed]
- Wasupalli, G.K.; Verma, D. Polysaccharides as Biomaterials. In Fundamental Biomaterials: Polymers; Thomas, S., Balakrishnan, P., Sreekala, M.R., Eds.; Woodhead Publishing: Cambridge, UK, 2018; pp. 37–70. [Google Scholar]
- Tafuro, G.; Costantini, A.; Baratto, G.; Francescato, S.; Busata, L.; Semenzato, A. Characterization of polysaccharidic associations for cosmetic use: Rheology and texture analysis. Cosmetics 2021, 8, 62. [Google Scholar] [CrossRef]
- Zhu, B.; Ni, F.; Sun, Y.; Zhu, X.; Yin, H.; Yao, Z.; Du, Y. Insight into carrageenases: Major review of sources, category, property, purification method, structure, and applications. Crit. Rev. Biotechnol. 2018, 38, 1261–1276. [Google Scholar] [CrossRef]
- Vilela, A.; Cosme, F.; Pinto, T. Emulsions, Foams, and Suspensions: The Microscience of the Beverage Industry. Beverages 2018, 4, 25. [Google Scholar] [CrossRef] [Green Version]
- Nilforoushzadeh, M.A.; Amirkhani, M.A.; Zarrintaj, P.; Salehi Moghaddam, A.; Mehrabi, T.; Alavi, S.; Mollapour Sisakht, M. Skin care and rejuvenation by cosmeceutical facial mask. J. Cosmet. Dermatol. 2018, 17, 693–702. [Google Scholar] [CrossRef]
- Graham, S.; Marina, P.F.; Blencowe, A. Thermoresponsive polysaccharides and their thermoreversible physical hydrogel networks. Carbohydr. Polym. 2019, 207, 143–159. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Moon, W.S.; Choi, J.N.; Do, K.H.; Moon, S.H.; Cho, K.K.; Han, C.J.; Choi, I.S. Effects of seaweed Laminaria japonica extracts on skin moisturizing activity in vivo. Int. J. Cosmet. Sci. 2013, 64, 193–205. [Google Scholar]
- Dolorosa, M.T.; Nurjanah; Purwaningsih, S.; Anwar, E.; Hidayat, T. Tyrosinase inhibitory activity of Sargassum plagyophyllum and Eucheuma cottonii methanol extracts. IOP Conf. Ser. Earth Environ. Sci. 2019, 278, 012020. [Google Scholar] [CrossRef]
- Cha, S.H.; Ko, S.C.; Kim, D.; Jeon, Y.J. Screening of marine algae for potential tyrosinase inhibitor: Those inhibitors reduced tyrosinase activity and melanin synthesis in zebrafish. J. Dermatol. 2011, 38, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.Y.; Kim, K.H.; Cheah, S.H. Inhibitory effects of Sargassum polycystum on tyrosinase activity and melanin formation in B16F10 murine melanoma cells. J. Ethnopharmacol. 2011, 137, 1183–1188. [Google Scholar] [CrossRef]
- Park, J.; Lee, H.; Choi, S.; Pandey, L.K.; Depuydt, S.; De Saeger, J.; Park, J.-T.; Han, T. Extracts of red seaweed, Pyropia yezoensis, inhibit melanogenesis but stimulate collagen synthesis. J. Appl. Phycol. 2021, 33, 653–662. [Google Scholar] [CrossRef]
- Jesumani, V.; Du, H.; Pei, P.; Aslam, M.; Huang, N. Comparative study on skin protection activity of polyphenol-rich extract and polysaccharide-rich extract from Sargassum vachellianum. PLoS ONE 2020, 15, e0227308. [Google Scholar]
- Thu, N.T.H.; Anh, H.T.L.; Hien, H.T.M.; Ha, N.C.; Tam, L.T.; Khoi, T.X.; Duc, T.M.; Hong, D.D. Preparation and evaluation of cream mask from Vietnamese seaweeds. J. Cosmet. Sci. 2018, 69, 447–462. [Google Scholar] [PubMed]
- Pratama, G.; Yanuarti, R.; Ilhamdy, A.F.; Suhana, M.P. Formulation of sunscreen cream from Eucheuma cottonii and Kaempferia galanga (zingiberaceae). IOP Conf. Ser. Earth Environ. Sci. 2019, 278, 012062. [Google Scholar] [CrossRef]
- Poulose, N.; Sajayan, A.; Ravindran, A.; Sreechithra, T.V.; Vardhan, V.; Selvin, J.; Kiran, G.S. Photoprotective effect of nanomelanin-seaweed concentrate in formulated cosmetic cream: With improved antioxidant and wound healing properties. J. Photochem. Photobiol. B 2020, 205, 111816. [Google Scholar] [CrossRef] [PubMed]
- Pallela, R. Antioxidants from Marine Organisms and Skin Care. In Systems Biology of Free Radicals and Antioxidants; Laher, I., Ed.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 3771–3783. [Google Scholar]
- Raikou, V.; Protopapa, E.; Kefala, V. Photo-protection from marine organisms. Rev. Clin. Pharmacol. Pharmacokinet. 2011, 25, 131–136. [Google Scholar]
- Riani Mansauda, K.L.; Anwar, E.; Nurhayati, T. Antioxidant and anti-collagenase activity of sargassum plagyophyllum extract as an anti-wrinkle cosmetic ingredient. Pharmacogn. Mag. 2018, 10, 932–936. [Google Scholar]
- Kasitowati, R.D.; Wahyudi, A.; Asmara, R.; Aliviyanti, D.; Iranawati, F.; Panjaitan, M.A.P.; Pratiwi, D.C.; Arsad, S. Identification photoprotective activity of marine seaweed: Eucheuma sp. IOP Conf. Ser. Earth Environ. Sci. 2021, 679, 012014. [Google Scholar] [CrossRef]
- Sami, F.J.; Soekamto, N.H.; Firdaus; Latip, J. Bioactivity profile of three types of seaweed as an antioxidant, uv-protection as sunscreen and their correlation activity. Food Res. 2021, 5, 441–447. [Google Scholar] [CrossRef]
- Pangestuti, R.; Siahaan, E.A.; Kim, S.-K. Photoprotective substances derived from marine algae. Mar. Drugs 2018, 16, 399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hameury, S.; Borderie, L.; Monneuse, J.-M.; Skorski, G.; Pradines, D. Prediction of skin anti-aging clinical benefits of an association of ingredients from marine and maritime origins: Ex vivo evaluation using a label-free quantitative proteomic and customized data processing approach. J. Cosmet. Dermatol. 2019, 18, 355–370. [Google Scholar] [CrossRef] [Green Version]
- Uji, Y.; Shibayama, J.; Shirakawa, Y. Oil-In-Water Type Emulsified Composition for External Skin Use. JPH0366281B2, 16 October 1991. [Google Scholar]
- Ando, H.; Ando, Y. Cosmetic Containing Agar Oligosaccharide and/or Its Esterified Substance. JP3223038B2, 29 October 2001. [Google Scholar]
- Kang, N.G.; Park, B.G. Solid-Type Cosmetic Composition for Moisturizing. KR20120019409A, 6 March 2012. [Google Scholar]
- Luo, Q.; Peng, Y. Powder-Containing Oil-in-Water Type Solid-State Cosmetic Composition and Preparation Method and Application Thereof. CN110664628A, 10 January 2020. [Google Scholar]
- Kawagishi, F.; Yamada, K.; Yokota, S. Pack Cosmetic. JPH11302124A, 2 November 1999. [Google Scholar]
- Hasunuma, K.; Saito, M. Powdery Pack Cosmetic. JPH08217631A, 27 August 1996. [Google Scholar]
- Nakagaki, E.; Suzuki, T. Hair Cosmetic. JP2779926B2, 23 July 1998. [Google Scholar]
- Sawaki, S.; Yamada, K. Water-Soluble Powdery Cosmetic. JP2889922B2, 10 May 1999. [Google Scholar]
- Pedroso De Oliveira, A.P. A Base Composition for Preparing Multi-Functional Formulations for Skin Care and Process for the Preparation Thereof. MXPA04009861A, 18 April 2005. [Google Scholar]
- Igarashi, Y.; Kobayashi, M.; Oka, S.; Takagaki, K. Scalp and Hair Cosmetic. JP2015030670A, 16 February 2015. [Google Scholar]
- Bi, L.; Pan, S.; Shao, X.; Sui, H.; Zhao, L.; Zou, P. Nano-Liposome Emulsion and Preparation Method Thereof. CN103876982A, 25 June 2014. [Google Scholar]
- Billiotte, J.-C.; Dampeirou, C. Cosmetic Skin Firming Compsn. For Combating Effects of Stress and Ageing. CH686997A5, 30 August 1996. [Google Scholar]
- Kim, K.B.; Ko, H.J.; Lee, D.H.; Lee, G.S.; Pyo, H.B. Ulva Spp Seaweed Hydrolysates That Have High Glucuronic Acid Cotent, Preparation Method Thereof and Antiaging Cosmetic Composition Containing the Same. KR101356535B1, 29 January 2014. [Google Scholar]
- Kong, Q.; Zhou, L. Anti-Aging Cosmetic Containing Seaweed Extract and Preparation Method Thereof. CN105030587A, 11 November 2015. [Google Scholar]
- Yao, Z. Marine Bioactive Cosmetic. CN112426381A, 2 March 2021. [Google Scholar]
- Yao, Z. Marine Bioactive Cosmetic. CN112451429A, 9 March 2021. [Google Scholar]
- Yang, H. Seaweed-Containing Cosmetic Additive and Preparation Method and Application Thereof. CN107669588A, 9 February 2018. [Google Scholar]
- Min, G.H. Functional Cosmetic Composition for Anti-Dust and Anti-Inflammatory Containing Natural Extract as an Effective Ingredient and Functional Cosmetic Including the Same. KR101988489B1, 12 June 2019. [Google Scholar]
- Kawashima, Y.; Uchibori, T. Antimicrobial Agent. JP2879590B2, 5 April 1999. [Google Scholar]
- Andre, G.; Pellegrini, L.; Pellegrini, M. Use of Undaria Pinnatifida Seaweed Extract in Cosmetic or Dermatological Compositions for Protecting the Skin and Visible Organs from the Harmful Effects of Oxygen Radicals and Atmospheric Pollution. FR2837383A1, 26 September 2003. [Google Scholar]
- Gwon, S.B.; Jo, J.H.; Joo, W.H.; Kim, E.H.; Lee, J.H.; Lee, J.H.; Park, S.H.; Ryu, G.Y. Antioxidant and Whitening Functional Cosmetics. KR101351187B1, 14 January 2014. [Google Scholar]
- Imada, K.; Mitsui, Y. Anti-Perspiration and Deodorization Cosmetic, and Method for Producing the Same. JP2008184395A, 14 August 2008. [Google Scholar]
- Park, D.I. Cosmetic Composition that Is Effective against Wrinkles. KR20120119797A, 31 October 2012. [Google Scholar]
- Hagino, H.; Saito, M. Cosmetics. US2004131580A1, 8 July 2004. [Google Scholar]
- Subuchi, H. Hair Shampoo, Hair Treatment, Hair-Growing Agent and Cosmetic Cream. JP2006008599A, 12 January 2006. [Google Scholar]
- Holtkoetter, O.; Scheunemann, V.; Schulze Zur Wiesche, E. Hair Treatment Agent. EP2457556A2, 23 November 2011. [Google Scholar]
- Aono, M.; Yamaoka, Y. Scalp and Hair Cosmetic. JP2012184220A, 27 September 2012. [Google Scholar]
- Cha, Y.J.; Hong, Y.C.; Hong, Y.K.; Kim, Y.J. Cosmetic Manufacturing Method Using Natural Originated Material and Cosmetic Thereof. KR20150063336A, 9 June 2015. [Google Scholar]
- Cheng, G.; Cheng, J. Nano-Element Shampoo. CN104739747A, 1 July 2015. [Google Scholar]
- Kim, K.E. Kelp, Deep Cleansing, Shampoo, Cosmetics. KR20110013849A, 10 February 2011. [Google Scholar]
- Egawa, M.; Nakamura, R. Skin Care Method. JP2015043973A, 12 March 2015. [Google Scholar]
- Courtin, O. Cosmetic Composition for Combating the Adverse Effects of Agents in the Atmosphere. FR2688137A1, 10 September 1993. [Google Scholar]
- Ishii, K. Combined Cosmetic. JP2011032200A, 17 February 2011. [Google Scholar]
- Wu, X. Cosmetic Hand Film. CN202682386U, 23 January 2013. [Google Scholar]
- Han, J.; Zhao, Y. Moisturizing and Oil-Controlling Cosmetic Composition and Preparation Method of Composition. CN111407684A, 14 July 2020. [Google Scholar]
- Bi, L.; Mou, W.; Sui, H.; Yang, J.; Yu, J.; Zhao, L.; Zou, P. Seaweed Composition with Oil-Control Function and Cosmetic Thereof. CN108904340A, 30 November 2018. [Google Scholar]
- Li, Y. Liquid Cosmetic Formula and Preparation Method Thereof. CN112022768A, 4 December 2020. [Google Scholar]
- Qian, F. Oil-Control Acne-Preventive Cosmetic with Pure Herbal Essence and Preparation Method Thereof. CN111888287A, 6 November 2020. [Google Scholar]
- Chen, J.; Jin, Z.; Ouyang, Z.; Quan, C.; Yu, G.; Zhang, F. Preparing Method of Traditional Chinese Medicine Anti-Acne Cosmetic. CN108434039A, 24 August 2018. [Google Scholar]
- Kim, D.S. Cosmetic Composition Comprising the Ozonized Oil and Femented Extract of Seaweed. KR20180013659A, 7 February 2018. [Google Scholar]
- Hua, C.; Huili, S.; Xin, C.; Zhigang, C. Marine Biological Function Cosmetic for Minimizing Pores. CN102178636A, 14 September 2011. [Google Scholar]
- Chen, Y.; Yang, S.; Zhou, Z. Production Process of Moisturizing Lotion with Pore Cleaning Function. CN111991324A, 28 August 2020. [Google Scholar]
- Nakamura, R. Pore-Shrinking Agent. JP2015030675A, 16 February 2015. [Google Scholar]
- Chun, J.U. Composition Having Anti-Wrinkle Effects Using the Natural Plant Extract and Cosmetic Composition Comprising the Same. KR101952695B1, 27 February 2019. [Google Scholar]
- Wang, J. Making Method of Anti-Wrinkle Beautifying Seaweed-Containing Flour. CN105685784A, 22 June 2016. [Google Scholar]
- Yu, K. Anti-Aging Composition and Cosmetic Product Containing Same. CN109431888A, 8 March 2019. [Google Scholar]
- Chen, H.; Chen, L.; Ge, X.; Wang, Y.; Yue, K. Composition Capable of Resisting Aging and Fading Spots and Cosmetic Containing Composition. CN108670927A, 19 October 2018. [Google Scholar]
- Osawa, Y.; Sawaki, S.; Tamaoki, S. Elastin-Like Agent and Cosmetic Containing the Same. JP2003342150A, 3 December 2003. [Google Scholar]
- Deng, Y. Vitamin B (VB) Face Cream Cosmetic Containing 24K Gold. CN102824285A, 19 December 2012. [Google Scholar]
- Gerčikovs, I.; Lando, O. Cosmetic Preparation for Taking Care of the Feet Skin. LV14321A, 20 April 2011. [Google Scholar]
- Gedouin, A.; Vallee, R. Production of Cosmetic Composition for Protecting Skin from Effects of Atmospheric Pollution. FR2779953A1, 24 December 1999. [Google Scholar]
- Hori, M.; Nishibe, Y.; Tanaka, K. Cosmetic Composition. JP2003104835A, 9 April 2003. [Google Scholar]
- Yang, Z. Seaweed Extraction Liquid, Preparation Method of Seaweed Extraction Liquid, Whitening Composition and Preparation Method of Whitening Composition. CN108324601A, 27 July 2018. [Google Scholar]
- Li, Y.; Pei, Q.; Shi, C.; Shi, H.; Shi, Z. Pearl Cosmetic Cellular Liquor and Preparation Method Thereof. CN106265463A, 4 January 2017. [Google Scholar]
- Choi, C.Y.; Choi, S.J.; Im, S.J.; Jeong, C.S.; Jeong, J.C.; Jo, A.; Kang, H.W.; Kim, H.G.; Kim, J.Y.; Kim, J.Y.; et al. Pharmaceutical Composition and Cosmetic Composition for Skin Regeneration Comprising Active Ingredient Extracted and Isolated from Porphyra Dentata. WO2020175754A1, 3 September 2020. [Google Scholar]
- Yang, G.; Zhao, J.; Zhao, Y. Facial Beautifying Composition and Preparation Method Thereof. CN111544369A, 18 August 2020. [Google Scholar]
- Takabayashi, M. Anti-Suntan Cosmetic and its Production. JP3568979B2, 22 September 2004. [Google Scholar]
- Paik, H.K. Cosmetic Compositions Containing Natural Ultraviolet Intercepting Agent Based Seaweed from Jeju Island. KR20140089997A, 16 July 2014. [Google Scholar]
- Han, J.S.; Lee, E.J.; Lee, J.W.; Oh, J.Y.; Park, S.G. Sunscreen Cosmetic Composition. KR20170004842A, 11 January 2017. [Google Scholar]
- Murakami, M.; Ota, K.; Saito, M.; Sumida, Y. Weight-Reducing Composition and Method for Reducing Weight. JPH08104618A, 23 April 1996. [Google Scholar]
- Azuma, T.; Hayashi, Y.; Ishihata, S.; Kuroda, A.; Sakai, K.; Sato, S. Sheetlike Pack Cosmetic for Body. JP2001019615A, 23 January 2001. [Google Scholar]
- Takabayashi, M. Beautifying and Whitening Cosmetic. JP2970767B2, 2 November 1999. [Google Scholar]
- Kawai, N.; Tanaka, K.; Wakamatsu, K. Cosmetic Composition. JP2001139419A, 22 May 2001. [Google Scholar]
- Kawai, N.; Tanaka, K.; Wakamatsu, K. Cosmetic Composition. JP2001302491A, 31 October 2001. [Google Scholar]
- Adachi, K.; Kotake, Y.; Suzuki, Y. Preparation for External Use for Skin. JP3460904B2, 27 October 2003. [Google Scholar]
- Kang, S.W.; Kim, E.J.; Kim, S.J. Skin Moisturizing and Funtional Composition Containing Fermented Seeweed. KR20190029897A, 21 March 2019. [Google Scholar]
- Han, S.H.; Hong, Y.J.; Kim, H.C.; Kim, Y.J. Cosmetic Composition Containing Gulfweed Extract, Sea Staghorn Extract, and Brown Seaweed Extract. WO2012070835A2, 31 May 2012. [Google Scholar]
- Ahn, D.H.; Kim, J.Y.; Moon, J.N.; Moon, W.S. Cosmetic Composition Containing Seaweed Extract. WO2015099280A1, 2 July 2015. [Google Scholar]
- Zhou, Y. Cosmetic Preparation with Anti-Allergic and Repairing Effects and Preparation Method Thereof. CN108578353A, 28 September 2018. [Google Scholar]
- Kang, D.H.; Kim, M.G.; Lee, B.H. Beauty Expenses Composite Containing Seaweed Extract. KR20150022365A, 4 March 2015. [Google Scholar]
- Cong, L.; Gao, H.; Li, C.; Lin, S.; Liu, D.; Liu, P.; Mao, Y.; Zhang, C.; Zhang, W. Sunscreen Cosmetic Composition and Method for Preparing Seaweed Sunscreen Components of Composition. CN104644511A, 27 May 2015. [Google Scholar]
- Cai, C.; Li, Y.; Wang, H. Facial Mask for Removing Freckles, Whitening and Relieving Sunburn and Preparation Method of Facial Mask. CN106619272A, 10 May 2017. [Google Scholar]
- Kawakubo, A.; Kouno, S.; Matsuka, S.; Motoyoshi, K.; Ninomiya, M.; Ota, Y. Photo-Aging Resister and Skin Cosmetic Containing the Resister. JP3432033B2, 28 July 2003. [Google Scholar]
- Gan, X.; Zhang, K. Eye-Beautifying Firming Eye Cream Cosmetic and Preparation Method Thereof. CN109875933A, 14 June 2019. [Google Scholar]
- Choi, J.B.; Jo, J.H.; Joo, W.H.; Kim, E.H.; Lee, J.H.; Lee, J.H.; Ryu, G.Y. Antioxidant And Whitening Functional Cosmetics. KR101413328B1, 1 July 2014. [Google Scholar]
- Li, K.; Liang, Y.; Wang, T.; Yue, Q.; Zhao, L. Method for Preparing Seaweed Fermentation Solution by Virtue of Probiotics Fermentation and Application of Seaweed Fermentation Solution in Cosmetics. CN108653059A, 16 October 2018. [Google Scholar]
- Choi, B.S.; Choi, J.H.; Chun, H.S.; Kim, S.; Kim, S.J.; Lee, H.J.; Park, E.T.; Park, S.E. Food Composition Comprising Algae Extracts and the Use Thereof. KR20130054518A, 27 May 2013. [Google Scholar]
- Hirose, K.; Hirose, Y. Composition Having Bleaching Activity and Cosmetic Containing the Same. JP2006036680A, 9 February 2006. [Google Scholar]
- Hirose, K.; Hirose, Y. Composition Having Hair Loss Preventing Action and Hair Loss Preventing Cosmetic Containing the Same. JP2006052151A, 23 February 2006. [Google Scholar]
- Zhang, R. Pure Natural Moisturizing Cosmetic. CN103690407A, 2 April 2014. [Google Scholar]
- Park, J.W. Cosmetic Composition for Spa Comprising Natural Mixture as Effective Component. KR20180024141A, 8 March 2018. [Google Scholar]
- Ku, W.L.; Lee, H.J. Cosmetic Composition Using Pine Tree and Seaweeds and Manufacturing Method of it. KR20200122677A, 28 October 2020. [Google Scholar]
- Park, S.L. Cosmetic Composition for Anti-Wrinkle Activity Comprising Fermented Soybean Paste and Seaweed Extracts as Active Ingredient. KR20200080500A, 7 July 2020. [Google Scholar]
- Oouma, T.; Takekoshi, Y.; Takahashi, T. Cosmetics. US2002009472A1, 24 January 2002. [Google Scholar]
- Choi, K.J. Cosmetics Composition. KR20090126670A, 9 December 2009. [Google Scholar]
- Guo, Z. Cosmetic Containing Active Peptide. CN105982831A, 5 October 2016. [Google Scholar]
- Qiu, H.Y.; BI, H.Y.; Chen, X.; Chen, W.Y.; Du, L.N.; Jin, Y.G. Curcumin Hydrogel Combined with Photodynamics to Treat Acne. J. Int. Pharm. Res. 2017, 44, 1125–1130. [Google Scholar]
- Cho, J.H. Cosmetic Composition Contained Seaweeds Extract with Silver Nano-Particles Colloid. KR20080035090A, 23 April 2008. [Google Scholar]
- Chen, J.; Chen, J. Anti-Inflammatory Sedation Patch Mask. CN112402339A, 26 February 2021. [Google Scholar]
- Kim, J.E.; Yang, Y.J. Mist Containing Seaweed Extract. KR20180080058A, 11 July 2018. [Google Scholar]
- Fukuhara, K.; Kamiyama, K. Hair Cosmetic. JP2005272396A, 6 October 2005. [Google Scholar]
- Kim, K.E. Kelp Shampco, Pock, Cosmetics. KR20110006337A, 20 January 2011. [Google Scholar]
- Chen, F.; Li, Y.; Wang, X. Washing Cosmetic Containing Seaweed Extract-Carrageen. CN104434696A, 25 March 2015. [Google Scholar]
- Sato, Y.; Sigihara, Y. Cosmetic Composition, Screening Method, and Cosmetic Method. WO2019098352A1, 23 May 2019. [Google Scholar]
- Huang, J.; Li, C.; Lin, S.; Liu, D.; Ma, J.; Qian, J.; Xiang, Q. Water-Free Washing-Free Gold Seaweed Cleaning Oil. CN109431878A, 8 March 2019. [Google Scholar]
- Chen, H.C. Diet Cosmetic Food and Its Production. CN101028075A, 5 September 2007. [Google Scholar]
- Takahashi, N. Production of Cosmetic or Bathing Agent. JPS62286907A, 12 December 1987. [Google Scholar]
- Hashimoto, H.; Hiraki, Y.; Ichioka, M.; Inami, M.; Kamiyama, S.; Kono, H.; Nagaoka, M.; Yoshikawa, S. Highly Pure Fucoidan and Preparation Thereof. JP2000351801A, 10 October 2000. [Google Scholar]
- Kusuoku, H.; Nishizawa, Y.; Shibuya, Y. Purification of Seaweed Extract. JP2000109407A, 18 April 2000. [Google Scholar]
- Kusuoku, H.; Nishizawa, Y.; Shibuya, Y. Method for Purifying Seaweed Extract. JP2005145983A, 9 June 2005. [Google Scholar]

| Component | Properties/Activities | Seaweed | References |
|---|---|---|---|
| Agar | Thickener; antioxidant | Pterocladia, Pterocladiella, Gelidium amansii, Gracilaria | [28,46,53,54,55] |
| Alginate | High stability, thickening agent, gelling agent | Brown seaweeds | [34,56,57] |
| Carrageenans | Antioxidant, antitumor, antiaging, thickeners properties, radiation protection | Red seaweeds, Porphyra haitanensis, Gracilaria chouae, Gracilaria blodgettii | [16,49,58,59,60,61,62] |
| Fucoidans | Photoaging inhibition; minimized elastase activity; antioxidant, anti-inflammatory collagenase and elastase inhibition, skin-whitening | Fucoidan (Sigma), Ascophyllum nodosum, Chnoospora minima, Ecklonia maxima, Hizikia fusiforme, Saccharina japonica, Sargassum hemiphyllum, Sargassum horneri, Sargassum polycystum, Sargassum vachellianum | [2,23,24,43,44,46,48,63,64,65,66,67] |
| Laminaran | Reconstructed dermis; skin cell anti-inflammation; antioxidant | Saccharina longicruris, Laminarin (Sigma) | [68,69] |
| Polysaccharides | Hydration | Saccharina japonica, Chondrus crispus, Codium tomentosum | [28] |
| Ulvan | Antiaging, antiherpetic | Ulva pertusa, Ulva sp. | [51,70] |
| Extract/Compound | Activity | Seaweed | Reference |
|---|---|---|---|
| Eleven mycosporine-like amino acids | UV-protective effect, antioxidant | Agarophyton chilense, Pyropia plicata and Champia novae-zelandiae | [147] |
| Mycosporine-like amino acids extract (with porphyra-334 and shinorine in a ratio of 2:1) | Anti-aging | Phorphyra umbilicalis | [151] |
| Mycosporine-like amino acids extract (mainly palythine and asterina-330) | Antioxidant, UV-protective effect, anti-aging | Curdieara covitzae, Iridaea cordata | [152] |
| Mycosporine-like amino acids extract (mainly porphyra-334, shinorine, palythine and asterina-330) | Antioxidant; UV-protective effect | Gracilaria vermiculophylla | [153] |
| Mycosporine-like amino acids extract (mainly palythine, asterina-330, shinorine, palythinol, porphyra-334 and usujirene) | Antioxidant, antiproliferative | Chondrus crispus, Mastocarpus stellatus, Palmaria palmata | [154] |
| Mycosporine-like amino acids extract (mainly deoxygadusol, palythene and usujirene) | Antioxidant | Rhodymenia pseudopalmata | [155] |
| Aqueous extract from freshwater macroalga (mainly polysaccharides and amino acids) | Skin moisturizing effect | Rhizoclonium hieroglyphicum | [156] |
| Peptide PPY1 | Anti-inflammatory | Pyropia yezoensis | [134] |
| Peptides PYP1-5 and porphyra 334 | Increase production of elastin and collagen | Porphyra yezoensis f. coreana Ueda | [135] |
| Methanol extract rich in proteins, vitamins, minerals, porphyra-334 and shinorine | Hydration, skin protective, anti-wrinkle, anti-roughness | Phorphyra umbilicalis | [148] |
| Phycobiliproteins (R-phycoerythrin allophycocyanin and phycocyanin) | Antioxidant | Gracilaria gracilis | [157] |
| Hydrolyzed extract | Antitumor | Porphyra haitanesis | [158] |
| Algae extract | Decrease of progerin production, anti-elastase, anti-collagenase | Alaria esculenta | [159] |
| Compound | Activity | Seaweed | References |
|---|---|---|---|
| Dioxinodehydroeckol | Preventive activity against UVB-induced apoptosis | Ecklonia cava | [190] |
| Dieckol | Adipogenesis inhibitory effect | Ecklonia cava | [197] |
| Eckol | Anti-inflammatory, anti-tyrosinase | Eisenia bicyclis, Ecklonia stolonifera | [192,193,198] |
| Eckol, 6,6′-bieckol, 8,8′-bieckol, dieckol, and phlorofucofuroeckol-A | Antiallergic | Ecklonia cava, E. stolonifera | [179] |
| Fucofuroeckol-A | Protective against UVB | Ecklonia stolonifera Okamura | [191] |
| Fuhalol | Antioxidant | Cystoseira compressa | [175] |
| Fucophloroethol (isomer) | Antioxidant | Fucus vesiculosus | [199] |
| Eckstolonol | Antioxidant enzymatic activities of catalase and superoxide dismutase | Ecklonia cava | [200] |
| Octaphlorethol A | Antioxidative effects | Ishige foliacea | [201] |
| Phlorofucofuroeckol A | Hepatoprotective effect against oxidative stress | Eisenia bicyclis | [93] |
| Tyrosinase inhibitory activity | Ecklonia stolonifera | [182] | |
| 2-phloroeckol and 2-O-(2,4,6-trihydroxyphenyl)-6,60-bieckol | Tyrosinase inhibitory activity | Ecklonia cava | [185] |
| Phlorofucofuroeckol B | Antiallergic | Eisenia arborea | [202] |
| Phlorotannins | Antioxidant, anticoagulant, antiinflammatory, antibacterial, antiviral, antitumor; antidiabetic, photoprotective | Brown algae, Ascophyllum nodosum, Fucus serratus, Himanthalia elongata, Halidrys siliquosa | [45,172,175,198,203,204] |
| Compound | Activity | Seaweed | References |
|---|---|---|---|
| E-10-oxooctadec-8-enoic acid, E-9-oxooctadec-10- enoic acid | Anti-inflammatory | Gracilaria verrucosa | [226] |
| Essential oil (tetradeconoic acid, hexadecanoic acid, (9Z, 12Z)-9,12-octadecadienoic acid, (9Z)-hexadec-9-enoic acid) | Antibacterial activity against Staphylococcus aureus and Bacillus cereus Antioxidant: radical scavenging (DPPH, superoxide, ABTS) | Laminaria japonica | [227] |
| Fucosterol | Antioxidant: increased antioxidative enzymes (superoxide dismutase, catalase, glutathione peroxidase) | Pelvetia siliquosa | [219,228] |
| Fucosterol | Anti-photodamage: decreased UVB-induced MMPs and increased procollagen Anti-inflammatory | Hizikia fusiformis | [60,188] |
| Phytosterol | Antitumoral | Commercial (Sigma) | [229] |
| Lipidic profile | Antioxidant, enzyme inhibition | Ulva rigida, Gracilaria sp., Fucus vesiculosus, Saccharina latissima | [211] |
| Unsaturated fatty acids | Antioxidant | Brown algae | [230] |
| Fatty acid profiling | Bioindicator of chemical stress | Pterocladia capillacea, Sargassum hornschuchii, Ulva lactuca | [231] |
| Element (Concentration) | Brown Algae | Green Algae | Red Algae |
|---|---|---|---|
| Ca (%) | 0.89–1.32 | 0.21–1.87 | 0.39–45.0 |
| Mg (%) | 0.22–1.2 | 0.12–2.8 | 0.20–167 |
| P (%) | 0.15–0.98 | 0.21–500 | 0.10–1.40 |
| K (%) | 3.8–11.5 | 1.1–8.1 | 0.33–10.2 |
| Na (%) | 1.3–7.1 | 0.52–8.9 | 1.1–4.3 |
| S (%) | 1.33–1.5 | 0.23–8.5 | 1.5–4.0 |
| Cu (ppm) | 1.1–11.0 | 1.6–12.1 | <0.4–35.0 |
| I (ppm) | 0.20–500 | 20–1000 | 0.24–1200 |
| Fe (ppm) | 15.8–270 | 17.7–2890 | 16–1820 |
| Mn (ppm) | <1–52.7 | <2–347 | <1–748 |
| Zn (ppm) | 2.5–52.3 | 1.98–84 | 7.2–714.4 |
| Extract/Compound | Activity | Seaweed | References |
|---|---|---|---|
| 97% fucoxanthin extract | Antioxidant (DPPH scavenging capacity, reducing power) | Himanthalia elongata | [265] |
| Fucoxanthin | Antioxidant, anti-melanogenesis | Brown seaweeds | [266,267] |
| Antiobesity | Undaria pinnatifida | [263] | |
| Skin protective (antiphotodamage, anti-pigmentary, antiphotoaging, anti-wrinkling | Sargassum siliquastrum | [268] | |
| Anti-inflammatory | Myagropsis myagroides | [269] | |
| Tyrosinase activity | Laminaria japonica | [266] | |
| Photoprotective | Undaria pinnatifida | [270] | |
| Antioxidant | Sargassum fusiforme, | [271] | |
| Lutein | Whitening; visual disorders and cognition diseases | Rhodophyta spp. | [28,272] |
| Activity | Applicant Company | References |
|---|---|---|
| Functional and sensorial | ||
| Emulsifying, water retention, gelling | Asahi Denka Kogyo Kk; Health Care Ltd.; Ichimaru Pharcos Inc; Iwasekenjiro Shoten Kk; Lg Household & Amp Lvxinyan Guangdong Bio Tech Co. Ltd. | [324,325,326,327] |
| Film forming | Kowa Techno Search Kk | [328] |
| Improved water solubility and imparting excellent feeling of use | Artnature Co. Ltd.; Kanebo Ltd.; Koosee Kk; Kyoei Kagaku Kogyo Kk; Natura Cosmeticos Sa; Toyo Shinyaku Co. Ltd.; | [329,330,331,332,333] |
| Stabilization system | Yantai New Era Health Industry Daily Chemical Co. Ltd. | [334] |
| Biological | ||
| Anti-aging and antistress | Givenchy Parfums; Hanbul Cosmetics Co. Ltd.; Hainan Hairun Biolog Technology Co. Ltd. Shengfeng Yantai Agricultural Tech Co. Ltd. | [335,336,337,338,339] |
| Anti-inflammatory | Explzn Inc; Yantai Yucheng Enterprise Man Consulting Co. Ltd. | [340,341] |
| Antimicrobial | Nippon Enu Yuu Esu Kk | [342] |
| Antioxidant | Gelyma; Jeollanamdo | [343,344] |
| Antiperspirant, desodorant | Japan Natural Lab Co. Ltd. | [345] |
| Anti-wrinkle | Mamachi Co. Ltd. | [346] |
| Bood circulation | Kowa Techno Search Kk | [328] |
| Hair and scalp care and treatment, hair growth | Clean Sea Co. Ltd.; Henkel Ag & Co Kgaa; Kose Corp; Nantong Snakebite Therapy Res Inst; Pinebio Co. Ltd.; Sako Kk; Shirako Co. Ltd.; Lion Corp | [347,348,349,350,351,352,353,354] |
| Moisturizing | Amazonebio Co. Ltd.; Clarins; Jingmen Nuoweiying New Material Tech Co. Ltd.; Kracie Home Products Ltd.; Qingdao Better Biolog Science & Technology Co. Ltd. | [308,355,356,357,358] |
| Oil control, acne prevention and removal of acne marks | Yantai New Era Health Ind Daily Chemical Co. Ltd.; Guangzhou Yuanmeisheng Cosmetic Co. Ltd.; Shanghai Bonaquan Cosmetics Co. Ltd.; Suzhou Cosmetic Materials Co. Ltd.; Tubio; Yantai New Era Health Ind Daily Chemical Co. Ltd. | [359,360,361,362,363] |
| Pore shrinking, cleaning and minimizing | Foshan Aai Cosmetic Health Care Product Co. Ltd.; South China Sea Inst Oceanology; Rongding Guangdong Biotechnology Co. Ltd.; Kose Corp | [364,365,366] |
| Prevention and amelioration of aged and rough skin | Anhui Shuanglu Flour Co. Ltd.; Dzintars As; Explzn Inc; Guangzhou Saliai Stemcell Science & Technology Co. Ltd.; Kyoei Chemical Ind; Nox Bellcow Zs Nonwoven Chemical Ltd.; Shanghai Bonaquan Cosmetics Co. Ltd.; Wuhu Chuanshi Information Tech Co. Ltd. | [360,367,368,369,370,371,372,373] |
| Protecting from pollution | Codif International Sa | [374] |
| Safe melanin production and whitenning | Ichimaru Pharcos Inc; Shenzhen Sanda Cosmetics Co. Ltd. | [375,376] |
| Skin regeneration and epidermal cell repair | Beihai Yuanlong Pearl Company Ltd.; Jeonnam Bioindustry Found; Hexie Tech Co. Ltd.; Mokpo Marin Food Industry Res Center; Yantai New Era Health Industry Daily Chemical Co. Ltd. | [334,377,378,379] |
| Sunscreen and anti-sun tan | Lg Household & Health Care Ltd.; Mikimoto Seiyaku Kk; Miin | [380,381,382] |
| Weight-reduction and slimming | Kanebo Ltd.; Sekisui Plastics | [383,384] |
| Whitening | Ichimaru Pharcos Inc; Lion Corp; Mikimoto Seiyaku Kk; World Costec Co. Ltd. | [385,386,387,388,389] |
| Mixed Effects, More Than One Of The Following Actions | ||
| Antiaging, anti-allergic, anti-inflammatory, antioxidant, anti-wrinkle; cleaning, moisturizing, repairing, sunscreen, whitenning | Amorepacific Corp; Baiyun Lianjia Fine Chemical Factory; Ecomine Co. Ltd.; Foshan Chancheng Relakongjian Biotechnology Co. Ltd.; Guangdong Danz Group Co. Ltd.; Guangzhou Baiyun Lianjia Fine Chemical Factory; Guangzhou Keneng Cosmetic Res Co. Ltd.; Guangzhou Xibao Daily Chemical Co. Ltd.; Hainan Shiboli Biotechnology Co. Ltd.; I2b Co. Ltd.; Jeollanamdo; Kaiso Shigen Kenkyusho Kk; Pola Chem Ind Inc | [344,390,391,392,393,394,395,396,397,398] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Hortas, L.; Flórez-Fernández, N.; Torres, M.D.; Ferreira-Anta, T.; Casas, M.P.; Balboa, E.M.; Falqué, E.; Domínguez, H. Applying Seaweed Compounds in Cosmetics, Cosmeceuticals and Nutricosmetics. Mar. Drugs 2021, 19, 552. https://doi.org/10.3390/md19100552
López-Hortas L, Flórez-Fernández N, Torres MD, Ferreira-Anta T, Casas MP, Balboa EM, Falqué E, Domínguez H. Applying Seaweed Compounds in Cosmetics, Cosmeceuticals and Nutricosmetics. Marine Drugs. 2021; 19(10):552. https://doi.org/10.3390/md19100552
Chicago/Turabian StyleLópez-Hortas, Lucía, Noelia Flórez-Fernández, Maria D. Torres, Tania Ferreira-Anta, María P. Casas, Elena M. Balboa, Elena Falqué, and Herminia Domínguez. 2021. "Applying Seaweed Compounds in Cosmetics, Cosmeceuticals and Nutricosmetics" Marine Drugs 19, no. 10: 552. https://doi.org/10.3390/md19100552
APA StyleLópez-Hortas, L., Flórez-Fernández, N., Torres, M. D., Ferreira-Anta, T., Casas, M. P., Balboa, E. M., Falqué, E., & Domínguez, H. (2021). Applying Seaweed Compounds in Cosmetics, Cosmeceuticals and Nutricosmetics. Marine Drugs, 19(10), 552. https://doi.org/10.3390/md19100552