Chemical Structure and Anticoagulant Property of a Novel Sulfated Polysaccharide from the Green Alga Cladophora oligoclada
Abstract
:1. Introduction
2. Results and Discussion
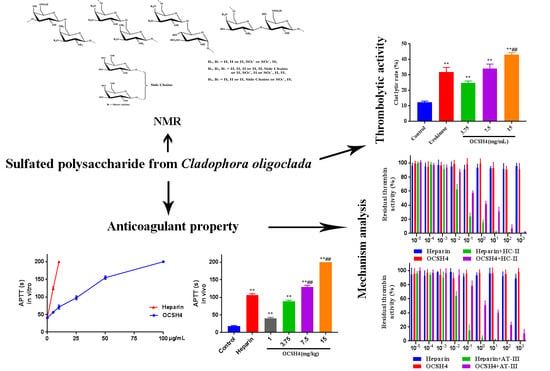
2.1. Structural Characteristics of the Sulfated Polysaccharide
2.2. Anticoagulant Activity In Vitro of OCSH4
2.3. Anticoagulant Activity In Vivo of OCSH4
2.4. Inhibition of Coagulation Factors II, V, X, VIII, IX, XI, and XII by OCSH4
2.5. Inhibition of Thrombin /Factor Xa by Heparin Cofactor-II (HC-II) or Antithrombin-III (AT-III) in the Presence of OCSH4
2.6. Thrombolytic Activity In Vitro of OCSH4
3. Materials and Methods
3.1. Materials
3.2. Animals
3.3. Isolation of the Sulfated Polysaccharide
3.4. Component Analysis of OCSH4
3.5. Methylation Analyses of OCSH4 and dsOCSH4
3.6. FTIR Spectroscopy
3.7. NMR Spectroscopy
3.8. Anticoagulant Activity In Vitro
3.9. Anticoagulant Activity In Vivo
3.10. Assay of Coagulation Factor II, V, X, VIII, IX, XI, or XII Activity
3.11. Inhibition of Thrombin/Factor Xa by HC-II or AT-III in the Presence of OCSH4
3.12. Thrombolytic Activity In Vitro
3.13. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Du, Z.; Shi, F.; Liu, D.; Ye, H.; Surhio, M.M.; Li, J.; Ye, M. Anticoagulant activity of a sulfated Lachnum polysaccharide in mice with a state of hypercoagulability. Bioorg. Med. Chem. Lett. 2016, 26, 5550–5556. [Google Scholar] [CrossRef]
- Mendis, S.; Davis, S.; Norrving, B. Organizational update: The world health organization global status report on noncommunicable diseases 2014, one more landmark step in the combat against stroke and vascular disease. Stroke 2015, 46, 121–122. [Google Scholar] [CrossRef] [PubMed]
- Kelton, J.G.; Warkentin, T.E. Heparin-induced thrombocytopenia: A historical perspective. Blood 2008, 112, 2607–2616. [Google Scholar] [CrossRef] [Green Version]
- Qiao, M.; Lin, L.; Xia, K.; Li, J.; Zhang, X.; Linhardt, R.J. Recent advances in biotechnology for heparin and heparan sulfate analysis. Talanta 2020, 219, 121270. [Google Scholar] [CrossRef] [PubMed]
- Lahaye, M.; Robic, A. Structure and functional properties of Ulvan, a polysaccharide from green seaweeds. Biomacromolecules 2007, 8, 1765–1773. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, E.V.; Byankina, A.O.; Kalitnik, A.A.; Kim, Y.H.; Bogdanovich, L.N.; Solov’eva, T.F.; Yermak, I.M. Influence of red algal sulfated polysaccharides on blood coagulation and platelets activation in vitro. J. Biomed. Mater. Res. A 2014, 102, 1431–1438. [Google Scholar] [CrossRef]
- Shang, F.; Mou, R.; Zhang, Z.; Gao, N.; Lin, L.; Li, Z.; Wu, M.; Zhao, J. Structural analysis and anticoagulant activities of three highly regular fucan sulfates as novel intrinsic factor Xase inhibitors. Carbohydr. Polym. 2018, 195, 257–266. [Google Scholar] [CrossRef]
- Wu, M.; Wen, D.; Gao, N.; Xiao, C.; Yang, L.; Xu, L.; Lian, W.; Peng, W.; Jiang, J.; Zhao, J. Anticoagulant and antithrombotic evaluation of native fucosylated chondroitin sulfates and their derivatives as selective inhibitors of intrinsic factor Xase. Eur. J. Med. Chem. 2015, 92, 257–269. [Google Scholar] [CrossRef]
- Yoon, S.J.; Pyun, Y.R.; Hwang, J.K.; Mourao, P.A.S. A sulfated fucan from the brown alga Laminaria cichorioides has mainly heparin cofactor II-dependent anticoagulant activity. Carbohydr. Res. 2007, 342, 2326–2330. [Google Scholar] [CrossRef]
- Cao, S.; He, X.; Qin, L.; He, M.; Yang, Y.; Liu, Z.; Mao, W. Anticoagulant and antithrombotic properties in vitro and in vivo of a novel sulfated polysaccharide from marine green alga Monostroma nitidum. Mar. Drugs 2019, 17, 247. [Google Scholar] [CrossRef] [Green Version]
- Harada, N.; Maeda, M. Chemical structure of antithrombin-active rhamnan sulfate from Monostroma nitidum. Biosci. Biotechnol. Biochem. 1998, 62, 1647–1652. [Google Scholar] [CrossRef]
- Mendes, M.; Presa, F.; Viana, R.; Costa, M.; Amorim, M.; Bellan, D.; Alves, M.; Costa, L.; Trindade, E.; Rocha, H. Anti-thrombin, anti-adhesive, anti-migratory, and anti-proliferative activities of sulfated galactans from the tropical green seaweed Udotea flabellum. Mar. Drugs 2018, 17, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adrien, A.; Bonnet, A.; Dufour, D.; Baudouin, S.; Maugard, T.; Bridiau, N. Anticoagulant activity of sulfated ulvan isolated from the green macroalga Ulva rigida. Mar. Drugs 2019, 17, 291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, L.; He, M.; Yang, Y.; Fu, Z.; Tang, C.; Shao, Z.; Zhang, J.; Mao, W. Anticoagulant-active sulfated arabinogalactan from Chaetomorpha linum: Structural characterization and action on coagulation factors. Carbohydr. Polym. 2020, 242, 116394. [Google Scholar] [CrossRef] [PubMed]
- Munir, M.; Qureshi, R.; Bibi, M.; Khan, A.M. Pharmaceutical aptitude of Cladophora: A comprehensive review. Algal Res. 2019, 39, 101476. [Google Scholar] [CrossRef]
- Arata, P.X.; Quintana, I.; Raffo, M.P.; Ciancia, M. Novel sulfated xylogalactoarabinans from green seaweed Cladophora falklandica: Chemical structure and action on the fibrin network. Carbohydr. Polym. 2016, 154, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Surayot, U.; Lee, J.H.; Park, W.; You, S. Structural characteristics of polysaccharides extracted from Cladophora glomerata Kützing affecting nitric oxide releasing capacity of RAW 264.7 cells. Bioact. Carbohydr. Diet. Fibre 2016, 7, 26–31. [Google Scholar] [CrossRef]
- Sun, X.; Wang, L.; Fu, R.; Yang, Y.; Cheng, R.; Li, J.; Wang, S.; Zhang, J. The chemical properties and hygroscopic activity of the exopolysaccharide lubcan from Paenibacillus sp. ZX1905. Int. J. Biol. Macromol. 2020, 164, 2641–2650. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Bi, S.; Li, H.; Li, J.; Li, C.; Yu, R.; Song, L.; Zhu, J. Purification and characterization of a novel mixed-linkage α, β-d-glucan from Arca subcrenata and its immunoregulatory activity. Int. J. Biol. Macromol. 2021, 182, 207–216. [Google Scholar] [CrossRef]
- Wagner, H.; Jordan, E. An immunologically active arabinogalactan from Viscum album ‘berries’. Phytochemistry 1988, 27, 2511–2517. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, T.; Wang, H.; Cui, Z.; Cheng, F.; Wang, K. Structural characterization and in vitro antitumor activity of an acidic polysaccharide from Angelica sinensis (Oliv.) Diels. Carbohydr. Polym. 2016, 147, 401–408. [Google Scholar] [CrossRef]
- Ciancia, M.; Fernandez, P.V.; Leliaert, F. Diversity of sulfated polysaccharides from cell walls of coenocytic green algae and their structural relationships in view of green algal evolution. Front. Plant Sci. 2020, 11, 554585. [Google Scholar] [CrossRef]
- Ramana, K.S.; Rao, E.V. Structural features of the sulphated polysaccharide from a green seaweed, Cladophora socialis. Phytochemistry 1991, 30, 259–262. [Google Scholar] [CrossRef]
- Yu, J.; Ji, H.; Yang, Z.; Liu, A. Relationship between structural properties and antitumor activity of Astragalus polysaccharides extracted with different temperatures. Int. J. Biol. Macromol. 2019, 124, 469–477. [Google Scholar] [CrossRef]
- Zayed, A.; Muffler, K.; Hahn, T.; Rupp, S.; Finkelmeier, D.; Burger-Kentischer, A.; Ulber, R. Physicochemical and biological characterization of fucoidan from Fucus vesiculosus purified by dye affinity chromatography. Mar. Drug 2016, 14, 79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandez, P.V.; Quintana, I.; Cerezo, A.S.; Caramelo, J.J.; Pol-Fachin, L.; Verli, H.; Estevez, J.M.; Ciancia, M. Anticoagulant activity of a unique sulfated pyranosic (1→3)-β-l-arabinan through direct interaction with thrombin. J. Biol. Chem. 2013, 288, 223–233. [Google Scholar] [CrossRef] [Green Version]
- Li, N.; Liu, X.; He, X.; Wang, S.; Cao, S.; Xia, Z. Structure and anticoagulant property of a sulfated polysaccharide isolated from the green seaweed Monostroma angicava. Carbohydr. Polym. 2017, 159, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Xu, H.; Xie, L.; Sun, J.; Sun, T.; Wu, X.; Fu, Q. Purification, characterization and in vitro anticoagulant activity of polysaccharides from Gentiana scabra Bunge roots. Carbohydr. Polym. 2016, 140, 308–313. [Google Scholar] [CrossRef]
- Madeira, J.C.; da Silva, G.V.L.; Batista, J.J.; Saraiva, G.D.; Santos, G.R.C.; Assreuy, A.M.; Mourão, P.A.S.; Pereira, M.G. An arabinogalactan-glycoconjugate from Genipa americana leaves present anticoagulant, antiplatelet and antithrombotic effects. Carbohydr. Polym. 2018, 202, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, A.P.; Gailani, D. The intrinsic pathway of coagulation as a target for antithrombotic therapy. Hematol. Oncol. Clin. N. Am. 2016, 30, 1099–1114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moran, C.S.; Seto, S.W.; Krishna, S.M.; Sharma, S.; Jose, R.J.; Biros, E.; Wang, Y.; Morton, S.K.; Golledge, J. Parenteral administration of factor Xa/IIa inhibitors limits experimental aortic aneurysm and atherosclerosis. Sci. Rep. 2017, 7, 1–12. [Google Scholar]
- Arata, P.X.; Quintana, I.; Canelón, D.J.; Vera, B.E.; Compagnone, R.S.; Ciancia, M. Chemical structure and anticoagulant activity of highly pyruvylated sulfated galactans from tropical green seaweeds of the order Bryopsidales. Carbohydr. Polym. 2015, 122, 376–386. [Google Scholar] [CrossRef]
- Melo, F.R.; Pereira, M.S.; Fogue, D.; Mourao, P.A.S. Antithrombin-mediated anticoagulant activity of sulfated polysaccharides. J. Biol. Chem. 2004, 279, 20824–20835. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Hao, J.; He, X.; Wang, S.; Cao, S.; Qin, L. A rhamnan-type sulfated polysaccharide with novel structure from Monostroma angicava Kjellm (Chlorophyta) and its bioactivity. Carbohydr. Polym. 2017, 173, 732–748. [Google Scholar] [CrossRef]
- Kuznetsova, T.A.; Besednova, N.N.; Mamaev, A.N.; Momot, A.P.; Shevchenko, N.M.; Zvyagintseva, T.N. Anticoagulant activity of fucoidan from brown algae Fucus evanescens of the Okhotsk Sea. Bull. Exp. Biol. Med. 2003, 136, 471–473. [Google Scholar] [CrossRef]
- Pozharitskaya, O.N.; Obluchinskaya, E.D.; Shikov, A.N. Mechanisms of bioactivities of fucoidan from the brown seaweed Fucus vesiculosus L. of the barents sea. Mar. Drugs 2020, 18, 275. [Google Scholar] [CrossRef]
- Dore, C.; Alves, M.; Will, L.; Costa, T.; Rêgo, L.; Accardo, C.; Rocha, H.; Filgueira, L.; Leite, E. A sulfated polysaccharide, fucans, isolated from brown algae Sargassum vulgare with anticoagulant, antithrombotic, antioxidant and anti-inflammatory effects. Carbohydr. Polym. 2013, 91, 467–475. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Therho, T.T.; Hartiala, K. Method for determination of the sulfate content of glycosaminoglycans. Anal. Biochem. 1971, 41, 471–476. [Google Scholar] [CrossRef]
- Bitter, T.; Muir, H.M. A modified uronic acid carbazole reaction. Anal. Biochem. 1962, 4, 330–334. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Chem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Tanaka, T.; Nakashima, T.; Ueda, T.; Tomii, K.; Kouno, I. Facilediscrimination of aldose enantiomers by reversed-phase HPLC. Chem. Pharm. Bull. 2007, 55, 899–901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, I.J.; Blunt, J.W. Desulfation of algal galactans. Carbohydr. Res. 1998, 309, 39–43. [Google Scholar] [CrossRef]
- Harris, P.J.; Henry, R.J.; Blakeney, A.B.; Stone, B.A. An improved procedure for the methylation analysis of oligosaccharides and polysaccharides. Carbohydr. Res. 1984, 127, 59–73. [Google Scholar] [CrossRef]
- Petersen, B.O.; Vinogradov, E.; Kay, W.; Würtz, P.; Nyberg, N.T.; Duus, J.Ø.; Sørensen, O.W. H2BC: A new technique for NMR analysis of complex carbohydrates. Carbohydr. Res. 2006, 341, 550–556. [Google Scholar] [CrossRef]
- Mackie, I.J.; Lawrie, A.S.; Kitchen, S.; Gaffney, P.J.; Howarth, D.; Lowe, G.D.O. A performance evaluation of commercial fibrinogen reference preparations and assays for clauss and PT-derived fibrinogen. Thromb. Haemost. 2002, 87, 997–1005. [Google Scholar]
- Mourao, P.A.S.; Pereira, M.S.; Pavao, M.S.G.; Mulloy, B. Tollefsen, D.M.; Mowinckel, M.C. Structure and anticoagulant activity of a fucosylated chondroitin sulfate from echinoderm-sulfated fucose branches on the polysaccharide account for its high anticoagulant action. J. Biol. Chem. 1996, 271, 23973–23984. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, O.; Hiraga, K. Haemorrhagic toxicosis in rats given butylated hydroxytoluene. Acta Pharmacol. Toxicol. 1981, 49, 14–20. [Google Scholar] [CrossRef]
- Colliec, S.; Fischer, A.M.; Tapon-Bretaudiere, J.; Boisson, C.; Durand, P.; Jozefonvicz, J. Anticoagulant properties of a fucoidan fraction. Thromb. Res. 1991, 64, 143–154. [Google Scholar] [CrossRef]
- Omura, K.; Hitosugi, M.; Zhu, X.; Ikeda, M.; Maeda, H.; Tokudome, S. A newly derived protein from Bacillus subtilis natto with both antithrombotic and fibrinolytic effects. J. Pharmacol. Sci. 2005, 99, 247–251. [Google Scholar] [CrossRef] [Green Version]










| Methylated Alditol Acetate | Molar Percent Ratio | Linkage Pattern | |
|---|---|---|---|
| OCSH4 | dsOCSH4 | ||
| 1,5-Di-O-acetyl-2,3,4,6-tetra-O-methyl-galactitol | 10.91 | 11.06 | Galp-(1→ |
| 1,3,5-Tri-O-acetyl-2,4,6-tri-O-methyl-galactitol | -- | 13.25 | →3)-Galp-(1→ |
| 1,5,6-Tri-O-acetyl-2,3,4-tri-O-methyl-glucitol | -- | 10.31 | →6)-Glcp-(1→ |
| 1,5,6-Tri-O-acetyl-2,3,4-tri-O-methyl-galactitol | 37.03 | 52.32 | →6)-Galp-(1→ |
| 1,4,5,6-Tetra-O-acetyl-2,3-di-O-methyl-galactitol | 10.44 | -- | →4,6)-Galp-(1→ |
| 1,3,5,6-Tetra-O-acetyl-2,4-di-O-methyl-glucitol | 10.33 | -- | →3,6)-Glcp-(1→ |
| 1,3,5,6-Tetra-O-acetyl-2,4-di-O-methyl-galactitol | 26.26 | 13.06 | →3,6)-Galp-(1→ |
| 1,2,5,6-Tetra-O-acetyl-3,4-di-O-methyl-galactitol | 5.03 | -- | →2,6)-Galp-(1→ |
| Sugar Residues | Chemical Shifts (ppm) | |||||
|---|---|---|---|---|---|---|
| H1/C1 | H2/C2 | H3/C3 | H4/C4 | H5/C5 | H6/C6 | |
| A→6)-α-d-Glcp-(1→ | 5.01/99.43 | 3.86/71.10 | 3.80/73.59 | 3.73/70.48 | 3.71/71.63 | 4.00;4.09/69.59 |
| B→3)-β-d-Galp-(1→ | 4.73/104.99 | 3.80/71.19 | 3.96/75.63 | 4.08/70.30 | 3.73/73.59 | 3.80;3.86/62.05 |
| C β-d-Galp-(1→ | 4.61/104.09 | 3.71/71.72 | 3.69/71.89 | 4.00/70.39 | 3.96/73.59 | 3.86/62.14 |
| D→3,6)-β-d-Galp-(1→ | 4.53/104.99 | 3.56/71.01 | 3.74/76.06 | 3.72/71.01 | 3.80/73.68 | 3.96/69.68 |
| E→6)-β-d-Galp-(1→ | 4.51/104.42 | 3.73/71.89 | 3.58/71.63 | 3.99/70.58 | 3.97/73.50 | 3.96/69.68 |
| Sugar Residues | Chemical Shifts (ppm) | |||||
|---|---|---|---|---|---|---|
| H1/C1 | H2/C2 | H3/C3 | H4/C4 | H5/C5 | H6/C6 | |
| A→6)-α-d-Glcp(3SO4)-(1→ | 5.09/99.57 | 3.99/70.31 | 4.48/79.55 | 3.74/72.26 | 3.72/72.31 | 4.94;4.14/70.31 |
| B→6)-β-d-Galp(2SO4)-(1→ | 4.76/103.03 | 4.40/79.64 | 3.69/70.48 | 3.99/70.31 | 3.95/74.03 | 3.94/70.40 |
| C β-d-Galp-(1→ | 4.62/104.30 | 3.72/73.95 | 3.69/71.32 | 3.98/70.40 | 3.96/74.63 | 3.80/62.43 |
| D→3)-β-d-Galp(6SO4)-(1→ | 4.60/104.30 | 3.69/72.26 | 3.73/77.34 | 4.05/70.31 | 3.57/72.26 | 4.23/72.26 |
| E→6)-β-d-Galp(4SO4)-(1→ | 4.59/104.30 | 3.57/73.87 | 3.59/72.26 | 4.43/77.34 | 3.99/74.03 | 3.94/70.23 |
| F→3,6)-β-d-Galp-(1→ | 4.53/104.88 | 3.56/72.18 | 3.73/77.34 | 3.57/71.32 | 3.59/73.95 | 3.95/70.23 |
| G→6)-β-d-Galp-(1→ | 4.50/104.88 | 3.72/72.26 | 3.57/72.26 | 3.99/70.31 | 3.95/74.03 | 3.94/70.40 |
| Samples | Concentration (mg/kg) | Clotting Time a (s) | ||
|---|---|---|---|---|
| APTT | TT | PT | ||
| OCSH4 | 0 | 18.2 ± 1.7 | 20.3 ± 0.9 | 15.3 ± 0.1 |
| 1 | 39.7 ± 3.3 ** | 35.5 ± 2.6 ** | 15.2 ± 0.7 | |
| 3.75 | 88.9 ± 2.7 ** | 42.1 ± 1.2 ** | 15.6 ± 0.4 | |
| 7.5 | 129.6 ± 4.5 **## | 60.4 ± 2.7 ** | 15.7 ± 0.9 | |
| 15 | >200 **## | 79.7 ± 3.4 **# | 15.7 ± 1.1 | |
| Heparin | 0 | 18.2 ± 1.7 | 20.3 ± 0.9 | 15.3 ± 0.1 |
| 1 | 106.4 ± 3.7 ** | 60.2 ± 0.8 ** | 55.3 ± 1.7 | |
| Coagulation Factors | Activities of Coagulation Factors (%) a | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| OCSH4 (μg/mL) | Heparin (μg/mL) | ||||||||
| 0 | 5 | 10 | 25 | 50 | 0 | 0.1 | 1 | 5 | |
| II | 101.8 ± 1.9 | 97.2 ± 2.9 | 98.4 ± 1.2 | 87.2 ± 1.2 | 63.7 ± 2.0 | 100.0 ± 0.9 | 90.7 ± 2.6 | 86.4 ± 2.1 | 75.2 ± 1.1 |
| V | 101.2 ± 2.21 | 98.7 ± 3.4 | 69.6 ± 1.8 | 35.5 ± 4.0 | 18.3 ± 1.1 | 99.9 ± 3.2 | 69.3 ± 4.7 | 41.6 ± 2.9 | <1.0 |
| VII | 100.0 ± 2.1 | 64.7 ± 2.2 | 41.4 ± 1.7 | 6.8 ± 4.0 | <1.0 | 100.4 ± 1.8 | 50.2 ± 2.7 | 1.4 ± 3.3 | <1.0 |
| IX | 100.0 ± 1.0 | 79.0 ± 3.2 | 53.5 ± 0.7 | 12.1 ± 0.9 | <1.0 | 100.9 ± 0.3 | 55.9 ± 0.7 | 2.3 ± 1.6 | <1.0 |
| X | 99.8 ± 1.1 | 97.1 ± 0.8 | 95.2 ± 1.5 | 94.4 ± 2.0 | 94.2 ± 2.6 | 100.5 ± 1.3 | 97.8 ± 3.1 | 95.2 ± 0.5 | 82.1 ± 3.3 |
| XI | 100.0 ± 0.2 | 72.5 ± 1.4 | 47.7 ± 0.4 | 9.1 ± 1.2 | <1.0 | 100.7 ± 1.2 | 42.0 ± 1.9 | 9.2 ± 1.1 | <1.0 |
| XII | 102.0 ± 2.4 | 41.3 ± 1.2 | 27.0 ± 0.3 | 7.9 ± 1.6 | <1.0 | 101.2 ± 1.9 | 59.7 ± 0.5 | 1.3 ± 0.2 | <1.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, M.; Yang, Y.; Shao, Z.; Zhang, J.; Feng, C.; Wang, L.; Mao, W. Chemical Structure and Anticoagulant Property of a Novel Sulfated Polysaccharide from the Green Alga Cladophora oligoclada. Mar. Drugs 2021, 19, 554. https://doi.org/10.3390/md19100554
He M, Yang Y, Shao Z, Zhang J, Feng C, Wang L, Mao W. Chemical Structure and Anticoagulant Property of a Novel Sulfated Polysaccharide from the Green Alga Cladophora oligoclada. Marine Drugs. 2021; 19(10):554. https://doi.org/10.3390/md19100554
Chicago/Turabian StyleHe, Meijia, Yajing Yang, Zhuling Shao, Junyan Zhang, Changning Feng, Lei Wang, and Wenjun Mao. 2021. "Chemical Structure and Anticoagulant Property of a Novel Sulfated Polysaccharide from the Green Alga Cladophora oligoclada" Marine Drugs 19, no. 10: 554. https://doi.org/10.3390/md19100554
APA StyleHe, M., Yang, Y., Shao, Z., Zhang, J., Feng, C., Wang, L., & Mao, W. (2021). Chemical Structure and Anticoagulant Property of a Novel Sulfated Polysaccharide from the Green Alga Cladophora oligoclada. Marine Drugs, 19(10), 554. https://doi.org/10.3390/md19100554