Posidonia oceanica (L.) Delile Dampens Cell Migration of Human Neuroblastoma Cells
Abstract
:1. Introduction
2. Results
2.1. Biochemical Properties and Antioxidant Activity of P. oceanica Leaf Extract (POE)
2.2. POE Does Not Affect SH-SY5Y Cell Viability
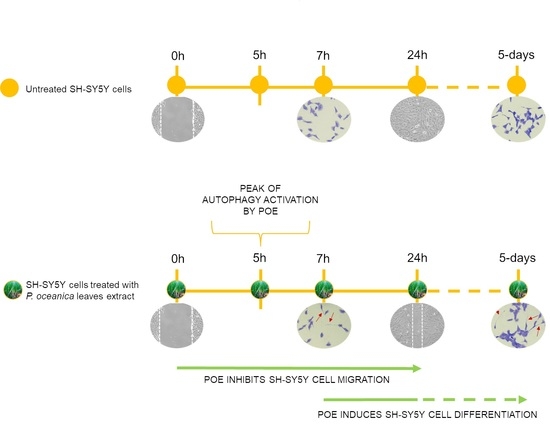
2.3. POE Blocks SH-SY5Y Cancer Cell Migration
2.4. POE Prevents Gelatinase Activity
2.5. POE Triggers Autophagy Activation
2.6. POE Induces a Morphological Change towards Cell Differentiation
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. P. oceanica Extraction Method
4.3. Cell Line and Culture Conditions
4.4. Cell Viability Assay
4.5. Wound Healing Assay
4.6. Gelatin Zymography
4.7. Western Blot Analysis
4.8. Cell Morphological Analysis
4.9. Immunofluorescence Staining
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Colon, N.C.; Chung, D.H. Neuroblastoma. Adv. Pediatr. 2011, 58, 297–311. [Google Scholar] [CrossRef] [Green Version]
- Park, J.R.; Eggert, A.; Caron, H. Neuroblastoma: Biology, prognosis, and treatment. Hematol. Oncol. Clin. N. Am. 2010, 24, 65–86. [Google Scholar] [CrossRef]
- Sokol, E.; Desai, A.V. The evolution of risk classification for neuroblastoma. Children 2019, 6, 27. [Google Scholar] [CrossRef] [Green Version]
- Neuroblastoma—Childhood: Statistics. Available online: https://www.cancer.net/cancer-types/neuroblastoma-childhood/statistics (accessed on 8 September 2021).
- Lambert, A.W.; Pattabiraman, D.R.; Weinberg, R.A. Emerging biological principles of metastasis. Cell 2017, 168, 670–691. [Google Scholar] [CrossRef] [Green Version]
- Fares, J.; Fares, M.Y.; Khachfe, H.H.; Salhab, H.A.; Fares, Y. Molecular principles of metastasis: A hallmark of cancer revisited. Signal Transduct. Target. Ther. 2020, 5, 28. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, Y.; Shimada, H.; Seeger, R.C.; Laug, W.E.; DeClerck, Y.A. Matrix metalloproteinases-2 and -9 are expressed in human neuroblastoma: Contribution of stromal cells to their production and correlation with metastasis. Cancer Res. 1998, 58, 2209–2216. [Google Scholar] [PubMed]
- Noujaim, D.; van Golen, C.M.; van Golen, K.L.; Grauman, A.; Feldman, E.L. N-Myc and Bcl-2 coexpression induces MMP-2 secretion and activation in human neuroblastoma cells. Oncogene 2002, 21, 4549–4557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, H.F.; Lai, K.C.; Hsu, S.C.; Lin, H.J.; Kuo, C.L.; Liao, C.L.; Yang, J.S.; Chung, J.G. Involvement of matrix metalloproteinases on the inhibition of cells invasion and migration by emodin in human neuroblastoma SH-SY5Y cells. Neurochem. Res. 2009, 34, 1575–1583. [Google Scholar] [CrossRef]
- Barletta, E.; Ramazzotti, M.; Fratianni, F.; Pessani, D.; Degl′Innocenti, D. Hydrophilic extract from Posidonia oceanica inhibits activity and expression of gelatinases and prevents HT1080 human fibrosarcoma cell line invasion. Cell Adhes. Migr. 2015, 9, 422–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, Z.; Wang, J.; Su, Q.; Luan, M.; Chen, X.; Xu, X. The role of MMP-2 and MMP-9 in the metastasis and development of hypopharyngeal carcinoma. Braz. J. Otorhinolaryngol. 2021, 87, 521–528. [Google Scholar] [CrossRef]
- Roy, R.; Yang, J.; Moses, M.A. Matrix metalloproteinases as novel biomarkers and potential therapeutic targets in human cancer. J. Clin. Oncol. 2009, 27, 5287–5297. [Google Scholar] [CrossRef] [Green Version]
- Björklund, M.; Koivunen, E. Gelatinase-mediated migration and invasion of cancer cells. Biochim. Biophys. Acta 2005, 1755, 37–69. [Google Scholar] [CrossRef] [Green Version]
- Cathcart, J.; Pulkoski-Gross, A.; Cao, J. Targeting matrix metalloproteinases in cancer: Bringing new life to old ideas. Genes Dis. 2015, 2, 26–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmer, T.D.; Ashby, W.J.; Lewis, J.D.; Zijlstra, A. Targeting tumor cell motility to prevent metastasis. Adv. Drug Deliv. Rev. 2011, 63, 568–581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomolonis, J.A.; Agarwal, S.; Shohet, J.M. Neuroblastoma pathogenesis: Deregulation of embryonic neural crest development. Cell Tissue Res. 2018, 372, 245–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bayeva, N.; Coll, E.; Piskareva, O. Differentiating neuroblastoma: A systematic review of the retinoic acid, its derivatives, and synergistic interactions. J. Pers. Med. 2021, 11, 211. [Google Scholar] [CrossRef] [PubMed]
- Hansen, L.A.; Sigman, C.C.; Andreola, F.; Ross, S.A.; Kelloff, G.J.; De Luca, L.M. Retinoids in chemoprevention and differentiation therapy. Carcinogenesis 2000, 21, 1271–1279. [Google Scholar] [CrossRef]
- Doldo, E.; Costanza, G.; Agostinelli, S.; Tarquini, C.; Ferlosio, A.; Arcuri, G.; Passeri, D.; Scioli, M.G.; Orlandi, A. Vitamin A, cancer treatment and prevention: The new role of cellular retinol binding proteins. Biomed. Res. Int. 2015, 2015, 624627. [Google Scholar] [CrossRef] [Green Version]
- Reynolds, C.P.; Matthay, K.K.; Villablanca, J.G.; Maurer, B.J. Retinoid therapy of high-risk neuroblastoma. Cancer Lett. 2003, 197, 185–192. [Google Scholar] [CrossRef]
- Cernaianu, G.; Brandmaier, P.; Scholz, G.; Ackermann, O.P.; Alt, R.; Rothe, K.; Cross, M.; Witzigmann, H.; Tröbs, R.B. All-trans retinoic acid arrests neuroblastoma cells in a dormant state. Subsequent nerve growth factor/brain-derived neurotrophic factor treatment adds modest benefit. J. Pediatr. Surg. 2008, 43, 1284–1294. [Google Scholar] [CrossRef]
- Ponthan, F.; Borgström, P.; Hassan, M.; Wassberg, E.; Redfern, C.P.F.; Kogner, P. The vitamin A analogues: 13-cis retinoic acid, 9-cis retinoic acid, and Ro 13-6307 inhibit neuroblastoma tumour growth in vivo. Med. Pediatr. Oncol. 2001, 36, 127–131. [Google Scholar] [CrossRef]
- Malve, H. Exploring the ocean for new drug developments: Marine pharmacology. J. Pharm. Bioallied Sci. 2016, 8, 83–91. [Google Scholar] [CrossRef]
- Batanouny, K.H. Wild Medicinal Plants in Egypt; Academy of Scientific Research and Technology: Cairo, Egypt, 1999. [Google Scholar]
- El-Mokasabi, F.M. Floristic composition and traditional uses of plant species in Wadi Alkuf, Al-Jabal Al-Akhder, Libya. Am. Eur. J. Agric. Environ. Sci. 2014, 14, 685–697. [Google Scholar] [CrossRef]
- Gokce, G.; Haznedaroglu, M.Z. Evaluation of antidiabetic, antioxidant and vasoprotective effects of Posidonia oceanica extract. J. Ethnopharmacol. 2008, 115, 122–130. [Google Scholar] [CrossRef]
- Vasarri, M.; De Biasi, A.M.; Barletta, E.; Pretti, C.; Degl′Innocenti, D. An overview of new insights into the benefits of the seagrass Posidonia oceanica for human health. Mar. Drugs 2021, 19, 476. [Google Scholar] [CrossRef]
- Vasarri, M.; Leri, M.; Barletta, E.; Ramazzotti, M.; Marzocchini, R.; Degl′Innocenti, D. Anti-inflammatory properties of the marine plant Posidonia oceanica (L.) delile. J. Ethnopharmacol. 2020, 247, 112252. [Google Scholar] [CrossRef]
- Vasarri, M.; Barletta, E.; Ramazzotti, M.; Degl′Innocenti, D. In vitro anti-glycation activity of the marine plant Posidonia oceanica (L.) Delile. J. Ethnopharmacol. 2020, 259, 112960. [Google Scholar] [CrossRef] [PubMed]
- Leri, M.; Ramazzotti, M.; Vasarri, M.; Peri, S.; Barletta, E.; Pretti, C.; Degl′Innocenti, D. Bioactive compounds from Posidonia oceanica (L.) delile impair malignant cell migration through autophagy modulation. Mar. Drugs 2018, 16, 137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piazzini, V.; Vasarri, M.; Degl′Innocenti, D.; Guastini, A.; Barletta, E.; Salvatici, M.C.; Bergonzi, M.C. Comparison of chitosan nanoparticles and soluplus micelles to optimize the bioactivity of Posidonia oceanica extract on human neuroblastoma cell migration. Pharmaceutics 2019, 11, 655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mouhieddine, T.H.; Nokkari, A.; Itani, M.M.; Chamaa, F.; Bahmad, H.; Monzer, A.; El-Merahbi, R.; Daoud, G.; Eid, A.; Kobeissy, F.H.; et al. Metformin and ara-a effectively suppress brain cancer by targeting cancer stem/progenitor cells. Front. Neurosci. 2015, 9, 442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiang, L.; Zhao, B.; Ming, M.; Wang, N.; He, T.C.; Hwang, S.; Thorburn, A.; He, Y.Y. Regulation of cell proliferation and migration by p62 through stabilization of Twist1. Proc. Natl. Acad. Sci. USA 2014, 111, 9241–9246. [Google Scholar] [CrossRef] [Green Version]
- Catalano, M.; D′Alessandro, G.; Lepore, F.; Corazzari, M.; Caldarola, S.; Valacca, C.; Faienza, F.; Esposito, V.; Limatola, C.; Cecconi, F.; et al. Autophagy induction impairs migration and invasion by reversing EMT in glioblastoma cells. Mol. Oncol. 2015, 9, 1612–1625. [Google Scholar] [CrossRef] [Green Version]
- Tuloup-Minguez, V.; Hamaï, A.; Greffard, A.; Nicolas, V.; Codogno, P.; Botti, J. Autophagy modulates cell migration and β1 integrin membrane recycling. Cell Cycle. 2013, 12, 3317–3328. [Google Scholar] [CrossRef] [Green Version]
- Weng, J.; Wang, C.; Wang, Y.; Tang, H.; Liang, J.; Liu, X.; Huang, H.; Hou, J. Beclin1 inhibits proliferation, migration and invasion in tongue squamous cell carcinoma cell lines. Oral Oncol. 2014, 50, 983–990. [Google Scholar] [CrossRef] [PubMed]
- Messi, E.; Florian, M.C.; Caccia, C.; Zanisi, M.; Maggi, R. Retinoic acid reduces human neuroblastoma cell migration and invasiveness: Effects on DCX, LIS1, neurofilaments-68 and vimentin expression. BMC Cancer 2008, 8, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nasser, T.I.N.; Spencer, G.E. Neurite outgrowth. In Reference Module in Biomedical Sciences; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar] [CrossRef]
- Willinger, M.; Schachner, M. GM1 ganglioside as a marker for neuronal differentiation in mouse cerebellum. Dev. Biol. 1980, 74, 101–117. [Google Scholar] [CrossRef]
- Lee, M.C.; Lee, W.S.; Park, C.S.; Juhng, S.W. The biologic role of ganglioside in neuronal differentiation: Effects of GM1 ganglioside on human neuroblastoma SH-SY5Y cells. J. Korean Med. Sci. 1994, 9, 179–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shipley, M.M.; Mangold, C.A.; Szpara, M.L. Differentiation of the SH-SY5Y human neuroblastoma cell line. J. Vis. Exp. 2016, 108, 53193. [Google Scholar] [CrossRef]
- Steeg, P.S.; Theodorescu, D. Metastasis: A therapeutic target for cancer. Nat. Clin. Pract. Oncol. 2008, 5, 206–219. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Kim, S.K. Matrix metalloproteinase inhibitors (MMPIs) from marine natural products: The current situation and future prospects. Mar. Drugs 2009, 7, 71–84. [Google Scholar] [CrossRef] [Green Version]
- Zou, Z.; Tao, T.; Li, H.; Zhu, X. mTOR signaling pathway and mTOR inhibitors in cancer: Progress and challenges. Cell Biosci. 2020, 10, 31. [Google Scholar] [CrossRef] [PubMed]
- Dwane, S.; Durack, E.; Kiely, P.A. Optimising parameters for the differentiation of SH-SY5Y cells to study cell adhesion and cell migration. BMC Res. Notes 2013, 6, 366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matthay, K.K.; Maris, J.M.; Schleiermacher, G.; Nakagawara, A.; Mackall, C.L.; Diller, L.; Weiss, W.A. Neuroblastoma. Nat. Rev. Dis. Primers 2016, 2, 16078. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.B.; Yoo, B.S. Propolis inhibits neurite outgrowth in differentiating SH-SY5Y human neuroblastoma cells. Toxicol. Res. 2016, 32, 239–243. [Google Scholar] [CrossRef] [Green Version]







| Phenolic Compound | Chemical Structure | Composition (%) |
|---|---|---|
| D-(+)-Catechin |  | 85 |
| Ferulic acid |  | 1.7 |
| (−)-Epicatechin |  | 1.4 |
| Chlorogenic acid |  | 0.6 |
| Gallic acid |  | 0.4 |
| Others | ̶ | 11 |
| TP | TC | Antioxidant | Radical Scavenging | |
|---|---|---|---|---|
| Method | Folin-Ciocalteau | Phenol/Sulfuric acid | Ferrozine® | DPPH |
| Reference control | Gallic acid | Glucose | Ascorbic acid | Ascorbic acid |
| POE | 3.2 ± 0.1 | 6.3 ± 1.4 | 1.2 ± 0.3 | 10.0 ± 2.0 |
| Primary Antibody | Target | Dilution | Host | Source |
|---|---|---|---|---|
| SQTSM1/p62 | SQTSM1/p62 protein | 1:1000 | Rabbit | Abcam |
| LC3 | Microtubule-associated protein light chain 3 | 1:1000 | Rabbit | Invitrogen |
| S6 | Ribosomal protein S6 | 1:1000 | Rabbit | Cell Signaling |
| p-S6 | Ribosomal protein S6 (Ser235/236) | 1:2000 | Rabbit | Cell Signaling |
| α-Tubulin | α-Tubulin protein | 1:1000 | Mouse | Genetex |
| Primary Antibody | Target | Dilution | Host | Source |
|---|---|---|---|---|
| β3-Tubulin | total β3-tubulin protein | 1:1000 | Rabbit | Cell Signaling |
| GAP43 | GAP43 protein | 1:1000 | Rabbit | Cell Signaling |
| NeuN | NeuN protein | 1:1000 | Rabbit | Cell Signaling |
| Synaptophysin | Synaptophysin protein | 1:2000 | Rabbit | Cell Signaling |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasarri, M.; Leri, M.; Barletta, E.; Pretti, C.; Degl’Innocenti, D. Posidonia oceanica (L.) Delile Dampens Cell Migration of Human Neuroblastoma Cells. Mar. Drugs 2021, 19, 579. https://doi.org/10.3390/md19100579
Vasarri M, Leri M, Barletta E, Pretti C, Degl’Innocenti D. Posidonia oceanica (L.) Delile Dampens Cell Migration of Human Neuroblastoma Cells. Marine Drugs. 2021; 19(10):579. https://doi.org/10.3390/md19100579
Chicago/Turabian StyleVasarri, Marzia, Manuela Leri, Emanuela Barletta, Carlo Pretti, and Donatella Degl’Innocenti. 2021. "Posidonia oceanica (L.) Delile Dampens Cell Migration of Human Neuroblastoma Cells" Marine Drugs 19, no. 10: 579. https://doi.org/10.3390/md19100579
APA StyleVasarri, M., Leri, M., Barletta, E., Pretti, C., & Degl’Innocenti, D. (2021). Posidonia oceanica (L.) Delile Dampens Cell Migration of Human Neuroblastoma Cells. Marine Drugs, 19(10), 579. https://doi.org/10.3390/md19100579