Eisenia bicyclis Extract Repairs UVB-Induced Skin Photoaging In Vitro and In Vivo: Photoprotective Effects
Abstract
:1. Introduction
2. Results
2.1. Identification of Phlorotannins in EEB by UPLC-PDA-ESI-MS
2.2. EEB Protects against UVB-Reduced Cell Viability in Hs68 Fibroblasts
2.3. EEB Ameliorates UVB-Induced MMP-1 Production and Pro-Collagen Type I Degradation in Hs68 Fibroblasts
2.4. EEB Promotes the Skin Moisturization Factors in HaCaT Keratinocytes
2.5. EEB Reduces UVB-Induced ROS and Enhances Antioxidant Enzymes Expression via the Nrf2 Signaling Pathway in Hs68 Fibroblasts
2.6. EEB Reduces UVB-Induced Wrinkle Formation in the Dorsal Skin of HR-1 Hairless Mice
2.7. EEB Inhibits UVB-Induced MMP-1 via the MAPK/AP-1 Signaling Pathway in the Dorsal Skin of HR-1 Hairless Mice
2.8. EEB Alleviates UVB-Induced Skin Thickening and Collagen Degradation in the Dorsal Skin of HR-1 Hairless Mice
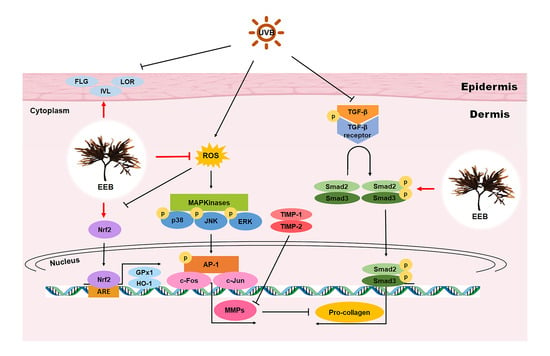
3. Discussion
4. Materials and Methods
4.1. Preparation and UPLC-PDA-ESI-MS Analysis of EEB
4.2. Cell Culture, UVB-Irradiation, and Sample Treatment
4.3. Cell Viability Assay
4.4. Analysis of MMP-1 and Pro-Collagen Type I Production
4.5. Analysis of Western Blots
4.6. Analysis of Quantitative qRT-PCR
4.7. Measurement of Intracellular ROS Production
4.8. Animals
4.9. Skin Photoaging Model
4.10. Analysis of Skin Wrinkle Formation
4.11. Histological Analysis
4.12. Measurement of Dorsal Thickness, Skin Hydration and Skin TEWL
4.13. Analyze of Hepatoxicity and Renal Toxicity of EEB
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Sanches Silveira, J.E.; Myaki Pedroso, D.M. UV light and skin aging. Rev. Environ. Health. 2014, 29, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Kageyama, H.; Waditee-Sirisattha, R. Antioxidative, Anti-Inflammatory, and Anti-Aging Properties of Mycosporine-Like Amino Acids: Molecular and Cellular Mechanisms in the Protection of Skin-Aging. Mar. Drugs 2019, 17, 222. [Google Scholar] [CrossRef] [Green Version]
- Deng, M.; Xu, Y.; Yu, Z.; Wang, X.; Cai, Y.; Zheng, H.; Li, W.; Zhang, W. Protective Effect of Fat Extract on UVB-Induced Photoaging In Vitro and In Vivo. Oxid. Med. Cell. Longev. 2019, 2019, 6146942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Wang, X.; Vikash, V.; Ye, Q.; Wu, D.; Liu, Y.; Dong, W. ROS and ROS-Mediated Cellular Signaling. Oxid. Med. Cell. Longev. 2016, 2016, 4350965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, E.; Gao, W.; Xiao, Y.K.; Ngo, H.T.T.; Yi, T.H. Helianthus annuus L. flower prevents UVB-induced photodamage in human dermal fibroblasts by regulating the MAPK/AP-1, NFAT, and Nrf2 signaling pathways. J. Cell. Biochem. 2019, 120, 601–612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, H.J.; Alam, M.B.; Baek, M.E.; Kwon, Y.G.; Lim, J.Y.; Lee, S.H. Protection against UVB-Induced Photoaging by Nypa fruticans via Inhibition of MAPK/AP-1/MMP-1 Signaling. Oxid. Med. Cell. Longev. 2020, 2020, 2905362. [Google Scholar] [CrossRef]
- Ho, C.C.; Ng, S.C.; Chuang, H.L.; Wen, S.Y.; Kuo, C.H.; Mahalakshmi, B.; Huang, C.Y.; Kuo, W.W. Extracts of Jasminum sambac flowers fermented by Lactobacillus rhamnosus inhibit H2 O2—And UVB-induced aging in human dermal fibroblasts. Environ. Toxicol. 2021, 36, 607–619. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.S.; Cho, J.G.; Hwang, E.S.; Yang, J.E.; Gao, W.; Fang, M.Z.; Zheng, S.D.; Yi, T.H. Enhancement of Protective Effects of Radix Scutellariae on UVB-induced Photo Damage in Human HaCaT Keratinocytes. Appl. Biochem. Biotechnol. 2018, 184, 1073–1093. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.L.; Zhong, Q.Y.; Lin, B.Q.; Liu, Y.H.; Huang, Y.F.; Chen, Y.; Yuan, J.; Su, Z.R.; Zhan, J.Y. Andrographolide sodium bisulfate attenuates UVinduced photodamage by activating the keap1/Nrf2 pathway and downregulating the NFkappaB pathway in HaCaT keratinocytes. Int. J. Mol. Med. 2020, 45, 343–352. [Google Scholar] [CrossRef] [Green Version]
- Han, H.S.; Shin, J.S.; Myung, D.B.; Ahn, H.S.; Lee, S.H.; Kim, H.J.; Lee, K.T. Hydrangea serrata (Thunb.) Ser. Extract Attenuate UVB-Induced Photoaging through MAPK/AP-1 Inactivation in Human Skin Fibroblasts and Hairless Mice. Nutrients 2019, 11, 533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorz, L.R.; Yoo, B.C.; Kim, M.Y.; Cho, J.Y. Anti-Wrinkling and Anti-Melanogenic Effect of Pradosia mutisii Methanol Extract. Int. J. Mol. Sci. 2019, 20, 1043. [Google Scholar] [CrossRef] [Green Version]
- Jeong, D.; Lee, J.; Jeong, S.G.; Hong, Y.H.; Yoo, S.; Han, S.Y.; Kim, J.H.; Kim, S.; Kim, J.S.; Chung, Y.S.; et al. Artemisia asiatica ethanol extract exhibits anti-photoaging activity. J. Ethnopharmacol. 2018, 220, 57–66. [Google Scholar] [CrossRef]
- Park, N.H.; Park, J.S.; Kang, Y.G.; Bae, J.H.; Lee, H.K.; Yeom, M.H.; Cho, J.C.; Na, Y.J. Soybean extract showed modulation of retinoic acid-related gene expression of skin and photo-protective effects in keratinocytes. Int. J. Cosmet. Sci. 2013, 35, 136–142. [Google Scholar] [CrossRef]
- Hwang, I.S.; Kim, J.E.; Choi, S.I.; Lee, H.R.; Lee, Y.J.; Jang, M.J.; Son, H.J.; Lee, H.S.; Oh, C.H.; Kim, B.H.; et al. UV radiation-induced skin aging in hairless mice is effectively prevented by oral intake of sea buckthorn (Hippophae rhamnoides L.) fruit blend for 6 weeks through MMP suppression and increase of SOD activity. Int. J. Mol. Med. 2012, 30, 392–400. [Google Scholar] [CrossRef] [Green Version]
- Sumiyoshi, M.; Kimura, Y. Effects of a turmeric extract (Curcuma longa) on chronic ultraviolet B irradiation-induced skin damage in melanin-possessing hairless mice. Phytomedicine 2009, 16, 1137–1143. [Google Scholar] [CrossRef] [PubMed]
- Afaq, F.; Malik, A.; Syed, D.; Maes, D.; Matsui, M.S.; Mukhtar, H. Pomegranate fruit extract modulates UV-B-mediated phosphorylation of mitogen-activated protein kinases and activation of nuclear factor kappa B in normal human epidermal keratinocytes paragraph sign. Photochem. Photobiol. 2005, 81, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Jesumani, V.; Du, H.; Pei, P.; Aslam, M.; Huang, N. Comparative study on skin protection activity of polyphenol-rich extract and polysaccharide-rich extract from Sargassum vachellianum. PLoS ONE. 2020, 15, e0227308. [Google Scholar] [CrossRef] [Green Version]
- Wiraguna, A.; Pangkahila, W.; Astawa, I.N.M. Antioxidant properties of topical Caulerpa sp. extract on UVB-induced photoaging in mice. Dermatol. Rep. 2018, 10, 7597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, W.; Wang, L.; Fu, X.; Ni, L.; Duan, D.; Xu, J.; Gao, X. Protective Effect of a Fucose-Rich Fucoidan Isolated from Saccharina japonica against Ultraviolet B-Induced Photodamage In Vitro in Human Keratinocytes and In Vivo in Zebrafish. Mar. Drugs 2020, 18, 316. [Google Scholar] [CrossRef]
- Wang, L.; Oh, J.Y.; Kim, Y.S.; Lee, H.G.; Lee, J.S.; Jeon, Y.J. Anti-Photoaging and Anti-Melanogenesis Effects of Fucoidan Isolated from Hizikia fusiforme and Its Underlying Mechanisms. Mar. Drugs 2020, 18, 427. [Google Scholar] [CrossRef]
- Li, Y.-X.; Wijesekara, I.; Li, Y.; Kim, S.-K. Phlorotannins as bioactive agents from brown algae. Process. Biochem. 2011, 46, 2219–2224. [Google Scholar] [CrossRef]
- Kang, H.S.; Chung, H.Y.; Kim, J.Y.; Son, B.W.; Jung, H.A.; Choi, J.S. Inhibitory phlorotannins from the edible brown alga Ecklonia stolonifera on total reactive oxygen species (ROS) generation. Arch. Pharm. Res. 2004, 27, 194–198. [Google Scholar] [CrossRef]
- Shibata, T.; Ishimaru, K.; Kawaguchi, S.; Yoshikawa, H.; Hama, Y. Antioxidant activities of phlorotannins isolated from Japanese Laminariaceae. In Nineteenth International Seaweed Symposium; Springer: Dordrecht, The Netherlands, 2007; pp. 255–261. [Google Scholar]
- Kim, M.M.; Ta, Q.V.; Mendis, E.; Rajapakse, N.; Jung, W.K.; Byun, H.G.; Jeon, Y.J.; Kim, S.K. Phlorotannins in Ecklonia cava extract inhibit matrix metalloproteinase activity. Life Sci. 2006, 79, 1436–1443. [Google Scholar] [CrossRef] [PubMed]
- Shibata, T.; Fujimoto, K.; Nagayama, K.; Yamaguchi, K.; Nakamura, T. Inhibitory activity of brown algal phlorotannins against hyaluronidase. Food Sci. Technol. Int. 2002, 37, 703–709. [Google Scholar] [CrossRef]
- Wang, L.; Je, J.G.; Yang, H.W.; Jeon, Y.J.; Lee, S. Dieckol, an Algae-Derived Phenolic Compound, Suppresses UVB-Induced Skin Damage in Human Dermal Fibroblasts and Its Underlying Mechanisms. Antioxidants 2021, 10, 352. [Google Scholar] [CrossRef]
- Ryu, B.; Qian, Z.-J.; Kim, M.-M.; Nam, K.W.; Kim, S.-K. Anti-photoaging activity and inhibition of matrix metalloproteinase (MMP) by marine red alga, Corallina pilulifera methanol extract. Radiat. Phys. Chem. 2009, 78, 98–105. [Google Scholar] [CrossRef]
- Kim, H.M.; Lee, D.E.; Park, S.D.; Kim, Y.T.; Kim, Y.J.; Jeong, J.W.; Lee, J.-H.; Jang, S.S.; Chung, D.K.; Sim, J.-H. Preventive effect of fermented Gelidium amansii and Cirsium japonicum extract mixture against UVB-induced skin photoaging in hairless mice. Food Sci. Biotechnol. 2014, 23, 623–631. [Google Scholar] [CrossRef]
- Piao, M.J.; Kang, H.K.; Yoo, E.S.; Koh, Y.S.; Kim, D.S.; Lee, N.H.; Hyun, J.W. Photo-protective effect of Polysiphonia morrowii Harvey against ultraviolet B radiation-induced keratinocyte damage. J. Korean Soc. Appl. Biol. Chem. 2012, 55, 149–158. [Google Scholar] [CrossRef]
- Jung, H.A.; Jin, S.E.; Ahn, B.R.; Lee, C.M.; Choi, J.S. Anti-inflammatory activity of edible brown alga Eisenia bicyclis and its constituents fucosterol and phlorotannins in LPS-stimulated RAW264.7 macrophages. Food Chem. Toxicol. 2013, 59, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Irfan, M.; Kwon, T.H.; Yun, B.S.; Park, N.H.; Rhee, M.H. Eisenia bicyclis (brown alga) modulates platelet function and inhibits thrombus formation via impaired P2Y12 receptor signaling pathway. Phytomedicine 2018, 40, 79–87. [Google Scholar] [CrossRef]
- Eom, S.H.; Lee, D.S.; Kang, Y.M.; Son, K.T.; Jeon, Y.J.; Kim, Y.M. Application of yeast Candida utilis to ferment Eisenia bicyclis for enhanced antibacterial effect. Appl. Biochem. Biotechnol. 2013, 171, 569–582. [Google Scholar] [CrossRef] [PubMed]
- Ahn, B.R.; Moon, H.E.; Kim, H.R.; Jung, H.A.; Choi, J.S. Neuroprotective effect of edible brown alga Eisenia bicyclis on amyloid beta peptide-induced toxicity in PC12 cells. Arch. Pharm. Res. 2012, 35, 1989–1998. [Google Scholar] [CrossRef] [PubMed]
- Ferreres, F.; Lopes, G.; Gil-Izquierdo, A.; Andrade, P.B.; Sousa, C.; Mouga, T.; Valentão, P. Phlorotannin extracts from fucales characterized by HPLC-DAD-ESI-MSn: Approaches to hyaluronidase inhibitory capacity and antioxidant properties. Mar. Drugs 2012, 10, 2766–2781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mezghani, S.; Csupor, D.; Bourguiba, I.; Hohmann, J.; Amri, M.; Bouaziz, M. Characterization of phenolic compounds of Ulva rigida (Chlorophycae) and its antioxidant activity. Eur. J. Med. Plants 2016, 12, 1–9. [Google Scholar] [CrossRef]
- Kim, S.M.; Kang, S.W.; Jeon, J.-S.; Jung, Y.-J.; Kim, W.-R.; Kim, C.Y.; Um, B.-H. Determination of major phlorotannins in Eisenia bicyclis using hydrophilic interaction chromatography: Seasonal variation and extraction characteristics. Food Chem. 2013, 138, 2399–2406. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, Y.; Matsuda, K.; Yamada, Y.; Nishikawa, M.; Shioya, K.; Katsuzaki, H.; Imai, K.; AMANO, H. Anti-allergic phlorotannins from the edible brown alga, Eisenia arborea. Food Sci. Technol. Res. 2007, 13, 54–60. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.M.; Kang, K.; Jeon, J.-S.; Jho, E.H.; Kim, C.Y.; Nho, C.W.; Um, B.-H. Isolation of phlorotannins from Eisenia bicyclis and their hepatoprotective effect against oxidative stress induced by tert-butyl hyperoxide. Appl. Biochem. Biotechnol. 2011, 165, 1296–1307. [Google Scholar] [CrossRef]
- Lu, J.; Guo, J.H.; Tu, X.L.; Zhang, C.; Zhao, M.; Zhang, Q.W.; Gao, F.H. Tiron Inhibits UVB-Induced AP-1 Binding Sites Transcriptional Activation on MMP-1 and MMP-3 Promoters by MAPK Signaling Pathway in Human Dermal Fibroblasts. PLoS ONE 2016, 11, e0159998. [Google Scholar] [CrossRef]
- Im, A.R.; Yeon, S.H.; Lee, J.S.; Um, K.A.; Ahn, Y.J.; Chae, S. Protective effect of fermented Cyclopia intermedia against UVB-induced damage in HaCaT human keratinocytes. BMC Complement. Altern. Med. 2016, 16, 261. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, Y.; Uchi, H.; Furue, M. Antioxidant cinnamaldehyde attenuates UVB-induced photoaging. J. Dermatol. Sci. 2019, 96, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Alam, M.B.; Lee, S.H. Protection of UVB-Induced Photoaging by Fuzhuan-Brick Tea Aqueous Extract via MAPKs/Nrf2-Mediated Down-Regulation of MMP-1. Nutrients 2018, 11, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, S.I.; Jung, T.D.; Cho, B.Y.; Choi, S.H.; Sim, W.S.; Han, X.; Lee, S.J.; Kim, Y.C.; Lee, O.H. Antiphotoaging effect of fermented agricultural byproducts on ultraviolet Birradiated hairless mouse skin. Int. J. Mol. Med. 2019, 44, 559–568. [Google Scholar] [CrossRef] [Green Version]
- Ansary, T.M.; Hossain, M.R.; Kamiya, K.; Komine, M.; Ohtsuki, M. Inflammatory Molecules Associated with Ultraviolet Radiation-Mediated Skin Aging. Int. J. Mol. Sci. 2021, 22, 3974. [Google Scholar] [CrossRef]
- Whitaker, D.M.; Carlson, G.P. Anti-inflammation mechanism of extract from Eisenia bicyclis (Kjellman) Setchell. J. Pharm. Sci. 1975, 64, 1258–1259. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.M.; Eom, S.H.; Kim, Y.M. Protective effect of phlorotannins from Eisenia bicyclis against lipopolysaccharide-stimulated inflammation in HepG2 cells. Environ. Toxicol. Pharmacol. 2013, 35, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, Y.; Usui, M.; Katsuzaki, H.; Imai, K.; Kakinuma, M.; Amano, H.; Miyata, M. Orally Administered Phlorotannins from Eisenia arborea Suppress Chemical Mediator Release and Cyclooxygenase-2 Signaling to Alleviate Mouse Ear Swelling. Mar. Drugs 2018, 16, 267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joe, M.J.; Kim, S.N.; Choi, H.Y.; Shin, W.S.; Park, G.M.; Kang, D.W.; Kim, Y.K. The inhibitory effects of eckol and dieckol from Ecklonia stolonifera on the expression of matrix metalloproteinase-1 in human dermal fibroblasts. Biol. Pharm. Bull. 2006, 29, 1735–1739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myung, D.B.; Han, H.S.; Shin, J.S.; Park, J.Y.; Hwang, H.J.; Kim, H.J.; Ahn, H.S.; Lee, S.H.; Lee, K.T. Hydrangenol Isolated from the Leaves of Hydrangea serrata Attenuates Wrinkle Formation and Repairs Skin Moisture in UVB-Irradiated Hairless Mice. Nutrients 2019, 11, 2354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, J.S.; Han, H.S.; Lee, S.B.; Myung, D.B.; Lee, K.; Lee, S.H.; Kim, H.J.; Lee, K.T. Chemical Constituents from Leaves of Hydrangea serrata and Their Anti-photoaging Effects on UVB-Irradiated Human Fibroblasts. Biol. Pharm. Bull. 2019, 42, 424–431. [Google Scholar] [CrossRef] [Green Version]
- Ahn, H.S.; Kim, H.J.; Na, C.; Jang, D.S.; Shin, Y.K.; Lee, S.H. The Protective Effect of Adenocaulon himalaicum Edgew. and Its Bioactive Compound Neochlorogenic Acid against UVB-Induced Skin Damage in Human Dermal Fibroblasts and Epidermal Keratinocytes. Plants 2021, 10, 1669. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.I.; Shin, H.C.; Kim, S.H.; Park, W.Y.; Lee, K.T.; Choi, J.H. 6,6′-Bieckol, isolated from marine alga Ecklonia cava, suppressed LPS-induced nitric oxide and PGE(2) production and inflammatory cytokine expression in macrophages: The inhibition of NFkappaB. Int. Immunopharmacol. 2012, 12, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.I.; Jung, S.H.; Lee, K.T.; Choi, J.H. 8,8’-Bieckol, isolated from edible brown algae, exerts its anti-inflammatory effects through inhibition of NF-kappaB signaling and ROS production in LPS-stimulated macrophages. Int. Immunopharmacol. 2014, 23, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.R.; Noh, E.M.; Han, J.H.; Kim, J.M.; Hwang, J.K.; Hwang, B.M.; Chung, E.Y.; Kim, B.S.; Lee, S.H.; Lee, S.J.; et al. Brazilin inhibits UVB-induced MMP-1/3 expressions and secretions by suppressing the NF-kappaB pathway in human dermal fibroblasts. Eur. J. Pharmacol. 2012, 674, 80–86. [Google Scholar] [CrossRef]
- Vermeulen, L.; Vanden Berghe, W.; Beck, I.M.; De Bosscher, K.; Haegeman, G. The versatile role of MSKs in transcriptional regulation. Trends Biochem. Sci. 2009, 34, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Walton, K.L.; Johnson, K.E.; Harrison, C.A. Targeting TGF-beta Mediated SMAD Signaling for the Prevention of Fibrosis. Front. Pharmacol. 2017, 8, 461. [Google Scholar] [CrossRef] [Green Version]
- Owens, P.; Han, G.; Li, A.G.; Wang, X.J. The role of Smads in skin development. J. Invest. Dermatol. 2008, 128, 783–790. [Google Scholar] [CrossRef] [Green Version]
- Rochette, L.; Mazini, L.; Meloux, A.; Zeller, M.; Cottin, Y.; Vergely, C.; Malka, G. Anti-Aging Effects of GDF11 on Skin. Int. J. Mol. Sci. 2020, 21, 2598. [Google Scholar] [CrossRef]
- Son, Y.; Cheong, Y.K.; Kim, N.H.; Chung, H.T.; Kang, D.G.; Pae, H.O. Mitogen-Activated Protein Kinases and Reactive Oxygen Species: How Can ROS Activate MAPK Pathways? J. Signal. Transduct. 2011, 2011, 792639. [Google Scholar] [CrossRef]
- Shi, Y.; Massague, J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell 2003, 113, 685–700. [Google Scholar] [CrossRef] [Green Version]
- Fessing, M.Y.; Atoyan, R.; Shander, B.; Mardaryev, A.N.; Botchkarev, V.V., Jr.; Poterlowicz, K.; Peng, Y.; Efimova, T.; Botchkarev, V.A. BMP signaling induces cell-type-specific changes in gene expression programs of human keratinocytes and fibroblasts. J. Investig. Dermatol. 2010, 130, 398–404. [Google Scholar] [CrossRef] [Green Version]
- Berthon, J.Y.; Nachat-Kappes, R.; Bey, M.; Cadoret, J.P.; Renimel, I.; Filaire, E. Marine algae as attractive source to skin care. Free Radic. Res. 2017, 51, 555–567. [Google Scholar] [CrossRef]
- Jang, J.; Ye, B.R.; Heo, S.J.; Oh, C.; Kang, D.H.; Kim, J.H.; Affan, A.; Yoon, K.T.; Choi, Y.U.; Park, S.C.; et al. Photo-oxidative stress by ultraviolet-B radiation and antioxidative defense of eckstolonol in human keratinocytes. Environ. Toxicol. Pharmacol. 2012, 34, 926–934. [Google Scholar] [CrossRef]
- Wang, L.; Oh, J.Y.; Lee, W.; Jeon, Y.J. Fucoidan isolated from Hizikia fusiforme suppresses ultraviolet B-induced photodamage by down-regulating the expressions of matrix metalloproteinases and pro-inflammatory cytokines via inhibiting NF-kappaB, AP-1, and MAPK signaling pathways. Int. J. Biol. Macromol. 2021, 166, 751–759. [Google Scholar] [CrossRef]
- Wang, L.; Lee, W.; Oh, J.Y.; Cui, Y.R.; Ryu, B.; Jeon, Y.J. Protective Effect of Sulfated Polysaccharides from Celluclast-Assisted Extract of Hizikia fusiforme Against Ultraviolet B-Induced Skin Damage by Regulating NF-kappaB, AP-1, and MAPKs Signaling Pathways In Vitro in Human Dermal Fibroblasts. Mar. Drugs 2018, 16, 239. [Google Scholar] [CrossRef] [Green Version]
- De La Fuente, G.; Fontana, M.; Asnaghi, V.; Chiantore, M.; Mirata, S.; Salis, A.; Damonte, G.; Scarfi, S. The Remarkable Antioxidant and Anti-Inflammatory Potential of the Extracts of the Brown Alga Cystoseira amentacea var. stricta. Mar. Drugs 2020, 19, 2. [Google Scholar] [CrossRef] [PubMed]
- Wagener, F.A.; Carels, C.E.; Lundvig, D.M. Targeting the redox balance in inflammatory skin conditions. Int. J. Mol. Sci. 2013, 14, 9126–9167. [Google Scholar] [CrossRef]
- Kensler, T.W.; Wakabayashi, N.; Biswal, S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 89–116. [Google Scholar] [CrossRef] [PubMed]
- Kezic, S.; Jakasa, I. Filaggrin and Skin Barrier Function. Curr. Probl. Dermatol. 2016, 49, 1–7. [Google Scholar] [CrossRef]
- Candi, E.; Schmidt, R.; Melino, G. The cornified envelope: A model of cell death in the skin. Nat. Rev. Mol. Cell. Biol. 2005, 6, 328–340. [Google Scholar] [CrossRef] [PubMed]
- Nithya, S.; Radhika, T.; Jeddy, N. Loricrin—An overview. J. Oral. Maxillofac. Pathol. 2015, 19, 64–68. [Google Scholar] [CrossRef]
- Jansen van Rensburg, S.; Franken, A.; Du Plessis, J.L. Measurement of transepidermal water loss, stratum corneum hydration and skin surface pH in occupational settings: A review. Skin Res. Technol. 2019, 25, 595–605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verdier-Sevrain, S.; Bonte, F. Skin hydration: A review on its molecular mechanisms. J. Cosmet. Dermatol. 2007, 6, 75–82. [Google Scholar] [CrossRef]
- Papakonstantinou, E.; Roth, M.; Karakiulakis, G. Hyaluronic acid: A key molecule in skin aging. Derm. Endocrinol. 2012, 4, 253–258. [Google Scholar] [CrossRef] [Green Version]
- Draelos, Z. Aquaporins: An introduction to a key factor in the mechanism of skin hydration. J. Clin. Aesthet. Dermatol. 2012, 5, 53–56. [Google Scholar]
- Perez-Sanchez, A.; Barrajon-Catalan, E.; Herranz-Lopez, M.; Micol, V. Nutraceuticals for Skin Care: A Comprehensive Review of Human Clinical Studies. Nutrients 2018, 10, 403. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.H.; Choi, S.I.; Jung, T.D.; Cho, B.Y.; Lee, J.H.; Kim, S.H.; Yoon, S.A.; Ham, Y.M.; Yoon, W.J.; Cho, J.H.; et al. Anti-Photoaging Effect of Jeju Putgyul (Unripe Citrus) Extracts on Human Dermal Fibroblasts and Ultraviolet B-induced Hairless Mouse Skin. Int. J. Mol. Sci. 2017, 18, 2052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, D.E.; Huh, C.S.; Ra, J.; Choi, I.D.; Jeong, J.W.; Kim, S.H.; Ryu, J.H.; Seo, Y.K.; Koh, J.S.; Lee, J.H.; et al. Clinical Evidence of Effects of Lactobacillus plantarum HY7714 on Skin Aging: A Randomized, Double Blind, Placebo-Controlled Study. J. Microbiol. Biotechnol. 2015, 25, 2160–2168. [Google Scholar] [CrossRef]








| Compound | Rt (min) | Precursor Ion (m/z) | Monoisotopic Mass (M, AMU) | Mass Difference (mmu) | UV λmax (nm) |
|---|---|---|---|---|---|
| 1. Eckol | 6.52 | 373.05449 [M+H]+ | 372.04813196 | −1.47 | 230, 291 |
| 2. Phloroeckol | 7.03 | 497.06954 [M+H]+ | 496.06417594 | −2.46 | 232 |
| 3. 6,6′-bieckol | 9.50 | 743.08752 [M+H]+ | 742.08061385 | −0.92 | 294 |
| 4. 6,8′-bieckol | 10.24 | 743.08724 [M+H]+ | 742.08061385 | −1.19 | 292 |
| 5. 8,8′-bieckol | 10.53 | 743.08790 [M+H]+ | 742.08061385 | −0.54 | 291 |
| 6. Dieckol | 19.55 | 743.08856 [M+H]+ | 742.08061385 | +0.12 | 234, 291 |
| 7. Phlorofucofuroeckol A | 23.07 | 603.07353 [M+H]+ | 602.06965524 | −3.95 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, S.-I.; Han, H.-S.; Kim, J.-M.; Park, G.; Jang, Y.-P.; Shin, Y.-K.; Ahn, H.-S.; Lee, S.-H.; Lee, K.-T. Eisenia bicyclis Extract Repairs UVB-Induced Skin Photoaging In Vitro and In Vivo: Photoprotective Effects. Mar. Drugs 2021, 19, 693. https://doi.org/10.3390/md19120693
Choi S-I, Han H-S, Kim J-M, Park G, Jang Y-P, Shin Y-K, Ahn H-S, Lee S-H, Lee K-T. Eisenia bicyclis Extract Repairs UVB-Induced Skin Photoaging In Vitro and In Vivo: Photoprotective Effects. Marine Drugs. 2021; 19(12):693. https://doi.org/10.3390/md19120693
Chicago/Turabian StyleChoi, Se-In, Hee-Soo Han, Jae-Min Kim, Geonha Park, Young-Pyo Jang, Yu-Kyong Shin, Hye-Shin Ahn, Sun-Hee Lee, and Kyung-Tae Lee. 2021. "Eisenia bicyclis Extract Repairs UVB-Induced Skin Photoaging In Vitro and In Vivo: Photoprotective Effects" Marine Drugs 19, no. 12: 693. https://doi.org/10.3390/md19120693
APA StyleChoi, S. -I., Han, H. -S., Kim, J. -M., Park, G., Jang, Y. -P., Shin, Y. -K., Ahn, H. -S., Lee, S. -H., & Lee, K. -T. (2021). Eisenia bicyclis Extract Repairs UVB-Induced Skin Photoaging In Vitro and In Vivo: Photoprotective Effects. Marine Drugs, 19(12), 693. https://doi.org/10.3390/md19120693