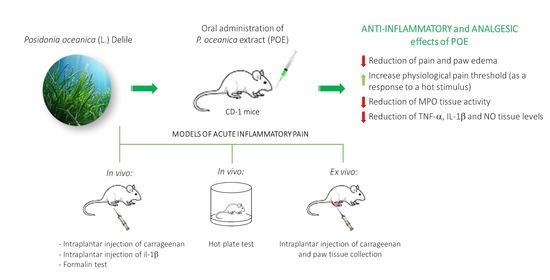
Efficacy of Posidonia oceanica Extract against Inflammatory Pain: In Vivo Studies in Mice
Abstract
:1. Introduction
2. Results and Discussion
2.1. Biochemical Characterization and Antioxidant Activity of POE
2.2. The Effect of POE Against Inflammatory Pain
2.3. Effect of POE on the Inflammatory and Oxidative Mediators
3. Materials and Methods
3.1. Animals
3.2. P. oceanica Extract (POE) Preparation
3.3. POE Administration
3.4. Carrageenan-Induced Pain and Paw Oedema in Mice
3.5. Formalin-Induced Pain
3.6. IL-1β-Induced Pain
3.7. Von Frey Test
3.8. Paw Pressure Test
3.9. Hot-Plate Test
3.10. Abdominal Constriction Test
3.11. Myeloperoxidase (MPO) Activity Assay
3.12. Tumor Necrosis Factor (TNF)-α and Interleukin (IL)-1β Assessment
3.13. Nitric Oxide (NO) Assay
3.14. Lipid Peroxidation (Thiobarbituric Acid-Reactive Substances (TBARS) Assay)
3.15. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Vacchi, M.; De Falco, G.; Simeone, S.; Montefalcone, M.; Morri, C.; Ferrari, M.; Bianchi, C.N. Biogeomorphology of the Mediterranean Posidonia oceanica seagrass meadows. Earth Surf. Process. Landf. 2016, 42, 42–54. [Google Scholar] [CrossRef] [Green Version]
- Batanouny, K.H. Wild Medicinal Plants in Egypt. Entrep. Sustain. Issues 2015, 3, 47. [Google Scholar]
- El-Mokasabi, F.M. Floristic composition and traditional uses of plant species at Wadi Alkuf, Al-Jabal Al-Akhder, Libya. Am.-Eurasian J. Sustain. Agric. 2014, 14, 685–697. [Google Scholar] [CrossRef]
- Gokce, G.; Haznedaroglu, M.Z. Evaluation of antidiabetic, antioxidant and vasoprotective effects of Posidonia oceanica extract. J. Ethnopharmacol. 2008, 115, 122–130. [Google Scholar] [CrossRef]
- Barletta, E.; Ramazzotti, M.; Fratianni, F.; Pessani, D.; Degl’Innocenti, D. Hydrophilic extract from Posidonia oceanica inhibits activity and expression of gelatinases and prevents HT1080 human fibrosarcoma cell line invasion. Cell Adhes. Migr. 2015, 9, 422–431. [Google Scholar] [CrossRef] [Green Version]
- Leri, M.; Ramazzotti, M.; Vasarri, M.; Peri, S.; Barletta, E.; Pretti, C.; Degl’Innocenti, D. Bioactive compounds from Posidonia oceanica (L.) Delile impair malignant cell migration through autophagy modulation. Mar. Drugs 2018, 16, 137. [Google Scholar] [CrossRef] [Green Version]
- Piazzini, V.; Vasarri, M.; Degl’Innocenti, D.; Guastini, A.; Barletta, E.; Salvatici, M.C.; Bergonzi, M.C. Comparison of Chitosan Nanoparticles and Soluplus Micelles to optimize the bioactivity of Posidonia oceanica extract on human neuroblastoma cell migration. Pharmaceutics 2019, 11, 655. [Google Scholar] [CrossRef] [Green Version]
- Vasarri, M.; Barletta, E.; Ramazzotti, M.; Degl’Innocenti, D. In vitro anti-glycation activity of the marine plant Posidonia oceanica (L.) Delile. J. Ethnopharmacol. 2020, 259, 112960. [Google Scholar] [CrossRef]
- Vasarri, M.; Leri, M.; Barletta, E.; Ramazzotti, M.; Marzocchini, R.; Degl’Innocenti, D. Anti-inflammatory properties of the marine plant Posidonia oceanica (L.) Delile. J. Ethnopharmacol. 2020, 247, 112252. [Google Scholar] [CrossRef]
- Wongrakpanich, S.; Wongrakpanich, A.; Melhado, K.; Rangaswami, J. A comprehensive review of non-steroidal anti-inflammatory drug use in the elderly. Aging Dis. 2018, 9, 143–150. [Google Scholar] [CrossRef] [Green Version]
- Rauf, A.; Jehan, N.; Ahmad, Z.; Mubarak, M.S. Analgesic potential of extracts and derived natural products from medicinal plants. In Pain Relief-From Analgesics to Alternative Therapies; Maldonado, C., Ed.; IntechOpen: Rijeka, Croatia, 2017. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2017, 9, 7204–7218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Q.; Yaksh, T.L. A brief comparison of the pathophysiology of inflammatory versus neuropathic pain. Curr. Opin. Anesthesiol. 2011, 24, 400–407. [Google Scholar] [CrossRef] [Green Version]
- Iannitti, T.; Graham, A.; Dolan, S. Adiponectin-mediated analgesia and anti-inflammatory effects in rat. PLoS ONE 2015, 10, e0136819. [Google Scholar] [CrossRef] [PubMed]
- Morales-Medina, J.C.; Flores, G.; Vallelunga, A.; Griffiths, N.H.; Iannitti, T. Cerebrolysin improves peripheral inflammatory pain: Sex differences in two models of acute and chronic mechanical hypersensitivity. Drug Dev. Res. 2019, 80, 513–518. [Google Scholar] [CrossRef]
- Crunkhorn, P.; Meacock, S.C. Mediators of the inflammation induced in the rat paw by carrageenin. Br. J. Pharmacol. 1971, 42, 392–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischer, M.; Carli, G.; Raboisson, P.; Reeh, P. The interphase of the formalin test. Pain 2014, 155, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Moriello, A.S.; Luongo, L.; Guida, F.; Christodoulou, M.S.; Perdicchia, D.; Maione, S.; Passarella, D.; Marzo, V.D.; Petrocellis, L. Chalcone derivatives activate and desensitize the transient receptor potential Ankyrin 1 cation channel, subfamily A, member 1 TRPA1 Ion channel: Structure-activity relationships in vitro and anti-nociceptive and anti-inflammatory activity in vivo. CNS Neurol. Disord. Drug Targets 2016, 15, 987–994. [Google Scholar] [CrossRef]
- Nakamura, H.; Imazu, C.; Ishii, K.; Yokoyama, Y.; Kadokawa, T.; Shimizu, M. Site of analgesic action of zomepirac sodium, a potent non-narcotic analgesic in experi- mental animals. Jpn. J. Pharmacol. 1983, 33, 875–883. [Google Scholar] [CrossRef]
- Boonyarikpunchai, W.; Sukrong, S.; Towiwat, P. Antinociceptive and anti-inflammatory effects of rosmarinic acid isolated from thunbergia laurifolia Lindl. Pharmacol. Biochem. Behav. 2014, 124, 67–73. [Google Scholar] [CrossRef]
- Di Cesare Mannelli, L.; Tenci, B.; Zanardelli, M.; Maidecchi, A.; Lugli, A.; Mattoli, L.; Ghelardini, C. Widespread pain reliever profile of a flower extract of Tanacetum parthenium. Phytomedicine 2015, 22, 7–8. [Google Scholar] [CrossRef]
- Moilanen, L.J.; Laavola, M.; Kukkonen, M.; Korhonen, R.; Leppänen, T.; Högestätt, E.D.; Zygmunt, P.M.; Nieminen, R.M.; Moilanen, E. TRPA1 contributes to the acute inflammatory response and mediates carrageenan-induced paw edema in the mouse. Sci. Rep. 2012, 2, 380. [Google Scholar] [CrossRef] [PubMed]
- Strzepa, A.; Pritchard, K.A.; Dittel, B.N. Myeloperoxidase: A new player in autoimmunity. Cell. Immunol. 2017, 317, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kadetoff, D.; Lampa, J.; Westman, M.; Andersson, M.; Kosek, E. Evidence of central inflammation in fibromyalgia-increased cerebrospinal fluid interleukin-8 levels. J. Neuroimmunol. 2012, 242, 33–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stejskal, V.; Ockert, K.; Bjørklund, G. Metal-induced inflammation triggers fibromyalgia in metal-allergic patients. Neuroendocrinol. Lett. 2013, 34, 559–565. [Google Scholar] [PubMed]
- Mizokami, S.S.; Hohmann, M.S.; Staurengo-Ferrari, L.; Carvalho, T.T.; Zarpelon, A.C.; Possebon, M.I.; de Souza, A.R.; Veneziani, R.C.; Arakawa, N.S.; Casagrande, R.; et al. Pimaradienoic acid inhibits Carrageenan-induced inflammatory leukocyte recruitment and edema in mice: Inhibition of oxidative stress, nitric oxide and cytokine production. PLoS ONE 2016, 11, e0149656. [Google Scholar] [CrossRef] [Green Version]
- Namgyal, D.; Sarwat, M. Saffron as a neuroprotective agent. In Saffron, 1st ed.; Sarwat, M., Sumaiya, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 93–102. [Google Scholar] [CrossRef]
- Sharma, B.; Kumar, H.; Kaushik, P.; Mirza, R.; Awasthi, R.; Kulkarni, G. Therapeutic benefits of Saffron in brain diseases: New lights on possible pharmacological mechanisms. In Saffron, 1st ed.; Sarwat, M., Sumaiya, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 117–130. [Google Scholar] [CrossRef]
- Haddadi, R.; Rashtiani, R. Anti-inflammatory and anti-hyperalgesic effects of milnacipran in inflamed rats: Involvement of myeloperoxidase activity, cytokines and oxidative/nitrosative stress. Inflammopharmacology 2020, 28, 903–913. [Google Scholar] [CrossRef]
- McGrath, J.C.; Lilley, E. Implementing guidelines on reporting research using animals (ARRIVE etc.): New requirements for publication in BJP. Br. J. Pharmacol. 2015, 172, 3189–3193. [Google Scholar] [CrossRef] [Green Version]
- Dallazen, J.L.; Maria-Ferreira, D.; da Luz, B.B.; Nascimento, A.M.; Cipriani, T.R.; de Souza, L.M.; Felipe, L.; Silva, B.; Nassini, R.; de Paula Werner, M.F. Pharmacological potential of alkylamides from acmella oleracea flowers and synthetic isobutylalkyl amide to treat inflammatory pain. Inflammopharmacology 2020, 28, 175–186. [Google Scholar] [CrossRef]
- Micheli, L.; Ghelardini, C.; Lucarini, E.; Parisio, C.; Trallori, E.; Cinci, L.; Di Cesare Mannelli, L. Intra-articular mucilages: Behavioural and histological evaluations for a new model of articular pain. J. Pharm. Pharmacol. 2019, 71, 971–981. [Google Scholar] [CrossRef] [Green Version]
- Abbott, F.V.; Guy, E.R. Effects of morphine, pentobarbital and amphetamine on formalin-induced behaviours in infant rats: Sedation versus specific suppression of pain. Pain 1995, 62, 303–312. [Google Scholar] [CrossRef]
- Ferreira, S.H.; Lorenzetti, B.B.; Bristow, A.F.; Poole, S. Interleukin-1 beta as a potent hyperalgesic agent antagonized by a tripeptide analogue. Nature 1998, 334, 698–700. [Google Scholar] [CrossRef]
- Sakurai, M.; Egashira, N.; Kawashiri, T.; Yano, T.; Ikesue, H.; Oishi, R. Oxaliplatin-induced neuropathy in the rat: Involvement of oxalate in cold hyperalgesia but not mechanical allodynia. Pain 2009, 147, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Di Cesare Mannelli, L.; Micheli, L.; Maresca, M.; Cravotto, G.; Bellumori, M.; Innocenti, M.; Mulinacci, N.; Ghelardini, C. Anti-neuropathic effects of rosmarinus officinalis L. terpenoid fraction: Relevance of nicotinic receptors. Sci. Rep. 2016, 6, 34832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Micheli, L.; Di Cesare Mannelli, L.; Del Bello, F.; Giannella, M.; Piergentili, A.; Quaglia, W.; Carrino, D.; Pacini, A.; Ghelardini, C. The use of the selective imidazoline I 1 receptor agonist carbophenyline as a strategy for neuropathic pain relief: Preclinical evaluation in a mouse model of oxaliplatin-induced neurotoxicity. Neurotherapeutics 2020, 17, 1005–1015. [Google Scholar] [CrossRef] [PubMed]
- Micheli, L.; Di Cesare Mannelli, L.; Lucarini, E.; Parisio, C.; Toti, A.; Fiorentino, B.; Rigamonti, M.A.; Calosi, L.; Ghelardini, C. Intranasal low-dose naltrexone against opioid side effects: A preclinical study. Front. Pharmacol. 2020, 11, 576624. [Google Scholar] [CrossRef]
- Koster, R.; Anderson, M.; De Beer, E.J. Acetic acid for analgesic screening. Fed. Proc. 1959, 18, 412–417. [Google Scholar]
- Haddadi, R.; Poursina, M.; Zeraati, F.; Nadi, F. Gastrodin microinjection suppresses 6-OHDA-induced motor impairments in parkinsonian rats: Insights into oxidative balance and microglial activation in SNc. Inflammopharmacology 2018, 26, 1305–1316. [Google Scholar] [CrossRef]
- Micheli, L.; Lucarini, E.; Trallori, E.; Avagliano, C.; De Caro, C.; Russo, R.; Calignano, A.; Ghelardini, C.; Pacini, A.; Di Cesare Mannelli, L. Phaseolus vulgaris L. Extract: Alpha-amylase inhibition against metabolic syndrome in mice. Nutrients 2019, 11, 1778. [Google Scholar] [CrossRef] [Green Version]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Micheli, L.; Vasarri, M.; Barletta, E.; Lucarini, E.; Ghelardini, C.; Degl’Innocenti, D.; Di Cesare Mannelli, L. Efficacy of Posidonia oceanica Extract against Inflammatory Pain: In Vivo Studies in Mice. Mar. Drugs 2021, 19, 48. https://doi.org/10.3390/md19020048
Micheli L, Vasarri M, Barletta E, Lucarini E, Ghelardini C, Degl’Innocenti D, Di Cesare Mannelli L. Efficacy of Posidonia oceanica Extract against Inflammatory Pain: In Vivo Studies in Mice. Marine Drugs. 2021; 19(2):48. https://doi.org/10.3390/md19020048
Chicago/Turabian StyleMicheli, Laura, Marzia Vasarri, Emanuela Barletta, Elena Lucarini, Carla Ghelardini, Donatella Degl’Innocenti, and Lorenzo Di Cesare Mannelli. 2021. "Efficacy of Posidonia oceanica Extract against Inflammatory Pain: In Vivo Studies in Mice" Marine Drugs 19, no. 2: 48. https://doi.org/10.3390/md19020048
APA StyleMicheli, L., Vasarri, M., Barletta, E., Lucarini, E., Ghelardini, C., Degl’Innocenti, D., & Di Cesare Mannelli, L. (2021). Efficacy of Posidonia oceanica Extract against Inflammatory Pain: In Vivo Studies in Mice. Marine Drugs, 19(2), 48. https://doi.org/10.3390/md19020048