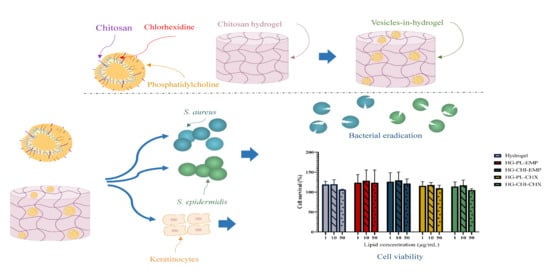
Chitosomes-In-Chitosan Hydrogel for Acute Skin Injuries: Prevention and Infection Control
Abstract
:1. Introduction
2. Results and Discussions
2.1. Vesicle Characteristics
2.1.1. Vesicle Characteristics
2.1.2. Surface-Available Chitosan
2.1.3. Vesicle Stability
2.2. Hydrogel Characterization
2.2.1. Hydrogel Characterization
2.2.2. Viscosity Evaluation
2.3. CHX Release
2.4. Evaluation of Potential Toxicity
2.5. Antimicrobial Evaluation
3. Materials and Methods
3.1. Materials
3.2. Vesicle Preparation
3.2.1. Vesicle Preparation
3.2.2. Size Reduction
3.3. Characterization of Chitosomes
3.3.1. Vesicle Size and Morphology
3.3.2. Zeta Potential and pH of the Vesicles
3.3.3. Separation and Entrapment Efficiency
3.3.4. Determination of Availability of Chitosan on the Surface
3.3.5. Chitosome and Vesicle Stability
3.4. Preparation and Characterization of Hydrogels
3.4.1. Preparation of Chitosan Hydrogel
3.4.2. Texture Properties and pH of Hydrogels
3.4.3. Viscosity Measurements
3.5. CHX Release Studies
3.6. Cell Viability Valuation
3.7. Antimicrobial Evaluation
3.8. Statistical Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salyer, C.E.; Bomholt, C.; Beckmann, N.; Bergmann, C.B.; Plattner, C.A.; Caldwell, C.C. Novel Therapeutics for the Treatment of Burn Infection. Surg. Infect. 2020, 22, 113–120. [Google Scholar] [CrossRef]
- Corcione, S.; Pensa, A.; Castiglione, A.; Lupia, T.; Bortolaso, B.; Romeo, M.R.; Stella, M.; Rosa, F.G.D. Epidemiology, prevalence and risk factors for infections in burn patients: Results from a regional burn centre’s analysis. J. Chemother. 2020, 33, 62–66. [Google Scholar] [CrossRef]
- van Duin, D.; Strassle, P.D.; DiBiase, L.M.; Lachiewicz, A.M.; Rutala, W.A.; Eitas, T.; Maile, R.; Kanamori, H.; Weber, D.J.; Cairns, B.A.; et al. Timeline of health care–associated infections and pathogens after burn injuries. Am. J. Infect. Control 2016, 44, 1511–1516. [Google Scholar] [CrossRef] [Green Version]
- Poulakou, G.; Lagou, S.; Tsiodras, S. What’s new in the epidemiology of skin and soft tissue infections in 2018? Curr. Opin. Infect. Dis. 2019, 32, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Jabalameli, F.; Mirsalehian, A.; Khoramian, B.; Aligholi, M.; Khoramrooz, S.S.; Asadollahi, P.; Taherikalani, M.; Emaneini, M. Evaluation of biofilm production and characterization of genes encoding type III secretion system among Pseudomonas aeruginosa isolated from burn patients. Burns 2012, 38, 1192–1197. [Google Scholar] [CrossRef] [PubMed]
- Brandenburg, K.S.; Weaver, A.J.; Karna, S.L.R.; You, T.; Chen, P.; Stryk, S.V.; Qian, L.; Pineda, U.; Abercrombie, J.J.; Leung, K.P. Formation of Pseudomonas aeruginosa Biofilms in Full-thickness Scald Burn Wounds in Rats. Sci. Rep. 2019, 9, 13627. [Google Scholar] [CrossRef] [PubMed]
- Smolle, C.; Cambiaso-Daniel, J.; Forbes, A.A.; Wurzer, P.; Hundeshagen, G.; Branski, L.K.; Huss, F.; Kamolz, L.-P. Recent trends in burn epidemiology worldwide: A systematic review. Burns 2017, 43, 249–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- James, S.L.; Lucchesi, L.R.; Bisignano, C.; Castle, C.D.; Dingels, Z.V.; Fox, J.T.; Hamilton, E.B.; Henry, N.J.; McCracken, D.; Roberts, N.L.S.; et al. Epidemiology of injuries from fire, heat and hot substances: Global, regional and national morbidity and mortality estimates from the Global Burden of Disease 2017 study. Inj. Prev. 2019, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Mofazzal Jahromi, M.A.; Sahandi Zangabad, P.; Moosavi Basri, S.M.; Sahandi Zangabad, K.; Ghamarypour, A.; Aref, A.R.; Karimi, M.; Hamblin, M.R. Nanomedicine and advanced technologies for burns: Preventing infection and facilitating wound healing. Adv. Drug Deliv. Rev. 2018, 123, 33–64. [Google Scholar] [CrossRef]
- Khan, F.; Pham, D.T.N.; Oloketuyi, S.F.; Manivasagan, P.; Oh, J.; Kim, Y.-M. Chitosan and their derivatives: Antibiofilm drugs against pathogenic bacteria. Colloids Surf. B 2020, 185, 110627. [Google Scholar] [CrossRef]
- Bakshi, P.S.; Selvakumar, D.; Kadirvelu, K.; Kumar, N.S. Chitosan as an environment friendly biomaterial—A review on recent modifications and applications. Int. J. Biol. Macromol. 2020, 150, 1072–1083. [Google Scholar] [CrossRef]
- Barbosa, A.I.; Coutinho, A.J.; Costa Lima, S.A.; Reis, S. Marine Polysaccharides in Pharmaceutical Applications: Fucoidan and Chitosan as Key Players in the Drug Delivery Match Field. Mar. Drugs 2019, 17, 654. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.M.; Shahruzzaman, M.; Biswas, S.; Nurus Sakib, M.; Rashid, T.U. Chitosan based bioactive materials in tissue engineering applications—A review. Bioact. Mater. 2020, 5, 164–183. [Google Scholar] [CrossRef]
- Jøraholmen, M.W.; Bhargava, A.; Julin, K.; Johannessen, M.; Škalko-Basnet, N. The Antimicrobial Properties of Chitosan Can be Tailored by Formulation. Mar. Drugs 2020, 18, 96. [Google Scholar] [CrossRef] [Green Version]
- Hu, Z.; Zhang, D.-Y.; Lu, S.-T.; Li, P.-W.; Li, S.-D. Chitosan-Based Composite Materials for Prospective Hemostatic Applications. Mar. Drugs 2018, 16, 273. [Google Scholar] [CrossRef] [Green Version]
- Desbrieres, J.; Peptu, C.; Ochiuz, L.; Savin, C.; Popa, M.; Vasiliu, S. Application of Chitosan-Based Formulations in Controlled Drug Delivery. In Sustainable Agriculture Reviews 36: Chitin and Chitosan: Applications in Food, Agriculture, Pharmacy, Medicine and Wastewater Treatment; Crini, G., Lichtfouse, E., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 241–314. [Google Scholar]
- Vanić, Ž.; Holsæter, A.M.; Škalko-Basnet, N. (Phospho) lipid-based Nanosystems for Skin Administration. Curr. Pharm. Des. 2015, 21, 4174–4192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hemmingsen, L.M.; Giordani, B.; Pettersen, A.K.; Vitali, B.; Basnet, P.; Škalko-Basnet, N. Liposomes-in-chitosan hydrogel boosts potential of chlorhexidine in biofilm eradication in vitro. Carbohydr. Polym. 2021, 262, 117939. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Dutta, J.; Dutta, P.K.; Koh, J. A systematic study on chitosan-liposome based systems for biomedical applications. Int. J. Biol. Macromol. 2020, 160, 470–481. [Google Scholar] [CrossRef] [PubMed]
- Andersen, T.; Mishchenko, E.; Flaten, G.E.; Sollid, J.U.E.; Mattsson, S.; Tho, I.; Škalko-Basnet, N. Chitosan-Based Nanomedicine to Fight Genital Candida Infections: Chitosomes. Mar. Drugs 2017, 15, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hurdle, J.G.; O’Neill, A.J.; Chopra, I.; Lee, R.E. Targeting bacterial membrane function: An underexploited mechanism for treating persistent infections. Nat. Rev. Microbiol. 2011, 9, 62–75. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Sayed, P.; Tornay, D.; Hirt-Burri, N.; de Buys Roessingh, A.; Raffoul, W.; Applegate, L.A. Implications of chlorhexidine use in burn units for wound healing. Burns 2020, 46, 1150–1156. [Google Scholar] [CrossRef]
- Hubbard, A.T.M.; Coates, A.R.; Harvey, R.D. Comparing the action of HT61 and chlorhexidine on natural and model Staphylococcus aureus membranes. J. Antibiot. 2017, 70, 1020–1025. [Google Scholar] [CrossRef]
- Drago, F.; Gariazzo, L.; Cioni, M.; Trave, I.; Parodi, A. The microbiome and its relevance in complex wounds. Eur. J. Dermatol. 2019, 29, 6–13. [Google Scholar] [CrossRef]
- Matica, M.A.; Aachmann, F.L.; Tøndervik, A.; Sletta, H.; Ostafe, V. Chitosan as a Wound Dressing Starting Material: Antimicrobial Properties and Mode of Action. Int. J. Mol. Sci. 2019, 20, 5889. [Google Scholar] [CrossRef] [Green Version]
- Vanić, Ž.; Škalko-Basnet, N. Chapter 11—Hydrogels as intrinsic antimicrobials. In Hydrogels Based on Natural Polymers; Chen, Y., Ed.; Elsevier BV: Amsterdam, The Netherlands, 2020; pp. 309–328. [Google Scholar]
- Billard, A.; Pourchet, L.; Malaise, S.; Alcouffe, P.; Montembault, A.; Ladavière, C. Liposome-loaded chitosan physical hydrogel: Toward a promising delayed-release biosystem. Carbohydr. Polym. 2015, 115, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Parhi, R. Chitin and Chitosan in Drug Delivery. In Sustainable Agriculture Reviews 36: Chitin and Chitosan: Applications in Food, Agriculture, Pharmacy, Medicine and Wastewater Treatment; Crini, G., Lichtfouse, E., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 175–239. [Google Scholar]
- Shariatinia, Z. Pharmaceutical applications of chitosan. Adv. Colloid Interface Sci. 2019, 263, 131–194. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Mao, J.; Cheng, Y.; Liu, H.; Lv, L.; Ge, M.; Li, S.; Huang, J.; Chen, Z.; Li, H.; et al. Recent Progress of Polysaccharide-Based Hydrogel Interfaces for Wound Healing and Tissue Engineering. Adv. Mater. Interfaces 2019, 6, 1900761. [Google Scholar] [CrossRef] [Green Version]
- Andersen, T.; Bleher, S.; Flaten, G.E.; Tho, I.; Mattsson, S.; Škalko-Basnet, N. Chitosan in mucoadhesive drug delivery: Focus on local vaginal therapy. Mar. Drugs 2015, 13, 222–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, M.; Kujundzic, M.; John, S.; Bismarck, A. Crab vs. Mushroom: A Review of Crustacean and Fungal Chitin in Wound Treatment. Mar. Drugs 2020, 18, 64. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Wang, C.; Li, C.; Qin, Y.; Wang, Z.; Yang, F.; Li, Z.; Wang, J. A functional chitosan-based hydrogel as a wound dressing and drug delivery system in the treatment of wound healing. RSC Adv. 2018, 8, 7533–7549. [Google Scholar] [CrossRef] [Green Version]
- Mathews, P.D.; Mertins, O. Chapter 9—Chitosan and lipid composites as versatile biomedical material. In Materials for Biomedical Engineering; Holban, A.-M., Grumezescu, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 259–291. [Google Scholar]
- Stoica, A.E.; Chircov, C.; Grumezescu, A.M. Hydrogel Dressings for the Treatment of Burn Wounds: An Up-To-Date Overview. Materials 2020, 13, 2853. [Google Scholar] [CrossRef]
- Amiri, N.; Ajami, S.; Shahroodi, A.; Jannatabadi, N.; Amiri Darban, S.; Fazly Bazzaz, B.S.; Pishavar, E.; Kalalinia, F.; Movaffagh, J. Teicoplanin-loaded chitosan-PEO nanofibers for local antibiotic delivery and wound healing. Int. J. Biol. Macromol. 2020, 162, 645–656. [Google Scholar] [CrossRef] [PubMed]
- El-Alfy, E.A.; El-Bisi, M.K.; Taha, G.M.; Ibrahim, H.M. Preparation of biocompatible chitosan nanoparticles loaded by tetracycline, gentamycin and ciprofloxacin as novel drug delivery system for improvement the antibacterial properties of cellulose based fabrics. Int. J. Biol. Macromol. 2020, 161, 1247–1260. [Google Scholar] [CrossRef] [PubMed]
- Hurler, J.; Žakelj, S.; Mravljak, J.; Pajk, S.; Kristl, A.; Schubert, R.; Škalko-Basnet, N. The effect of lipid composition and liposome size on the release properties of liposomes-in-hydrogel. Int. J. Pharm. 2013, 456, 49–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ternullo, S.; Schulte Werning, L.V.; Holsæter, A.M.; Škalko-Basnet, N. Curcumin-In-Deformable Liposomes-In-Chitosan-Hydrogel as a Novel Wound Dressing. Pharmaceutics 2019, 12, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paulsen, M.H.; Ausbacher, D.; Bayer, A.; Engqvist, M.; Hansen, T.; Haug, T.; Anderssen, T.; Andersen, J.H.; Sollid, J.U.E.; Strøm, M.B. Antimicrobial activity of amphipathic α,α-disubstituted β-amino amide derivatives against ESBL—CARBA producing multi-resistant bacteria; effect of halogenation, lipophilicity and cationic character. Eur. J. Med. Chem. 2019, 183, 111671. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.-Y.; van der Mei, H.C.; Ren, Y.; Busscher, H.J.; Shi, L. Lipid-Based Antimicrobial Delivery-Systems for the Treatment of Bacterial Infections. Front. Chem. 2020, 7, 1–15. [Google Scholar] [CrossRef]
- Ahani, E.; Montazer, M.; Toliyat, T.; Mahmoudi Rad, M.; Harifi, T. Preparation of nano cationic liposome as carrier membrane for polyhexamethylene biguanide chloride through various methods utilizing higher antibacterial activities with low cell toxicity. J. Microencapsul. 2017, 34, 121–131. [Google Scholar] [CrossRef] [Green Version]
- Chang, S.-H.; Lin, Y.-Y.; Wu, G.-J.; Huang, C.-H.; Tsai, G.J. Effect of chitosan molecular weight on anti-inflammatory activity in the RAW 264.7 macrophage model. Int. J. Biol. Macromol. 2019, 131, 167–175. [Google Scholar] [CrossRef]
- Hurler, J.; Engesland, A.; Kermany, B.P.; Škalko-Basnet, N. Improved texture analysis for hydrogel characterization: Gel cohesiveness, adhesiveness, and hardness. J. Appl. Polym. Sci. 2012, 125, 180–188. [Google Scholar] [CrossRef]
- Jøraholmen, M.W.; Basnet, P.; Tostrup, M.J.; Moueffaq, S.; Škalko-Basnet, N. Localized Therapy of Vaginal Infections and Inflammation: Liposomes-In-Hydrogel Delivery System for Polyphenols. Pharmaceutics 2019, 11, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Djekic, L.; Martinović, M.; Ćirić, A.; Fraj, J. Composite chitosan hydrogels as advanced wound dressings with sustained ibuprofen release and suitable application characteristics. Pharm. Dev. Technol. 2020, 25, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Wallace, L.A.; Gwynne, L.; Jenkins, T. Challenges and opportunities of pH in chronic wounds. Ther. Deliv. 2019, 10, 719–735. [Google Scholar] [CrossRef]
- Kruse, C.R.; Singh, M.; Targosinski, S.; Sinha, I.; Sørensen, J.A.; Eriksson, E.; Nuutila, K. The effect of pH on cell viability, cell migration, cell proliferation, wound closure, and wound reepithelialization: In vitro and in vivo study. Wound Repair Regen. 2017, 25, 260–269. [Google Scholar] [CrossRef]
- Schneider, L.A.; Korber, A.; Grabbe, S.; Dissemond, J. Influence of pH on wound-healing: A new perspective for wound-therapy? Arch. Dermatol. Res. 2007, 298, 413–420. [Google Scholar] [CrossRef]
- Dalisson, B.; Barralet, J. Bioinorganics and Wound Healing. Adv. Healthc. Mater. 2019, 8, 1900764. [Google Scholar] [CrossRef] [PubMed]
- Szymaǹska, E.; Sosnowska, K.; Miltyk, W.; Rusak, M.; Basa, A.; Winnicka, K. The Effect of β-Glycerophosphate Crosslinking on Chitosan Cytotoxicity and Properties of Hydrogels for Vaginal Application. Polymers 2015, 7, 2223–2244. [Google Scholar] [CrossRef] [Green Version]
- Kaplan, M.; Tuğcu-Demiröz, F.; Vural, İ.; Çelebi, N. Development and characterization of gels and liposomes containing ovalbumin for nasal delivery. J. Drug Deliv. Sci. Technol. 2018, 44, 108–117. [Google Scholar] [CrossRef]
- Szuhaj, B.F. PHOSPHOLIPIDS | Properties and Occurrence. In Encyclopedia of Food Sciences and Nutrition (Second Edition); Caballero, B., Ed.; Academic Press: Oxford, UK, 2003; pp. 4514–4519. [Google Scholar]
- Thirumaleshwar, S.K.; Kulkarni, P.V.; Gowda, D. Liposomal Hydrogels: A Novel Drug Delivery System for Wound Dressing. Curr. Drug Ther. 2012, 7, 212–218. [Google Scholar] [CrossRef]
- Souto, E.B.; Ribeiro, A.F.; Ferreira, M.I.; Teixeira, M.C.; Shimojo, A.A.M.; Soriano, J.L.; Naveros, B.C.; Durazzo, A.; Lucarini, M.; Souto, S.B.; et al. New Nanotechnologies for the Treatment and Repair of Skin Burns Infections. Int. J. Mol. Sci. 2020, 21, 393. [Google Scholar] [CrossRef] [Green Version]
- Ciobanu, B.C.; Cadinoiu, A.N.; Popa, M.; Desbrières, J.; Peptu, C.A. Modulated release from liposomes entrapped in chitosan/gelatin hydrogels. Mater. Sci. Eng. C 2014, 43, 383–391. [Google Scholar] [CrossRef]
- Heimbuck, A.M.; Priddy-Arrington, T.R.; Padgett, M.L.; Llamas, C.B.; Barnett, H.H.; Bunnell, B.A.; Caldorera-Moore, M.E. Development of Responsive Chitosan–Genipin Hydrogels for the Treatment of Wounds. ACS Appl. Bio Mater. 2019, 2, 2879–2888. [Google Scholar] [CrossRef]
- Mengoni, T.; Adrian, M.; Pereira, S.; Santos-Carballal, B.; Kaiser, M.; Goycoolea, F.M. A Chitosan—Based Liposome Formulation Enhances the In Vitro Wound Healing Efficacy of Substance P Neuropeptide. Pharmaceutics 2017, 9, 56. [Google Scholar] [CrossRef] [Green Version]
- Phetdee, M.; Polnok, A.; Viyoch, J. Development of chitosan-coated liposomes for sustained delivery of tamarind fruit pulp’s extract to the skin. Int. J. Cosmet. Sci. 2008, 30, 285–295. [Google Scholar] [CrossRef]
- Howling, G.I.; Dettmar, P.W.; Goddard, P.A.; Hampson, F.C.; Dornish, M.; Wood, E.J. The effect of chitin and chitosan on the proliferation of human skin fibroblasts and keratinocytes in vitro. Biomaterials 2001, 22, 2959–2966. [Google Scholar] [CrossRef]
- Hidalgo, E.; Dominguez, C. Mechanisms underlying chlorhexidine-induced cytotoxicity. Toxicol. In Vitro 2001, 15, 271–276. [Google Scholar] [CrossRef]
- Boyce, S.T.; Warden, G.D.; Holder, I.A. Cytotoxicity Testing of Topical Antimicrobial Agents on Human Keratinocytes and Fibroblasts for Cultured Skin Grafts. J. Burn Care Rehabil. 1995, 16, 97–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Intini, C.; Elviri, L.; Cabral, J.; Mros, S.; Bergonzi, C.; Bianchera, A.; Flammini, L.; Govoni, P.; Barocelli, E.; Bettini, R.; et al. 3D-printed chitosan-based scaffolds: An in vitro study of human skin cell growth and an in-vivo wound healing evaluation in experimental diabetes in rats. Carbohydr. Polym. 2018, 199, 593–602. [Google Scholar] [CrossRef]
- Lauto, A.; Hook, J.; Doran, M.; Camacho, F.; Poole-Warren, L.A.; Avolio, A.; Foster, L.J.R. Chitosan adhesive for laser tissue repair: In vitro characterization. Lasers Surg. Med. 2005, 36, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.P.; Espiga, A.; Silva, D.; Baptista, P.; Henriques, J.; Ferreira, C.; Silva, J.C.; Borges, J.P.; Pires, E.; Chaves, P.; et al. Development of a new chitosan hydrogel for wound dressing. Wound Repair Regen. 2009, 17, 817–824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hurler, J.; Sørensen, K.K.; Fallarero, A.; Vuorela, P.; Škalko-Basnet, N. Liposomes-in-hydrogel delivery system with mupirocin: In vitro antibiofilm studies and in vivo evaluation in mice burn model. BioMed Res. Int. 2013, 498485. [Google Scholar] [CrossRef] [Green Version]
- Chatelet, C.; Damour, O.; Domard, A. Influence of the degree of acetylation on some biological properties of chitosan films. Biomaterials 2001, 22, 261–268. [Google Scholar] [CrossRef]
- Gomathysankar, S.; Halim, A.S.; Makhtar, W.R.W.; Saad, A.Z.M.; Yaacob, N.S. Skin Substitutes in Wound Healing and the Stimulatory Effects of Adipose-Derived Stem Cells for the Proliferation of Keratinocytes on Chitosan. In Chronic Wounds, Wound Dressings and Wound Healing; Shiffman, M.A., Low, M., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 379–394. [Google Scholar]
- Seeger, M.A.; Paller, A.S. The Roles of Growth Factors in Keratinocyte Migration. Adv. Wound Care 2014, 4, 213–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oishi, Y.; Manabe, I. Macrophages in inflammation, repair and regeneration. Int. Immunol. 2018, 30, 511–528. [Google Scholar] [CrossRef] [PubMed]
- Kloc, M.; Ghobrial, R.M.; Wosik, J.; Lewicka, A.; Lewicki, S.; Kubiak, J.Z. Macrophage functions in wound healing. J. Tissue Eng. Regen. Med. 2019, 13, 99–109. [Google Scholar] [CrossRef]
- Ouyang, Q.-Q.; Hu, Z.; Lin, Z.-P.; Quan, W.-Y.; Deng, Y.-F.; Li, S.-D.; Li, P.-W.; Chen, Y. Chitosan hydrogel in combination with marine peptides from tilapia for burns healing. Int. J. Biol. Macromol. 2018, 112, 1191–1198. [Google Scholar] [CrossRef]
- Parthasarathy, A.; Vijayakumar, S.; Malaikozhundan, B.; Thangaraj, M.P.; Ekambaram, P.; Murugan, T.; Velusamy, P.; Anbu, P.; Vaseeharan, B. Chitosan-coated silver nanoparticles promoted antibacterial, antibiofilm, wound-healing of murine macrophages and antiproliferation of human breast cancer MCF 7 cells. Polym. Test. 2020, 90, 106675. [Google Scholar] [CrossRef]
- Xiao, J.; Zhou, Y.; Ye, M.; An, Y.; Wang, K.; Wu, Q.; Song, L.; Zhang, J.; He, H.; Zhang, Q.; et al. Freeze-Thawing Chitosan/Ions Hydrogel Coated Gauzes Releasing Multiple Metal Ions on Demand for Improved Infected Wound Healing. Adv. Healthc. Mater. 2021, 10, 2001591. [Google Scholar] [CrossRef]
- Sen, C.K. Human Wounds and Its Burden: An Updated Compendium of Estimates. Adv. Wound Care 2019, 8, 39–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goy, R.C.; Morais, S.T.B.; Assis, O.B.G. Evaluation of the antimicrobial activity of chitosan and its quaternized derivative on E. coli and S. aureus growth. Rev. Bras. Farmacogn. 2016, 26, 122–127. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.W.; Thomas, R.L.; Lee, C.; Park, H.J. Antimicrobial activity of native chitosan, degraded chitosan, and O-carboxymethylated chitosan. J. Food Prot. 2003, 66, 1495–1498. [Google Scholar] [CrossRef] [PubMed]
- Arkoun, M.; Daigle, F.; Heuzey, M.-C.; Ajji, A. Mechanism of Action of Electrospun Chitosan-Based Nanofibers against Meat Spoilage and Pathogenic Bacteria. Molecules 2017, 22, 585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alshamsan, A.; Aleanizy, F.S.; Badran, M.; Alqahtani, F.Y.; Alfassam, H.; Almalik, A.; Alosaimy, S. Exploring anti-MRSA activity of chitosan-coated liposomal dicloxacillin. J. Microbiol. Methods 2019, 156, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Laverde-Rojas, V.; Liscano, Y.; Rivera-Sánchez, S.P.; Ocampo-Ibáñez, I.D.; Betancourt, Y.; Alhajj, M.J.; Yarce, C.J.; Salamanca, C.H.; Oñate-Garzón, J. Antimicrobial Contribution of Chitosan Surface-Modified Nanoliposomes Combined with Colistin against Sensitive and Colistin-Resistant Clinical Pseudomonas aeruginosa. Pharmaceutics 2021, 13, 41. [Google Scholar] [CrossRef] [PubMed]
- Sacco, P.; Travan, A.; Borgogna, M.; Paoletti, S.; Marsich, E. Silver-containing antimicrobial membrane based on chitosan-TPP hydrogel for the treatment of wounds. J. Mater. Sci. Mater. Med. 2015, 26, 128. [Google Scholar] [CrossRef]
- Wang, X.; Cheng, F.; Wang, X.; Feng, T.; Xia, S.; Zhang, X. Chitosan decoration improves the rapid and long-term antibacterial activities of cinnamaldehyde-loaded liposomes. Int. J. Biol. Macromol. 2021, 168, 59–66. [Google Scholar] [CrossRef]
- Wu, I.Y.; Bala, S.; Škalko-Basnet, N.; di Cagno, M.P. Interpreting non-linear drug diffusion data: Utilizing Korsmeyer-Peppas model to study drug release from liposomes. Eur. J. Pharm. Sci. 2019, 138, 105026. [Google Scholar] [CrossRef]
- Maqbool, F.; Moyle, P.M.; Tan, M.S.A.; Thurecht, K.J.; Falconer, J.R. Preparation of albendazole-loaded liposomes by supercritical carbon dioxide processing. Artif. Cells Nanomed. Biotechnol. 2018, 46, S1186–S1192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muzzarelli, R.A.A. Colorimetric Determination of Chitosan. Anal. Biochem. 1998, 260, 255–257. [Google Scholar] [CrossRef]
- Hirsjärvi, S.; Qiao, Y.; Royere, A.; Bibette, J.; Benoit, J.-P. Layer-by-layer surface modification of lipid nanocapsules. Eur. J. Pharm. Biopharm. 2010, 76, 200–207. [Google Scholar] [CrossRef]
- Jøraholmen, M.W.; Škalko-Basnet, N.; Acharya, G.; Basnet, P. Resveratrol-loaded liposomes for topical treatment of the vaginal inflammation and infections. Eur. J. Pharm. Sci. 2015, 79, 112–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cauzzo, J.; Nystad, M.; Holsæter, A.M.; Basnet, P.; Škalko-Basnet, N. Following the Fate of Dye-Containing Liposomes In Vitro. Int. J. Mol. Sci. 2020, 21, 4847. [Google Scholar] [CrossRef] [PubMed]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [Green Version]
- Ternullo, S.; Gagnat, E.; Julin, K.; Johannessen, M.; Basnet, P.; Vanić, Ž.; Škalko-Basnet, N. Liposomes augment biological benefits of curcumin for multitargeted skin therapy. Eur. J. Pharm. Biopharm. 2019, 144, 154–164. [Google Scholar] [CrossRef] [PubMed]







| Size (nm) | PI 1 | Zeta Potential (mV) | EE 2 % | pH | |||
|---|---|---|---|---|---|---|---|
| Peak 1 % | Peak 2 % | Peak 3 % | |||||
| PL-EMP | 31 ± 9 5 ± 3 | 62 13 | 169 ± 18 90 ± 4 | 0.18 ± 0.01 | 0.6 ± 0.0 | - | 5.6 ± 0.0 |
| CHI-EMP | 14 ± 4 5 ± 4 | 41 ± 4 30 ± 12 | 150 ± 3 65 ± 16 | 0.22 ± 0.01 | 11.5 ± 0.3 | - | 4.4 ± 0.0 |
| PL-CHX | 16 ± 7 2 ± 1 | 66 ± 15 16 ± 5 | 243 ± 13 81 ± 6 | 0.32 ± 0.03 | 53.6 ± 2.0 | 68 ± 5 | 7.0 ± 0.3 |
| CHI-CHX | 14 ± 1 3 ± 1 | 79 ± 5 29 ± 15 | 260 ± 3 69 ± 16 | 0.30 ± 0.00 | 79.0 ± 3.7 | 74 ± 2 | 5.5 ± 0.1 |
| Surface-Available Chitosan (%) 3 | |
|---|---|
| CHI-EMP | 50.2 ± 2.9 |
| CHI-CHX | 48.5 ± 5.6 |
| Week | Size (nm) | PI 1 | Zeta Potential (mV) | pH | |||
|---|---|---|---|---|---|---|---|
| Peak 1 % | Peak 2 % | Peak 3 % | |||||
| PL-EMP | 2 | 33 ± 3 6 ± 2 | 133 ± 36 72 ± 35 | 331 ± 246 33 ± 39 | 0.20 ± 0.02 | −1.7 ± 0.4 | 5.6 ± 0.1 |
| 4 | 17 ± 1 2 ± 1 | 69 ± 21 26 ± 27 | 229 ± 49 62 ± 31 | 0.21 ± 0.02 | −3.1 ± 1.0 | 5.6 ± 0.4 | |
| CHI-EMP | 2 | 18 ± 2 3 ± 1 | 58 ± 9 15 ± 2 | 152 ± 3 82 ± 1 | 0.22 ± 0.01 | 12.0 ± 0.2 | 4.4 ± 0.0 |
| 4 | 18 ± 5 4 ± 1 | 56 ± 6 23 ± 23 | 144 ± 24 86 ± 1 | 0.22 ± 0.01 | 14.4 ± 0.5 | 4.5 ± 0.1 | |
| PL-CHX | 2 | 11 ± 0 1 ± 1 | 64 ± 8 18 ± 4 | 254 ± 21 81 ± 4 | 0.33 ± 0.03 | 55.9 ± 0.9 | 6.9 ± 0.2 |
| 4 | 22 ± 13 4 ± 3 | 101 ± 76 43 ± 43 | 225 ± 11 82 ± 5 | 0.32 ± 0.03 | 55.7 ± 1.0 | 7.2 ± 0.1 | |
| CHI-CHX | 2 | 14 ± 3 3 ± 3 | 54 ± 9 21 ± 20 | 222 ± 41 75 ± 22 | 0.30 ± 0.01 | 79.8 ± 4.5 | 5.5 ± 0.1 |
| 4 | 12 ± 1 2 ± 1 | 64 ± 17 21 ± 7 | 215 ± 49 76 ± 8 | 0.30 ± 0.02 | 83.0 ± 1.7 | 5.5 ± 0.1 | |
| Lipid Concentration (mg/mL) S. aureus | Lipid Concentration (mg/mL) S. epidermidis | |
|---|---|---|
| PL-EMP | - | - |
| CHI-EMP | 1.25 | 0.625 |
| PL-CHX | 0.32 | 0.039 |
| CHI-CHX | 0.078 | <0.005 |
| Lipid Concentration (mg/mL) 4 S. aureus | Lipid Concentration (mg/mL) 4 S. epidermidis | |
|---|---|---|
| Hydrogel | 1.56 × 10−2 | 0.10 × 10−2 |
| HG-PL-EMP | 1.56 × 10−2 | 0.10 × 10−2 |
| HG-CHI-EMP | 1.56 × 10−2 | 0.025 × 10−2 |
| HG-PL-CHX | 1.56 × 10−2 | 0.0063 × 10−2 |
| HG-CHI-CHX | 0.78 × 10−2 | 0.0031 × 10−2 |
| Composition | |
|---|---|
| PL-EMP | Lipoid S100 |
| CHI-EMP | Lipoid S100 Chitosan |
| PL-CHX | Lipoid S100 CHX |
| CHI-CHX | Lipoid S100 Chitosan CHX |
| Composition | |
|---|---|
| Hydrogel | Chitosan Glycerol |
| HG-PL-EMP | Chitosan Glycerol PL-EMP vesicles |
| HG-CHI-EMP | Chitosan Glycerol CHI-EMP vesicles |
| HG-PL-CHX | Chitosan Glycerol PL-CHX vesicles |
| HG-CHI-CHX | Chitosan Glycerol CHI-CHX vesicles |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hemmingsen, L.M.; Julin, K.; Ahsan, L.; Basnet, P.; Johannessen, M.; Škalko-Basnet, N. Chitosomes-In-Chitosan Hydrogel for Acute Skin Injuries: Prevention and Infection Control. Mar. Drugs 2021, 19, 269. https://doi.org/10.3390/md19050269
Hemmingsen LM, Julin K, Ahsan L, Basnet P, Johannessen M, Škalko-Basnet N. Chitosomes-In-Chitosan Hydrogel for Acute Skin Injuries: Prevention and Infection Control. Marine Drugs. 2021; 19(5):269. https://doi.org/10.3390/md19050269
Chicago/Turabian StyleHemmingsen, Lisa Myrseth, Kjersti Julin, Luqman Ahsan, Purusotam Basnet, Mona Johannessen, and Nataša Škalko-Basnet. 2021. "Chitosomes-In-Chitosan Hydrogel for Acute Skin Injuries: Prevention and Infection Control" Marine Drugs 19, no. 5: 269. https://doi.org/10.3390/md19050269
APA StyleHemmingsen, L. M., Julin, K., Ahsan, L., Basnet, P., Johannessen, M., & Škalko-Basnet, N. (2021). Chitosomes-In-Chitosan Hydrogel for Acute Skin Injuries: Prevention and Infection Control. Marine Drugs, 19(5), 269. https://doi.org/10.3390/md19050269