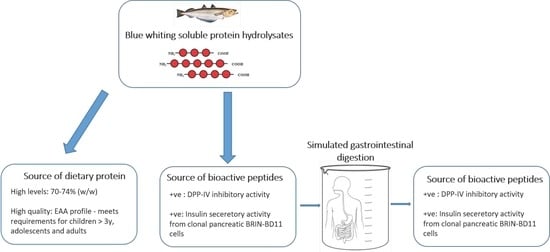
Physicochemical, Nutritional and In Vitro Antidiabetic Characterisation of Blue Whiting (Micromesistiuspoutassou) Protein Hydrolysates
Abstract
:1. Introduction
2. Results
2.1. Protein and Amino Acid Analysis of BW-SPH-A_F
2.2. Physicochemical Properties of BW-SPHs Pre- and Post-Simulated Gastrointestinal Digestion (SGID)
2.3. Changes in Amino Acid Profile of the BW-SPHs as a Result of Simulated Gastrointestinal Digestion (SGID)
2.4. DPP-IV Inhibitory Activity
2.5. Insulin Secretory Activity
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Generation of Blue Whiting Soluble Protein Hydrolysates (BW-SPHs)
4.3. Protein and Amino Acid Analysis
4.4. Simulated Gastrointestinal Digestion (SGID)
4.5. Physicochemical Characterisation
4.6. In Vitro DPP-IV Inhibitory Activity Assessment
4.7. Insulin Secretion Studies in Clonal Pancreatic Cells
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ariño, A.; Beltrán, J.A.; Roncalés, P. FISH/Dietary Importance of Fish and Shellfish. In Encyclopaedia of Food Sciences and Nutrition, 2nd ed.; Caballero, B., Ed.; Academic Press: London, UK, 2003; pp. 2471–2478. [Google Scholar]
- Harnedy, P.A.; FitzGerald, R.J. Bioactive peptides from marine processing waste and shellfish: A review. J. Funct. Foods 2012, 4, 6–24. [Google Scholar] [CrossRef]
- FAO—Food and Agriculture Organization of the United Nations. The State of World Fisheries and Aquaculture. Meeting the Sustainable Development Goals; FAO: Rome, Italy, 2018; pp. 1–217. [Google Scholar]
- Paulrud, A.; de Carvalho Gaspar, N.D.; Borrello, A. The 2014 Annual Economic Report on the EU Fishing Fleet; Annual Economic Report Brussels, Scientific Technical and Economic Committee for Fisheries (STECF), Publications Office of the European Union: Luxembourg, 2014; pp. 1–368. [Google Scholar]
- Egerton, S.; Culloty, S.; Whooley, J.; Stanton, C.; Ross, P.R. Boarfish (Capros aper): Review of a new capture fishery and its valorization potential. ICES J. Mar. Sci. 2017, 74, 2059–2068. [Google Scholar] [CrossRef]
- Harnedy, P.A.; Parthsarathy, V.; McLaughlin, C.M.; O’Keeffe, M.B.; Allsopp, P.J.; McSorley, E.M.; O’Harte, F.P.M.; FitzGerald, R.J. Blue whiting (Micromesistius poutassou) muscle protein hydrolysate with in vitro and in vivo antidiabetic properties. J. Funct. Foods 2018, 40, 137–145. [Google Scholar] [CrossRef] [Green Version]
- Cudennec, B.; Fouchereau-Peron, M.; Ferry, F.; Duclos, E.; Ravallec, R. In vitro and in vivo evidence for a satiating effect of fish protein hydrolysate obtained from blue whiting (Micromesistius poutassou) muscle. J. Funct. Foods 2012, 4, 271–277. [Google Scholar] [CrossRef]
- Duclos, E. Fish-Protein Hydrolysates Used in Preventing and/or Treating Metabolic Disorders such as Metabolic Syndrome Particularly Associated with Obesity. European Patent EP 2753194 A1, 16 July 2014. [Google Scholar]
- La Rochelle, H.D.; Courois, E.; Cudennec, B.; Fouchereau-Peron, M.; Ravallec-Ple, R. Fish Protein Hydrolysate Having Satietogenic Activity, Nutraceutical and Pharmacological Compositions Comprising such a Hydrolysate and Method for Obtaining Same. U.S. Patent US20150025001 A1, 17 February 2011. [Google Scholar]
- Nobile, V.; Duclos, E.; Michelotti, A.; Bizzaro, G.; Negro, M.; Soisson, F. Supplementation with a fish protein hydrolysate (Micromesistius poutassou): Effects on body weight, body composition, and CCK/GLP-1 secretion. Food Nutr. Res. 2016, 60, 29857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- FAO—Food and Agriculture Organization of the United Nations. Report of an FAO Expert Consultation. Dietary protein quality evaluation in human nutrition. FAO Food Nutr. Pap. 2013, 92, 1–79. [Google Scholar]
- Caruso, G.; Floris, R.; Serangeli, C.; Di Paola, L. Fishery wastes as a yet undiscovered treasure from the sea: Biomolecules sources, extraction methods and valorization. Mar. Drugs 2020, 18, 622. [Google Scholar] [CrossRef] [PubMed]
- WHO/FAO/UNU. Protein and amino acid requirement in human nutrition: Report of joint WHO/FAO/UNU expert consultation. WHO Tech. Rep. Ser. 2007, 935, 1–284. [Google Scholar]
- Mariotti, F.; Tomé, D.; Mirand, P.P. Converting nitrogen into protein—Beyond 6.25 and Jones’ factors. Crit. Rev. Food Sci. Nutr. 2008, 48, 177–184. [Google Scholar] [CrossRef]
- Aspevik, T.; Egede-Nissen, H.; Oterhals, Ĺ. A systematic approach to the comparison of cost efficiency of endopeptidases for the hydrolysis of atlantic salmon (Salmo salar) by-products. Food Technol. Biotechnol. 2016, 54, 421–431. [Google Scholar] [CrossRef]
- Steinsholm, S.; Oterhals, A.; Underhaug, J.; Måge, I.; Malmendal, A.; Aspevik, T. Sensory assessment of fish and chicken protein hydrolysates. Evaluation of NMR metabolomics profiling as a new prediction tool. J. Agric. Food Chem. 2020, 68, 3881–3890. [Google Scholar] [CrossRef] [PubMed]
- Newsholme, P.; Krause, M. Nutritional regulation of insulin secretion: Implications for diabetes. Clin. Biochem. Rev. 2012, 33, 35–47. [Google Scholar]
- Oseguera-Toledo, M.E.; de Mejía, E.G.; Reynoso-Camacho, R.; Cardador-Martínez, A.; Amaya-Llano, S.L. Proteins and bioactive peptides Mechanisms of action on diabetes management. Nutrafoods 2014, 13, 147–157. [Google Scholar] [CrossRef]
- Ranawana, V.; Kaur, B. Role of proteins in insulin secretion and glycemic control. Adv. Food Nutr. Res. 2013, 70, 1–47. [Google Scholar]
- NMIC Bulletin. Update on Type 2 Diabetes Mellitus, 2017, Volume 23, pp. 1–9. Available online: http://www.stjames.ie/media/NMIC%20Bulletin%20vol%2023%20No%201%20April%202017%20-%20Update%20On%20Type%202%20Diabetes.pdf (accessed on 10 April 2021).
- Harnedy-Rothwell, P.A.; McLaughlin, C.M.; O’Keeffe, M.B.; Le Gouic, A.V.; Allsopp, P.J.; McSorley, E.M.; Sharkey, S.; Whooley, J.; McGovern, B.; O’Harte, F.P.M.; et al. Identification and characterisation of peptides from a boarfish (Capros aper) protein hydrolysate displaying in vitro dipeptidyl peptidase-IV (DPP-IV) inhibitory and insulinotropic activity. Food Res. Int. 2020, 31, 108989. [Google Scholar] [CrossRef] [PubMed]
- Li-Chan, E.C.; Hunag, S.L.; Jao, C.L.; Ho, K.P.; Hsu, K.C. Peptides derived from Atlantic salmon skin gelatin as dipeptidyl-peptidase IV inhibitors. J. Agric. Food Chem. 2012, 60, 973–978. [Google Scholar] [CrossRef] [PubMed]
- Harnedy, P.A.; FitzGerald, R.J. In vitro assessment of the cardioprotective, anti-diabetic and antioxidant potential of Palmaria palmata protein hydrolysates. J. App. Phycol. 2013, 2, 1793–1803. [Google Scholar] [CrossRef]
- Harnedy, P.A.; Parthsarathy, V.; McLaughlin, C.M.; O’Keeffe, M.B.; Allsopp, P.J.; McSorley, E.M.; O’Harte, F.P.M.; FitzGerald, R.J. Atlantic salmon (Salmo salar) co-product-derived protein hydrolysates: A source of antidiabetic peptides. Food Res. Int. 2018, 106, 598–606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parthsarathy, V.; McLaughlin, C.M.; Harnedy, P.A.; Allsopp, P.J.; Crowe, W.; McSorley, E.M.; FitzGerald, R.J.; O’Harte, F.P.M. Boarfish (Capros aper) protein hydrolysate has potent insulinotropic and GLP-1 secretory activity in vitro and acute glucose lowering effects in mice. Int. J. Food Sci. Technol. 2019, 54, 271–281. [Google Scholar] [CrossRef] [Green Version]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Balance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; FitzGerald, R.J. Features of dipeptidyl peptidase IV (DPP-IV) inhibitory peptides from dietary proteins. J. Food Biochem. 2017, 43, e12451. [Google Scholar] [CrossRef] [Green Version]
- Harnedy-Rothwell, P.A.; McLaughlin, C.M.; Le Gouic, A.V.; Mullen, C.; Parthsarathy, V.; Allsopp, P.J.; McSorley, E.M.; FitzGerald, R.J.; O’Harte, F.P.M. In vitro and in vivo effects of Palmaria palmata derived peptides on glucose metabolism. Int. J. Pept. Res. Ther. 2021. [Google Scholar] [CrossRef]
- Neves, A.C.; Harnedy, P.A.; O’Keeffe, M.B.; Alashi, M.A.; Aluko, R.E.; FitzGerald, R.J. Antihypertensive, dipeptidyl peptidase IV inhibitory and antioxidant activities of salmon gelatin derived peptides. Food Res. Int. 2017, 100, 112–120. [Google Scholar] [CrossRef]
- Neves, A.C.; Harnedy, P.A.; O’Keeffe, M.B.; FitzGerald, R.J. Bioactive peptides from Atlantic salmon (Salmo salar) with angiotensin converting enzyme and dipeptidyl peptidase IV inhibitory, and antioxidant activities. Food Chem. 2017, 218, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Dixon, G.; Nolan, J.; McClenaghan, N.; Flatt, P.R.; Newsholme, P. A comparative study of amino acid consumption by rat islet cells and the clonal beta-cell line BRIN-BD11—The functional significance of L-alanine. J. Endocrin. 2003, 179, 447–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morifuji, M.; Ishizaka, M.; Baba, S.; Fukuda, K.; Matsumoto, H.; Koga, J.; Kanegae, M.; Higuchi, M. Comparison of different sources and degrees of hydrolysis of dietary protein: Effect on plasma amino acids, dipeptides, and insulin responses in human subjects. J. Agric. Food Chem. 2010, 58, 8788–8797. [Google Scholar] [CrossRef] [PubMed]
- Newsholme, P.; Brennan, L.; Bender, K. Amino acid metabolism, β-cell function, and diabetes. Diabetes 2006, 55, 39–47. [Google Scholar] [CrossRef] [Green Version]
- Power-Grant, O.; Bruen, C.; Brennan, L.; Giblin, L.; Jakeman, P.; FitzGerald, R.J. In vitro bioactive properties of intact and enzymatically hydrolysed whey protein: Targeting the enteroinsular axis. Food Funct. 2015, 6, 972–980. [Google Scholar] [CrossRef]
- Connolly, A.; Piggott, C.O.; FitzGerald, R.J. Characterisation of protein-rich isolates and antioxidative phenolic extracts from pale and black brewers’ spent grain. Int. J. Food Sci. Technol. 2013, 48, 1670–1681. [Google Scholar] [CrossRef]
- Sriperm, N.; Pesti, G.M.; Tillman, P.B. Evaluation of the fixed nitrogen-to-protein (N:P) conversion factor (6.25) versus ingredient specific N:P conversion factors in feedstuffs. J. Sci. Food Agric. 2011, 91, 1182–1186. [Google Scholar] [CrossRef]
- Walsh, D.J.; Bernard, H.; Murray, B.A.; MacDonald, J.; Pentzien, A.K.; Wright, G.A.; Wal, J.M.; Struthers, A.D.; Meisel, H.; FitzGerald, R.J. In vitro generation and stability of the lactokinin beta-lactoglobulin fragment (142–148). J. Dairy Sci. 2004, 87, 3845–3857. [Google Scholar] [CrossRef] [Green Version]
- Alder-Nissen, J. Determination of the degree of hydrolysis of food protein hydrolysates by trinitrobenzenesulfonic acid. J. Agric. Food Chem. 1979, 27, 1256–1262. [Google Scholar] [CrossRef] [PubMed]
- Spellman, D.; Kenny, P.; O’Cuinn, G.; FitzGerald, R.J. Aggregation properties of whey protein hydrolysates generated with Bacillus licheniformis proteinase activities. J. Agric. Food Chem. 2005, 53, 1258–1265. [Google Scholar] [CrossRef] [PubMed]
- Harnedy-Rothwell, P.A.; McLaughlin, C.M.; Crowe, W.; Allsopp, P.J.; McSorley, E.M.; Devaney, M.; Whooley, J.; McGovern, B.; Parthsarath, V.; O’Harte, F.P.M.; et al. Stability to thermal treatment of dipeptidyl peptidase IV (DPP-IV) inhibitory activity of a boarfish (Capros aper) protein hydrolysate when incorporated into tomato-based products. Int. J. Food Sci. Technol. 2020, 56, 158–165. [Google Scholar] [CrossRef]
- McClenaghan, N.H.; Barnett, C.R.; Ah-Sing, E.; Abdel-Wahab, Y.; O’Harte, F.P.; Yoon, T.W.; Swanston-Flatt, S.; Flatt, P. Characterization of a novel glucose-responsive insulin-secreting cell line, BRIN-BD11, produced by electrofusion. Diabetes 1996, 45, 1132–1140. [Google Scholar] [CrossRef] [PubMed]
- Flatt, P.R.; Bailey, C.J. Plasma glucose and insulin responses to glucagon and arginine in Aston ob/ob mice: Evidence for a selective defect in glucose-mediated insulin release. Horm. Met. Res. 1982, 14, 127–130. [Google Scholar] [CrossRef]
| Sample | Nitrogen Content (g/100 g) | N:P Conversion Factor | Protein Content (g/100 g) |
|---|---|---|---|
| BW-SPH-A | 14.05 ± 0.10 | 5.24 | 73.60 ± 0.53 |
| BW-SPH-B | 13.92 ± 0.05 | 5.19 | 72.24 ± 0.24 |
| BW-SPH-C | 14.45 ± 0.16 | 5.04 | 72.77 ± 0.80 |
| BW-SPH-D | 13.76 ± 0.06 | 5.12 | 70.37 ± 0.33 |
| BW-SPH-E | 13.87 ± 0.03 | 5.12 | 71.02 ± 0.14 |
| BW-SPH-F | 14.20 ± 0.06 | 5.15 | 73.03 ± 0.30 |
| BW-SPH-A | BW-SPH-B | BW-SPH-C | BW-SPH-D | BW-SPH-E | BW-SPH-F | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TAA | FAA | FAA-GI | TAA | FAA | FAA-GI | TAA | FAA | FAA-GI | TAA | FAA | FAA-GI | TAA | FAA | FAA-GI | TAA | FAA | FAA-GI | |
| Asx | 8.56 | 0.31 | 0.33 | 8.45 | 0.37 | 0.37 | 8.31 | 0.25 | 0.21 | 8.10 | 0.18 | 0.18 | 8.13 | 0.40 | 0.38 | 8.52 | 0.30 | 0.28 |
| Ser | 4.10 | 0.91 | 0.88 | 4.04 | 0.75 | 0.76 | 4.07 | 0.45 | 0.45 | 4.00 | 0.40 | 0.42 | 3.93 | 0.82 | 0.85 | 4.07 | 0.53 | 0.55 |
| Glx | 12.50 | 1.02 | 1.11 | 12.60 | 0.90 | 1.03 | 12.80 | 0.65 | 0.68 | 12.00 | 0.52 | 0.62 | 12.40 | 0.84 | 0.98 | 12.90 | 0.65 | 0.70 |
| Gly | 5.52 | 0.22 | 0.26 | 5.43 | 0.23 | 0.28 | 5.85 | 0.16 | 0.18 | 5.10 | 0.21 | 0.25 | 5.55 | 0.23 | 0.30 | 5.85 | 0.21 | 0.24 |
| Arg | 5.94 | 2.48 | 3.92 | 6.15 | 2.45 | 3.99 | 6.31 | 2.08 | 3.86 | 6.15 | 1.94 | 3.48 | 6.31 | 2.54 | 2.77 | 6.34 | 1.97 | 3.78 |
| Pro | 3.41 | <0.050 | <0.005 | 3.30 | <0.050 | <0.005 | 3.31 | <0.050 | <0.005 | 3.14 | <0.050 | <0.005 | 3.33 | <0.050 | <0.005 | 3.49 | <0.050 | <0.005 |
| Ala | 5.41 | 0.82 | 0.86 | 5.54 | 0.79 | 0.81 | 5.71 | 0.63 | 0.62 | 5.28 | 0.49 | 0.56 | 5.30 | 0.81 | 0.88 | 5.53 | 0.68 | 0.68 |
| Cys | 0.61 | nd | nd | 0.61 | 0.63 | nd | 0.59 | 0.41 | nd | 0.60 | 0.58 | nd | 0.57 | 0.23 | nd | 0.57 | nd | nd |
| Tyr | 3.23 | 0.82 | 2.06 | 2.37 | 0.66 | 1.42 | 2.04 | 0.44 | 1.68 | 2.47 | 0.46 | 1.70 | 2.25 | 0.81 | 1.75 | 2.31 | 0.60 | 1.61 |
| Hyp | 0.79 | - | - | 0.76 | - | - | 0.87 | - | - | 0.61 | - | - | 0.79 | - | - | 0.88 | - | - |
| Val | 3.89 | 0.97 | 0.81 | 3.81 | 1.12 | 0.99 | 3.39 | 0.42 | 0.41 | 3.50 | 0.43 | 0.34 | 3.70 | 1.22 | 1.09 | 3.57 | 0.65 | 0.60 |
| Ile | 3.35 | 0.85 | 0.95 | 3.34 | 1.17 | 1.23 | 2.78 | 0.39 | 0.45 | 2.96 | 0.48 | 0.39 | 3.32 | 1.25 | 1.33 | 3.05 | 0.66 | 0.85 |
| Leu | 5.78 | 2.71 | 2.88 | 5.63 | 3.08 | 3.08 | 5.49 | 1.28 | 2.00 | 5.51 | 1.62 | 1.88 | 5.40 | 3.41 | 3.50 | 5.61 | 2.21 | 2.49 |
| Thr | 3.70 | 0.91 | 0.98 | 3.57 | 0.89 | 1.01 | 3.41 | 0.72 | 0.72 | 3.38 | 0.57 | 0.59 | 3.43 | 0.95 | 1.19 | 3.58 | 0.72 | 0.96 |
| Met | 1.94 | 1.02 | 1.01 | 1.76 | 0.87 | 0.92 | 1.79 | 0.49 | 0.71 | 1.93 | 0.60 | 0.78 | 1.65 | 0.91 | 1.03 | 1.79 | 0.67 | 0.83 |
| Lys | 7.44 | 2.15 | 3.53 | 7.65 | 1.98 | 3.38 | 7.48 | 0.19 | 2.61 | 7.35 | 0.94 | 2.95 | 7.48 | 0.31 | 3.37 | 7.54 | 1.13 | 3.21 |
| His | 1.53 | 0.30 | 0.48 | 1.55 | 0.25 | 0.45 | 1.46 | 0.10 | 0.41 | 1.56 | 0.19 | 0.38 | 1.46 | 0.29 | 0.57 | 1.60 | 0.22 | 0.46 |
| Phe | 2.87 | 1.46 | 1.63 | 2.80 | 1.26 | 1.50 | 2.54 | 0.64 | 1.16 | 2.74 | 0.80 | 1.18 | 2.50 | 1.37 | 1.52 | 2.55 | 1.00 | 1.30 |
| Trp | 0.56 | 0.18 | 0.20 | 0.58 | 0.15 | 0.16 | 0.45 | 0.06 | 0.10 | 0.59 | 0.11 | 0.14 | 0.50 | 0.16 | 0.17 | 0.57 | 0.11 | 0.17 |
| ΣEAAs | 31.06 | 10.55 | 12.47 | 30.69 | 10.77 | 12.71 | 28.79 | 4.28 | 8.56 | 29.52 | 5.74 | 8.64 | 29.44 | 9.87 | 13.75 | 29.86 | 7.36 | 10.88 |
| ΣAAs | 81.03 | 17.13 | 21.87 | 79.94 | 17.55 | 21.37 | 78.65 | 9.34 | 16.24 | 76.97 | 10.52 | 15.85 | 78.00 | 16.54 | 21.68 | 80.32 | 12.31 | 18.73 |
| Sample Code | Degree of Hydrolysis (DH%) | Molecular Mass Distribution (%) | ||||
|---|---|---|---|---|---|---|
| >10 kDa | 5–10 kDa | 2–5 kDa | 1–2 kDa | <1 kDa | ||
| BW-SPH-A | 43.19 ± 2.16 a | 0.32 ± 0.08 | 1.47 ± 0.08 | 6.26 ± 0.04 | 14.11 ± 0.01 | 77.86 ± 0.16 |
| BW-SPH-A_GI | 57.48 ± 2.72 AB* | 0.00 ± 0.00 | 0.04 ± 0.06 | 1.22 ± 0.02 | 6.88 ± 0.23 | 91.87 ± 0.30 |
| BW-SPH-B | 45.78 ± 2.91 a | 0.99 ± 0.04 | 2.79 ± 0.03 | 9.10 ± 0.15 | 15.94 ± 0.12 | 71.19 ± 0.33 |
| BW-SPH-B_GI | 61.51 ± 2.66 AB* | 0.00 ± 0.00 | 0.13 ± 0.03 | 1.82 ± 0.02 | 8.36 ± 0.02 | 89.70 ± 0.07 |
| BW-SPH-C | 27.82 ± 1.11 c | 2.45 ± 0.05 | 5.56 ± 0.06 | 15.97 ± 0.07 | 20.48 ± 0.04 | 55.55 ± 0.13 |
| BW-SPH-C_GI | 55.37 ± 1.83 B* | 0.00 ± 0.00 | 0.27 ± 0.01 | 2.77 ± 0.02 | 11.38 ± 0.03 | 85.58 ± 0.01 |
| BW-SPH-D | 36.59 ± 0.87 b | 0.25 ± 0.05 | 1.49 ± 0.04 | 7.02 ± 0.03 | 16.67 ± 0.06 | 74.57 ± 0.08 |
| BW-SPH-D_GI | 56.13 ± 3.95 B* | 0.00 ± 0.00 | 0.09 ± 0.03 | 1.35 ± 0.06 | 7.99 ± 0.02 | 90.58 ± 0.11 |
| BW-SPH-E | 42.97 ± 3.30 a | 1.49 ± 0.02 | 3.31 ± 0.02 | 9.85 ± 0.05 | 15.62 ± 0.06 | 69.74 ± 0.13 |
| BW-SPH-E_GI | 65.23 ± 1.05 A* | 0.00 ± 0.00 | 0.19 ± 0.04 | 2.03 ± 0.05 | 8.54 ± 0.09 | 89.54 ± 0.15 |
| BW-SPH-F | 36.53 ± 2.05 b | 1.33 ± 0.02 | 3.71 ± 0.03 | 12.57 ± 0.06 | 19.45 ± 0.02 | 62.94 ± 0.11 |
| BW-SPH-F_GI | 57.90 ± 4.25 AB* | 0.00 ± 0.00 | 0.24 ± 0.04 | 2.40 ± 0.07 | 10.02 ± 0.07 | 87.34 ± 0.18 |
| Sample Code | Fish:Water | Enzyme Preparation | E:S (% (w/w)) | Temperature (°C) | Time (min) |
|---|---|---|---|---|---|
| BW-SPH-A | 2:1 | 1 and 2 | 0.169 and 0.169 | 50 | 120 |
| BW-SPH-B | 2:1 | 2 and 3 | 0.169 and 0.169 | 50 | 120 |
| BW-SPH-C | 1.7:1 | 4 and 5 | 0.005 and 0.005 | 50 | 45 |
| BW-SPH-D | 1.7:1 | 4 and 5 | 0.900 and 0.290 | 50 | 60 |
| BW-SPH-E | 2:1 | 2 and 6 | 0.169 and 0.169 | 50 | 120 |
| BW-SPH-F | 2:1 | 7 | 0.340 | 50 | 120 |
| Amino Acid | BW-SPH-A | BW-SPH-B | BW-SPH-C | BW-SPH-D | BW-SPH-E | BW-SPH-F |
|---|---|---|---|---|---|---|
| Amino acid ratio (infants (birth to 6 months)) | ||||||
| SAA | 1.05 | 0.99 | 0.99 | 1.09 | 0.95 | 0.98 |
| Trp | 0.45 * | 0.47 * | 0.36 * | 0.49 * | 0.41 * | 0.46 * |
| Thr | 1.14 | 1.12 | 1.06 | 1.09 | 1.10 | 1.11 |
| Val | 0.96 | 0.96 | 0.85 | 0.90 | 0.95 | 0.89 |
| Ile | 0.83 | 0.84 | 0.69 | 0.76 | 0.85 | 0.76 |
| Leu | 0.82 | 0.81 | 0.79 | 0.82 | 0.79 | 0.80 |
| AAA | 0.88 | 0.76 | 0.67 | 0.79 | 0.71 | 0.71 |
| Lys | 1.47 | 1.53 | 1.49 | 1.51 | 1.53 | 1.50 |
| His | 0.99 | 1.02 | 0.96 | 1.06 | 0.98 | 1.04 |
| Amino acid ratio (child (6 months to 3 years)) | ||||||
| SAA | 1.28 | 1.22 | 1.21 | 1.33 | 1.16 | 1.20 |
| Trp | 0.90 * | 0.94 * | 0.73 * | 0.99 * | 0.83 * | 0.92 * |
| Thr | 1.62 | 1.59 | 1.51 | 1.55 | 1.56 | 1.58 |
| Val | 1.23 | 1.23 | 1.08 | 1.16 | 1.21 | 1.14 |
| Ile | 1.42 | 1.44 | 1.19 | 1.31 | 1.46 | 1.31 |
| Leu | 1.19 | 1.18 | 1.14 | 1.19 | 1.15 | 1.16 |
| AAA | 1.59 | 1.38 | 1.21 | 1.42 | 1.29 | 1.28 |
| Lys | 1.77 | 1.86 | 1.80 | 1.83 | 1.85 | 1.81 |
| His | 1.04 | 1.07 | 1.00 | 1.11 | 1.03 | 1.10 |
| Amino acid ratio (older child, adolescent, adult) | ||||||
| SAA | 1.51 | 1.43 | 1.42 | 1.56 | 1.36 | 1.41 |
| Trp | 1.15 * | 1.22 * | 0.94 * | 1.27 * | 1.07 * | 1.18 * |
| Thr | 2.01 | 1.98 | 1.87 | 1.92 | 1.93 | 1.96 |
| Val | 1.32 | 1.32 | 1.16 | 1.24 | 1.30 | 1.22 |
| Ile | 1.52 | 1.54 | 1.27 | 1.40 | 1.56 | 1.39 |
| Leu | 1.29 | 1.28 | 1.24 | 1.28 | 1.25 | 1.26 |
| AAA | 2.02 | 1.75 | 1.54 | 1.81 | 1.63 | 1.62 |
| Lys | 2.11 | 2.21 | 2.14 | 2.18 | 2.19 | 2.15 |
| His | 1.30 | 1.34 | 1.25 | 1.39 | 1.28 | 1.37 |
| Sample Code | DPP-IV Inhibitory Activity IC50 (mg protein/mL) | Insulin Secretion (Fold Change Compared to Control) |
|---|---|---|
| BW-SPH-A | 2.42 ± 0.04 b | 4.47 ± 0.27 d |
| BW-SPH-A_GI | 2.49 ± 0.07 AB | 4.80 ± 0.18 BC |
| BW-SPH-B | 2.37 ± 0.05 b | 6.00 ± 0.25 ab |
| BW-SPH-B_GI | 2.54 ± 0.05 AB* | 5.38 ± 0.19 A |
| BW-SPH-C | 2.90 ± 0.07 c | 4.66 ± 0.53 cd |
| BW-SPH-C_GI | 2.80 ± 0.04 C | 4.96 ± 0.21 AB |
| BW-SPH-D | 2.12 ± 0.03 a | 6.31 ± 0.21 ab |
| BW-SPH-D_GI | 2.39 ± 0.07 A* | 4.48 ± 0.14 C* |
| BW-SPH-E | 2.46 ± 0.03 b | 5.49 ± 0.20 bc |
| BW-SPH-E_GI | 2.63 ± 0.08 B* | 4.86 ± 0.10 BC |
| BW-SPH-F | 2.41 ± 0.03 b | 6.42 ± 0.30 a |
| BW-SPH-F_GI | 2.56 ± 0.07 AB* | 4.68 ± 0.13 BC* |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harnedy-Rothwell, P.A.; Khatib, N.; Sharkey, S.; Lafferty, R.A.; Gite, S.; Whooley, J.; O’Harte, F.P.; FitzGerald, R.J. Physicochemical, Nutritional and In Vitro Antidiabetic Characterisation of Blue Whiting (Micromesistiuspoutassou) Protein Hydrolysates. Mar. Drugs 2021, 19, 383. https://doi.org/10.3390/md19070383
Harnedy-Rothwell PA, Khatib N, Sharkey S, Lafferty RA, Gite S, Whooley J, O’Harte FP, FitzGerald RJ. Physicochemical, Nutritional and In Vitro Antidiabetic Characterisation of Blue Whiting (Micromesistiuspoutassou) Protein Hydrolysates. Marine Drugs. 2021; 19(7):383. https://doi.org/10.3390/md19070383
Chicago/Turabian StyleHarnedy-Rothwell, Pádraigín A, Neda Khatib, Shaun Sharkey, Ryan A Lafferty, Snehal Gite, Jason Whooley, Finbarr PM O’Harte, and Richard J FitzGerald. 2021. "Physicochemical, Nutritional and In Vitro Antidiabetic Characterisation of Blue Whiting (Micromesistiuspoutassou) Protein Hydrolysates" Marine Drugs 19, no. 7: 383. https://doi.org/10.3390/md19070383
APA StyleHarnedy-Rothwell, P. A., Khatib, N., Sharkey, S., Lafferty, R. A., Gite, S., Whooley, J., O’Harte, F. P., & FitzGerald, R. J. (2021). Physicochemical, Nutritional and In Vitro Antidiabetic Characterisation of Blue Whiting (Micromesistiuspoutassou) Protein Hydrolysates. Marine Drugs, 19(7), 383. https://doi.org/10.3390/md19070383