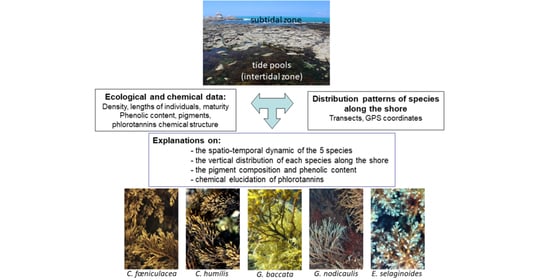
Phlorotannin and Pigment Content of Native Canopy-Forming Sargassaceae Species Living in Intertidal Rockpools in Brittany (France): Any Relationship with Their Vertical Distribution and Phenology?
Abstract
:1. Introduction
2. Results
2.1. Distribution of the Five Species along the Intertidal Rockyshore
2.2. Phenological Variables
2.3. Pigment Composition and Inter-Species Variability
2.4. Inter-Species and Inter-Seasonal Variabilities of Phenolic Contents
2.5. Purification of Phlorotannins and NMR Analyses
3. Discussion
3.1. Photo-Adaptation along the Shore but No Photoprotective Pigments in the Upper Shore
3.2. What Are the Drivers of the Variability of Phenolic Content in the Five Species?
3.3. Do the Five Species Produced the Same Phlorotannins?
3.4. Do Chemical Characteristics Could Help to Understand the Distribution of the Five Species along Brittany Rockyshores?
4. Materials and Methods
4.1. Native Canopy-Forming Sargassaceae Species and Sampled Rockpools
4.2. Extraction and Analysis of Pigments
4.3. Extraction of Phlorotannins and Determination of Phenolic Content
4.4. NMR Analyses of Phlorotannins
4.5. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Underwood, A.J. Structure of a rocky intertidal community in New South Wales: Patterns of vertical distribution and seasonal changes. J. Exp. Mar. Biol. Ecol. 1981, 51, 57–85. [Google Scholar] [CrossRef]
- Underwood, A.J.; Skilleter, G.A. Effects of patch-size on the structure of assemblages in rock pools. J. Exp. Mar. Biol. Ecol. 1996, 197, 63–90. [Google Scholar] [CrossRef]
- Daniel, M.J.; Boyden, C.R. Diurnal variations in physico-chemical conditions within intertidal rockpools. Field Stud. 1975, 4, 161–176. [Google Scholar]
- Morris, S.; Taylor, A.C. Diurnal and seasonal variation in physico-chemical conditions within intertidal rock pools. Estuar. Coast. Shelf Sci. 1983, 17, 339–355. [Google Scholar] [CrossRef]
- Huggett, J.; Griffiths, C.L. Some relationships between elevation, physico-chemical variables and biota of intertidal rock pools. Mar. Ecol. Prog. Ser. 1986, 29, 189–197. [Google Scholar] [CrossRef]
- Noël, L.M.-L.J.; Griffin, J.N.; Thompson, R.C.; Hawkins, S.J.; Burrows, M.T.; Crowe, T.P.; Jenkins, S.R. Assessment of a field incubation method estimating primary productivity in rockpool communities. Estuar. Coast. Shelf Sci. 2010, 88, 153–159. [Google Scholar] [CrossRef]
- Legrand, E.; Riera, P.; Pouliquen, L.; Bohner, O.; Cariou, T.; Martin, S. Ecological characterization of intertidal rockpools: Seasonal and diurnal monitoring of physico-chemical parameters. Reg. Stud. Mar. Sci. 2018, 17, 1–10. [Google Scholar] [CrossRef]
- Kooistra, W.H.C.F.; Joosten, A.M.T.; van den Hoek, C. Zonation patterns in intertidal pools and their possible causes: A multivariate approach. Bot. Mar. 1989, 32, 9–26. [Google Scholar] [CrossRef]
- Araújo, R.; Sousa-Pinto, I.; Bárbara, I.; Quintino, V. Macroalgal communities of intertidal rock pools in the northwest coast of Portugal. Acta Oecol. 2006, 30, 192–202. [Google Scholar] [CrossRef]
- Wallenstein, F.M.; Peres, S.D.; Xavier, E.D.; Neto, A.I. Phytobenthic communities of intertidal rock pools in the eastern islands of Azores and their relation to position on shore and pool morphology. Arquipélago—Life Mar. Sci. 2010, 27, 9–20. [Google Scholar]
- Dethier, M.N. Disturbance and recovery in intertidal pools: Maintenance of mosaic patterns. Ecol. Monogr. 1984, 54, 99–118. [Google Scholar] [CrossRef]
- Astles, K.L. Patterns of abundance and distribution of species in intertidal rock pools. J. Mar. Biol. Assoc. U. K. 1993, 73, 555–569. [Google Scholar] [CrossRef]
- Cabioc’h, J.; Floc’h, J.Y.; Le Toquin, A.; Boudouresque, C.F.; Meinesz, A.; Verlaque, M. Guide des Algues des Mers d’Europe; Delachaux et Niestlé: Paris, France, 2006. [Google Scholar]
- Connan, S.; Goulard, F.; Stiger, V.; Deslandes, E.; Ar Gall, E. Interspecific and temporal variation in phlorotannin levels in an assemblage of brown algae. Bot. Mar. 2004, 47, 410–416. [Google Scholar] [CrossRef]
- Dizerbo, A.; Herpe, E. Liste et Répartition des Algues Marines des Côtes Françaises de la Manche et de L’atlantique, Iles Anglo-Normandes Incluses; Éditions Scientifiques Anaximandre: Landerneau, France, 2007. [Google Scholar]
- Legrand, E.; Riera, P.; Bohner, O.; Coudret, J.; Schlicklin, F.; Derrien, M.; Martin, S. Impact of ocean acidification and warming on the productivity of a rock pool community. Mar. Environ. Res. 2018, 136, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Plouguerné, E.; Le Lann, K.; Connan, S.; Jechoux, G.; Deslandes, E.; Stiger-Pouvreau, V. Spatial and seasonal variation in density, reproductive status, length and phenolic content of the invasive brown macroalga Sargassum muticum (Yendo) Fensholt along the coast of Western Brittany (France). Aquat. Bot. 2006, 85, 337–344. [Google Scholar] [CrossRef]
- Billard, E.; Serrão, E.; Pearson, G.; Destombe, C.; Valero, M. Fucus vesiculosus and spiralis species complex: A nested model of local adaptation at the shore level. Mar. Ecol. Prog. Ser. 2010, 405, 163–174. [Google Scholar] [CrossRef] [Green Version]
- Le Lann, K.; Connan, S.; Stiger-Pouvreau, V. Phenology, TPC and size-fractioning phenolics variability in temperate Sargassaceae (Phaeophyceae, Fucales) from Western Brittany: Native versus introduced species. Mar. Environ. Res. 2012, 80, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Jégou, C. Étude du Genre Cystoseira des Côtes Bretonnes: Taxinomie, Ecologie et Caractérisation de Substances Naturelles. Doctoral Dissertation, Université de Bretagne Occidentale, Brest, France, 2011. [Google Scholar]
- Pardi, G.; Piazzi, L.; Cinelli, F. Demographic study of a Cystoseira humilis Kützing (Fucales: Cystoseiraceae) population in the Western Mediterranean. Bot. Mar. 2000, 43, 81–86. [Google Scholar] [CrossRef]
- Jódar-Pérez, A.B.; Terradas-Fernández, M.; López-Moya, F.; Asensio-Berbegal, L.; López-Llorca, L.V. Multidisciplinary Analysis of Cystoseira sensu lato (SE Spain) Suggest a Complex Colonization of the Mediterranean. J. Mar. Sci. Eng. 2020, 8, 961. [Google Scholar] [CrossRef]
- Karsten, U. Defense Strategies of Algae and Cyanobacteria against Solar UVR. In Algal Chemical Ecology; Amsler, C.D., Ed.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 273–296. [Google Scholar]
- Goss, R.; Jakob, T. Regulation and function of xanthophyll cycle-dependent photoprotection in algae. Photosynth. Res. 2010, 106, 103–122. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Adams, W.W., III. The role of xanthophyll cycle carotenoids in the protection of photosynthesis. Trends Plant Sci. 1996, 1, 21–26. [Google Scholar] [CrossRef]
- Heo, S.-J.; Jeon, Y.-J. Protective effect of fucoxanthin isolated from Sargassum siliquastrum on UV-B induced cell damage. J. Photochem. Photobiol. B 2009, 95, 101–107. [Google Scholar] [CrossRef]
- Burton, G.; Ingold, K. beta-Carotene: An unusual type of lipid antioxidant. Science 1984, 224, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Gévaert, F.; Créach, A.; Davoult, D.; Holl, A.-C.; Seuront, L.; Lemoine, Y. Photo-inhibition and seasonal photosynthetic performance of the seaweed Laminaria saccharina during a simulated tidal cycle: Chlorophyll fluorescence measurements and pigment analysis. Plant Cell Environ. 2002, 25, 859–872. [Google Scholar] [CrossRef] [Green Version]
- Gévaert, F.; Créach, A.; Davoult, D.; Migné, A.; Levavasseur, G.; Arzel, P.; Holl, A.-C.; Lemoine, Y. Laminaria saccharina photosynthesis measured in situ: Photoinhibition and xanthophyll cycle during a tidal cycle. Mar. Ecol. Prog. Ser. 2003, 247, 43–50. [Google Scholar] [CrossRef] [Green Version]
- Harker, M.; Berkaloff, C.; Lemoine, Y.; Britton, G.; Young, A.J.; Duval, J.-C.; Rmiki, N.-E.; Rousseau, B. Effects of high light and desiccation on the operation of the xanthophyll cycle in two marine brown algae. Eur. J. Phycol. 1999, 34, 35–42. [Google Scholar] [CrossRef]
- Uhrmacher, S.; Hanelt, D.; Nultsch, W. Zeaxanthin content and the degree of photoinhibition are linearly correlated in the brown alga Dictyota dichotoma. Mar. Biol. 1995, 123, 159–165. [Google Scholar] [CrossRef]
- Amsler, C.D.; Fairhead, V.A. Defensive and Sensory Chemical Ecology of Brown Algae. Adv. Bot. Res. 2006, 43, 1–91. [Google Scholar]
- Schoenwaelder, M.E. The occurrence and cellular significance of physodes in brown algae. Phycologia 2002, 41, 125–139. [Google Scholar] [CrossRef]
- Schoenwaelder, M.E. The biology of phenolic containing vesicles. Algae 2008, 23, 163–175. [Google Scholar] [CrossRef]
- Arnold, T.M.; Targett, N.M. Marine tannins: The importance of a mechanistic framework for predicting ecological roles. J. Chem. Ecol. 2002, 28, 1919–1934. [Google Scholar] [CrossRef] [PubMed]
- Meslet-Cladière, L.; Delage, L.; Leroux, C.J.J.; Goulitquer, S.; Leblanc, C.; Creis, E.; Ar Gall, E.; Stiger-Pouvreau, V.; Czjzek, M.; Potin, P. Structure/function analysis of a type III polyketide synthase in the brown alga Ectocarpus siliculosus reveals a biochemical pathway in phlorotannin monomer biosynthesis. Plant Cell 2013, 25, 3089–3103. [Google Scholar] [CrossRef] [Green Version]
- Arnold, T.M.; Targett, N.M. Quantifying in situ rates of phlorotannin synthesis and polymerization in marine brown algae. J. Chem. Ecol. 1998, 24, 577–595. [Google Scholar] [CrossRef]
- Ragan, M.; Glombitza, K.-W. Phlorotannins, brown algal polyphenols. Prog. Phycol. Res. 1986, 4, 129–241. [Google Scholar]
- Li, S.M.; Glombitza, K.W. Carmalols and phlorethofuhalols from the brown alga Carpophyllum maschalocarpum. Phytochemistry 1991, 30, 3417–3421. [Google Scholar] [CrossRef]
- Li, Y.; Qian, Z.-J.; Ryu, B.; Lee, S.-H.; Kim, M.-M.; Kim, S.-K. Chemical components and its antioxidant properties in vitro: An edible marine brown alga, Ecklonia cava. Bioorgan. Med. Chem. 2009, 17, 1963–1973. [Google Scholar] [CrossRef]
- Stiger-Pouvreau, V.; Jegou, C.; Cerantola, S.; Guérard, F.; Le Lann, K. Phlorotannins in Sargassaceae species from Brittany (France): Interesting molecules for ecophysiological and valorisation purposes. Adv. Bot. Res. 2014, 71, 379–411. [Google Scholar]
- Koch, M.; Gregson, R.P. Brominated phlorethols and nonhalogenated phlorotannins from the brown alga Cystophora congesta. Phytochemistry 1984, 23, 2633–2637. [Google Scholar] [CrossRef]
- Glombitza, K.W.; Knöss, W. Sulphated phlorotannins from the brown alga Pleurophycus gardneri. Phytochemistry 1992, 31, 279–281. [Google Scholar] [CrossRef]
- Le Lann, K.; Surget, G.; Couteau, C.; Coiffard, L.; Cérantola, S.; Gaillard, F.; Larnicol, M.; Zubia, M.; Guérard, F.; Poupart, N.; et al. Sunscreen, antioxidant, and bactericide capacities of phlorotannins from the brown macroalga Halidrys siliquosa. J. Appl. Phycol. 2016, 28, 3547–3559. [Google Scholar] [CrossRef] [Green Version]
- Lalegerie, F.; Gager, L.; Stiger-Pouvreau, V.; Connan, S. The stressful life of red and brown seaweeds on the temperate intertidal zone: Effect of abiotic and biotic parameters on the physiology of macroalgae and content variability of particular metabolites. Adv. Bot. Res. 2020, 95, 247–287. [Google Scholar]
- Gager, L.; Lalegerie, F.; Connan, S.; Stiger-Pouvreau, V. Marine Algal Derived Phenolic Compounds and their Biological Activities for Medicinal and Cosmetic Applications. In Recent Advances in Micro and Macroalgal Processing: Food and Health Perspectives; Rajauria, G., Yuan, Y.V., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2021; pp. 278–334. [Google Scholar]
- Alberte, R.S.; Friedman, A.L.; Gustafson, D.L.; Rudnick, M.S.; Lyman, H. Light-harvesting systems of brown algae and diatoms. Isolation and characterization of chlorophyll a/c and chlorophyll a/fucoxanthin pigment-protein complexes. BBA-Bioenergetics 1981, 635, 304–316. [Google Scholar] [CrossRef]
- De Martino, A.; Douady, D.; Rousseau, B.; Duval, J.-C.; Caron, L. Characterization of two light-harvesting subunits isolated from the brown alga Pelvetia canaliculata: Heterogeneity of xanthophyll distribution. Photochem. Photobiol. 1997, 66, 190–197. [Google Scholar] [CrossRef]
- De Martino, A.; Douady, D.; Quinet-Szely, M.; Rousseau, B.; Crépineau, F.; Apt, K.; Caron, L. The light-harvesting antenna of brown algae. Eur. J. Biochem. 2000, 267, 5540–5549. [Google Scholar] [CrossRef]
- Stengel, D.; Dring, M. Seasonal variation in the pigment content and photosynthesis of different thallus regions of Ascophyllum nodosum (Fucales, Phaeophyta) in relation to position in the canopy. Phycologia 1998, 37, 259–268. [Google Scholar] [CrossRef]
- Aguilera, J.; Bischof, K.; Karsten, U.; Hanelt, D.; Wiencke, C. Seasonal variation in ecophysiological patterns in macroalgae from an Arctic fjord. II. Pigment accumulation and biochemical defence systems against high light stress. Mar. Biol. 2002, 140, 1087–1095. [Google Scholar]
- Ramus, J.; Lemons, F.; Zimmerman, C. Adaptation of light-harvesting pigments to downwelling light and the consequent photosynthetic performance of the eulittoral rockweeds Ascophyllum nodosum and Fucus vesiculosus. Mar. Biol. 1977, 42, 293–303. [Google Scholar] [CrossRef]
- Döhler, G.; Hagmeier, E.; David, C. Effects of solar and artificial UV irradiation on pigments and assimilation of 15N ammonium and 15N nitrate by macroalgae. J. Photochem. Photobiol. B 1995, 30, 179–187. [Google Scholar] [CrossRef]
- Gager, L.; Connan, S.; Molla, M.; Couteau, C.; Arbona, J.-F.; Coiffard, L.; Cérantola, S.; Stiger-Pouvreau, V. Temporal variation of active phlorotannins determined by 1H NMR and in vitro assays. J. Appl. Phycol. 2020, 32, 2375–2386. [Google Scholar] [CrossRef]
- Ar Gall, E.; Lelchat, F.; Hupel, M.; Jégou, C.; Stiger-Pouvreau, V. Extraction and purification of phlorotannins from brown algae. In Natural Products from Marine Algae; Stengel, D.B., Connan, S., Eds.; Humana Press: New York, NY, USA, 2015; pp. 131–143. [Google Scholar]
- Surget, G.; Stiger-Pouvreau, V.; Le Lann, K.; Kervarec, N.; Couteau, C.; Coiffard, L.J.; Gaillard, F.; Cahier, K.; Guérard, F.; Poupart, N. Structural elucidation, in vitro antioxidant and photoprotective capacities of a purified polyphenolic-enriched fraction from a saltmarsh plant. J. Photochem. Photobiol. B 2015, 143, 52–60. [Google Scholar] [CrossRef]
- Surget, G.; Roberto, V.P.; Le Lann, K.; Mira, S.; Guérard, F.; Laizé, V.; Poupart, N.; Cancela, M.L.; Stiger-Pouvreau, V. Marine green macroalgae: A source of natural compounds with mineralogenic and antioxidant activities. J. Appl. Phycol. 2017, 29, 575–584. [Google Scholar] [CrossRef] [Green Version]
- Pavia, H.; Brock, E. Extrinsic factors influencing phlorotannin production in the brown alga Ascophyllum nodosum. Mar. Ecol. Prog. Ser. 2000, 193, 285–294. [Google Scholar] [CrossRef]
- Pavia, H.; Cervin, G.; Lindgren, A.; Åberg, P. Effects of UV-B radiation and simulated herbivory on phlorotannins in the brown alga Ascophyllum nodosum. Mar. Ecol. Prog. Ser. 1997, 157, 139–146. [Google Scholar] [CrossRef]
- Swanson, A.K.; Druehl, L.D. Induction, exudation and the UV protective role of kelp phlorotannins. Aquat. Bot. 2002, 73, 241–253. [Google Scholar] [CrossRef]
- Jennings, J.G.; Steinberg, P.D. In situ exudation of phlorotannins by the sublittoral kelp Ecklonia radiata. Mar. Biol. 1994, 121, 349–354. [Google Scholar] [CrossRef]
- Abdala-Díaz, R.T.; Cabello-Pasini, A.; Pérez-Rodríguez, E.; Álvarez, R.C.; Figueroa, F.L. Daily and seasonal variations of optimum quantum yield and phenolic compounds in Cystoseira tamariscifolia (Phaeophyta). Mar. Biol. 2006, 148, 459–465. [Google Scholar] [CrossRef]
- Cerantola, S.; Breton, F.; Ar Gall, E.; Deslandes, E. Co-occurrence and antioxidant activities of fucol and fucophlorethol classes of polymeric phenols in Fucus spiralis. Bot. Mar. 2006, 49, 347–351. [Google Scholar] [CrossRef]
- Glombitza, K.W.; Schnabel, C.; Koch, M. Antibiotica aus Algen, 27. Mitt. Niedermolekulare Phlorotannine der Braunalge Cystoseira baccata (Gmelin) Silva, Teil II. Arch. Pharm. 1981, 314, 602–608. [Google Scholar] [CrossRef]
- Glombitza, K.W.; Wegner-Hambloch, S.; Schulten, H.R. Antibiotics from algae, XXXVI. 1, 2 Phlorotannins from the brown alga Cystoseira granulata. Planta Med. 1985, 51, 116–120. [Google Scholar] [CrossRef]
- Glombitza, K.W.; Rösener, H.U.; Müller, D. Bifuhalol und diphlorethol aus Cystoseira tamariscifolia. Phytochemistry 1975, 14, 1115–1116. [Google Scholar] [CrossRef]
- Glombitza, K.W.; Rösener, H.U. Bifuhalol: Ein Diphenyläther aus Bifurcaria bifurcata. Phytochemistry 1974, 13, 1245–1247. [Google Scholar] [CrossRef]
- Jégou, C.; Kervarec, N.; Cérantola, S.; Bihannic, I.; Stiger-Pouvreau, V. NMR use to quantify phlorotannins: The case of Cystoseira tamariscifolia, a phloroglucinol-producing brown macroalga in Brittany (France). Talanta 2015, 135, 1–6. [Google Scholar] [CrossRef]
- Ferreres, F.; Lopes, G.; Gil-Izquierdo, A.; Andrade, P.B.; Sousa, C.; Mouga, T.; Valentão, P. Phlorotannin extracts from Fucales characterized by HPLC-DAD-ESI-MSn: Approaches to hyaluronidase inhibitory capacity and antioxidant properties. Mar. Drugs 2012, 10, 2766–2781. [Google Scholar] [CrossRef] [Green Version]
- de Sousa, C.B.; Gangadhar, K.N.; Macridachis, J.; Pavao, M.; Morais, T.R.; Campino, L.; Varela, J.; Lago, J.H.G. Cystoseira algae (Fucaceae): Update on their chemical entities and biological activities. Tetrahedron-Asymmetry 2017, 28, 1486–1505. [Google Scholar] [CrossRef]
- Kemp, J. Effects of temperature and salinity on resting metabolism in two South African rock pool Fish: The resident gobiid Caffrogobius caffer and the transient sparid Diplodus Sargus Capensis. Afr. Zool. 2009, 44, 151–158. [Google Scholar] [CrossRef]
- Sauvageau, C. A propos des Cystoseira de Banyuls et de Guéthary. Bull. Stn. Biol. Arcachon 1912, 14, 133–556. [Google Scholar]
- Roberts, M. Studies on marine algae of the British Isles. 6. Cystoseira foeniculacea (Linnaeus) Greville. Eur. J. Phycol. 1968, 3, 547–564. [Google Scholar]
- Roberts, M. Studies on marine algae of the British Isles. 8. Cystoseira tamariscifolia (Hudson) Papenfuss. Eur. J. Phycol. 1970, 5, 201–210. [Google Scholar] [CrossRef]
- Roberts, M. Studies on marine algae of the British Isles. 9. Cystoseira nodicaulis (Withering) M. Roberts. Eur. J. Phycol. 1977, 12, 175–199. [Google Scholar]
- Engelen, A.W.; Espirito-Santo, C.; Simões, T.; Monteiro, C.; Serrão, E.A.; Pearson, G.A.; Santos, R.O.P. Periodicity of propagule expulsion and settlement in the competing native and invasive brown seaweeds, Cystoseira humilis and Sargassum muticum (Phaeophyta). Eur. J. Phycol. 2008, 43, 275–282. [Google Scholar] [CrossRef] [Green Version]
- Le Lann, K.; Stiger-Pouvreau, V. Spatio-temporal phenologies of temperate Sargassaceae: Coexistence of invasive and native species. Phycologia 2009, 48, 74. [Google Scholar]
- Jégou, C.; Culioli, G.; Kervarec, N.; Simon, G.; Stiger-Pouvreau, V. LC/ESI-MSn and 1H HR-MAS NMR analytical methods as useful taxonomical tools within the genus Cystoseira C. Agardh (Fucales; Phaeophyceae). Talanta 2010, 83, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Underwood, A.J. Experimental ecology of rocky intertidal habitats: What are we learning? J. Exp. Mar. Biol. Ecol. 2000, 250, 51–76. [Google Scholar] [CrossRef]
- Wright, S.W.; Jeffrey, S.W.; Mantoura, R.F.C.; Llewellyn, C.A.; Bjornland, T.; Repeta, D.; Welschmeyer, N. Improved HPLC method for the analysis of chlorophylls and carotenoids in marine phytoplankton. Mar. Ecol. Prog. Ser. 1991, 77, 183–196. [Google Scholar] [CrossRef]
- Bidigare, R.; Van Heukelem, L.; Trees, C. Analysis of algal pigments by High-Performance Liquid Chromatography. In Algal Culturing Techniques; Andersen, R.A., Ed.; Academic Press: London, UK, 2005; pp. 327–345. [Google Scholar]
- Connan, S.; Delisle, F.; Deslandes, E.; Ar Gall, E. Intra-thallus phlorotannin content and antioxidant activity in Phaeophyceae of temperate waters. Bot. Mar. 2006, 49, 39–46. [Google Scholar] [CrossRef]
- Le Lann, K.; Jegou, C.; Stiger-Pouvreau, V. Effect of different conditioning treatments on total phenolic content and antioxidant activities in two Sargassacean species: Comparison of the frondose Sargassum muticum (Yendo) Fensholt and the cylindrical Bifurcaria bifurcata R. Ross. Phycol. Res. 2008, 56, 238–245. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: http://www.r-project.org (accessed on 25 June 2021).
- Siegel, S.; Castellan, N.J. Nonparametric Statistics for the Behavioral Sciences; McGraw-Hill: New York, NY, USA, 1988. [Google Scholar]




| Position of the Rockpool along the Intertidal Zone | ||||||
|---|---|---|---|---|---|---|
| Upper Position | Median Position | Lower Position | ||||
| C. humilis | C. fœniculacea | G. nodicaulis | G. baccata | E. selaginoides | ||
| Density (ind/0.25 m2) | Min. | 2 (W1) | 1.33 (W1) | 1 (W1) | 1 | 1 (Sp1/A1/W1) |
| Max. | 8.25 (Su2) | 5.33 (Su2) | 4.33 (Su1) | 2 (A1) | 1.33 (Su1) | |
| Percentage cover | Min. | 12.5% (W1) | 17.5% (Sp1) | 13.33% (W1) | 26.67% (W1) | 10% (Sp2) |
| Max. | 60% (A1) | 56.67% (A1/W1) | 50% (Su2) | 90% (Sp1) | 91.67% (Su1) | |
| Maturity | Min. | 16% (W1) | 0% (A) | 22.36% (Su1) | 100% all year round | 0% (W/Sp) |
| Max. | 100% (Sp/Su2) | 100% (Sp) | 100% (W/Sp1) | 100% (Su/A) | ||
| Mean length of individuals | Min. | 21.63 cm (Su2) | 15.68 cm (Su2) | 22.36 cm (Su1) | 53.67 cm (Su2) | 25.66 cm (W2) |
| Max. | 33.05 cm (W2) | 48.40 cm (W1) | 58.33 cm (Sp1) | 102.33 cm (Sp1) | 56.67 cm (Sp1/A1) | |
| Number of macroalgal taxa in the rockpool | 7 | 7–13 | 7–13 | 10–35 | 13–35 | |
| Mean TPC (% DW) | Type of Phlorotannins (from 2D NMR Results) | ||||
|---|---|---|---|---|---|
| Phloroglucinol | Fucol | Phlorethol | Fucophlorethol | ||
| C. humilis | 0.19 ± 0.09 | Yes | No | Yes | No |
| C. fœniculacea | 0.13 ± 0.06 | / | / | / | / |
| G. nodicaulis | 0.25 ± 0.16 | No | Yes | Yes | Yes |
| G. baccata | 0.21 ± 0.10 | Traces | Yes | Yes | Yes |
| E. selaginoides | 0.42 ± 0.21 | Yes | No | No | No |
| Examples of chemical structures of phlorotannins |  |  |  |  | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jégou, C.; Connan, S.; Bihannic, I.; Cérantola, S.; Guérard, F.; Stiger-Pouvreau, V. Phlorotannin and Pigment Content of Native Canopy-Forming Sargassaceae Species Living in Intertidal Rockpools in Brittany (France): Any Relationship with Their Vertical Distribution and Phenology? Mar. Drugs 2021, 19, 504. https://doi.org/10.3390/md19090504
Jégou C, Connan S, Bihannic I, Cérantola S, Guérard F, Stiger-Pouvreau V. Phlorotannin and Pigment Content of Native Canopy-Forming Sargassaceae Species Living in Intertidal Rockpools in Brittany (France): Any Relationship with Their Vertical Distribution and Phenology? Marine Drugs. 2021; 19(9):504. https://doi.org/10.3390/md19090504
Chicago/Turabian StyleJégou, Camille, Solène Connan, Isabelle Bihannic, Stéphane Cérantola, Fabienne Guérard, and Valérie Stiger-Pouvreau. 2021. "Phlorotannin and Pigment Content of Native Canopy-Forming Sargassaceae Species Living in Intertidal Rockpools in Brittany (France): Any Relationship with Their Vertical Distribution and Phenology?" Marine Drugs 19, no. 9: 504. https://doi.org/10.3390/md19090504
APA StyleJégou, C., Connan, S., Bihannic, I., Cérantola, S., Guérard, F., & Stiger-Pouvreau, V. (2021). Phlorotannin and Pigment Content of Native Canopy-Forming Sargassaceae Species Living in Intertidal Rockpools in Brittany (France): Any Relationship with Their Vertical Distribution and Phenology? Marine Drugs, 19(9), 504. https://doi.org/10.3390/md19090504