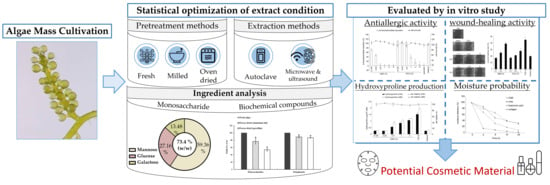
Extraction Procedure, Characteristics, and Feasibility of Caulerpa microphysa (Chlorophyta) Polysaccharide Extract as a Cosmetic Ingredient
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effects of Drying, Milling, and Extraction Procedures, and the Freeze-Drying Preservation of the Polysaccharide Yield
2.2. Monosaccharide, Bioactive Ingredient, and Molecular Composition of CME
2.3. In Vitro β-Hexosaminidase Secretion Inhibition Assay
2.4. In Vitro Wound-Healing Activity Assay
2.5. Hydroxyproline Production and In Vitro Permeation Assay
2.6. Moisture Absorption and Retention Assay
3. Materials and Methods
3.1. Cultivation Conditions
3.2. Pretreatment and Extraction
3.2.1. Extraction of Polysaccharides
3.2.2. Analysis of Total Polysaccharide Content
3.2.3. Analysis of Sugar Composition
3.2.4. Analysis of Molecular Weight
3.2.5. Analysis of Polyphenols
3.2.6. Analysis of Preservation Losses
3.3. Analysis of In Vitro Immunostimulatory Activity
3.3.1. Reagents
3.3.2. Analysis of MTT Cytotoxicity
3.3.3. Analysis of Sensitization and Stimulation for Degranulation
3.3.4. Analysis of Wound Healing
3.3.5. Analysis of Hydroxyproline
3.3.6. In Vitro Permeation Studies
3.3.7. Analysis of Moisture Absorption and Retention Capacity
3.4. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wells, M.L.; Potin, P.; Craigie, J.S.; Raven, J.A.; Merchant, S.S.; Helliwell, K.E.; Smith, A.G.; Camire, M.E.; Brawley, S.H. Algae as nutritional and functional food sources: Revisiting our understanding. J. Appl. Phycol. 2017, 29, 949–982. [Google Scholar] [CrossRef]
- De Jesus Raposo, M.F.; De Morais, A.M.B.; De Morais, R.M.S.C. Marine Polysaccharides from Algae with Potential Biomedical Applications. Mar. Drugs 2015, 13, 2967–3028. [Google Scholar] [CrossRef]
- Berthon, J.-Y.; Nachat-Kappes, R.; Bey, M.; Cadoret, J.-P.; Renimel, I.; Filaire, E. Marine algae as attractive source to skin care. Free Radic Res. 2017, 51, 555–567. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Xue, Y.T. Optimization of microwave assisted extraction, chemical characterization and antitumor activities of polysaccharides from Porphyra haitanensis. Carbohydr. Polym. 2019, 206, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Guidara, M.; Yaich, H.; Amor, I.B.; Fakhfakh, J.; Gargouri, J.; Lassoued, S.; Blecker, C.; Richel, A.; Attia, H.; Garna, H. Effect of extraction procedures on the chemical structure, antitumor and anticoagulant properties of ulvan from Ulva lactuca of Tunisia coast. Carbohydr. Polym. 2021, 253, 117283. [Google Scholar] [CrossRef]
- Pandeirada, C.O.; Maricato, É.; Ferreira, S.S.; Correia, V.G.; Pinheiro, B.A.; Evtuguin, D.V.; Palma, A.S.; Correia, A.; Vilanova, M.; Coimbra, M.A.; et al. Structural analysis and potential immunostimulatory activity of Nannochloropsis oculata polysaccharides. Carbohydr. Polym. 2019, 222, 114962. [Google Scholar] [CrossRef]
- Ansari, F.A.; Shriwastav, A.; Gupta, S.K.; Rawat, I.; Guldhe, A.; Bux, F. Lipid extracted algae as a source for protein and reduced sugar: A step closer to the biorefinery. Bioresour. Technol. 2015, 179, 559–564. [Google Scholar] [CrossRef]
- Fu, C.-C.; Hung, T.-C.; Chen, J.-Y.; Su, C.-H.; Wu, W.-T. Hydrolysis of microalgae cell walls for production of reducing sugar and lipid extraction. Bioresour. Technol. 2010, 101, 8750–8754. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.-T.; Shimamura, T.; Ishida, N.; Takahashi, H. Investigation of utilization of the algal biomass residue after oil extraction to lower the total production cost of biodiesel. J. Biosci. Bioeng. 2012, 114, 330–333. [Google Scholar] [CrossRef] [PubMed]
- Hung, W.-T.; Chen, Y.-T.; Chen, C.-H.; Lee, Y.C.; Fang, J.-M.; Yang, W.-B. Flow Chemistry System for Carbohydrate Analysis by Rapid Labeling of Saccharides after Glycan Hydrolysis. SLAS Technol. Transl. Life Sci. Innov. 2020, 25, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.B.; Hayashi, K.; Maeda, M.; Hayashi, T. Antiherpetic activities of sulfated polysaccharides from green algae. Planta Med. 2004, 70, 813–817. [Google Scholar] [CrossRef]
- Chen, Y.-T.; Hung, W.-T.; Wang, s.-h.; Fang, J.-M.; Yang, W.-B. Quantitative analysis of sugar ingredients in beverages and food crops by an effective method combining naphthimidazole derivatization and 1H-NMR spectrometry. Funct. Foods Health Dis. 2017, 7, 494. [Google Scholar] [CrossRef]
- Park, Y.K.; Rasmussen, H.E.; Ehlers, S.J.; Blobaum, K.R.; Lu, F.; Schlegal, V.L.; Carr, T.P.; Lee, J.Y. Repression of proinflammatory gene expression by lipid extract of Nostoc commune var sphaeroides Kützing, a blue-green alga, via inhibition of nuclear factor-kappaB in RAW 264.7 macrophages. Nutr. Res. 2008, 28, 83–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimiya, T.; Ohtani, K.; Satoh, S.; Abe, Y.; Ogita, Y.; Kawakita, H.; Hamada, H.; Konishi, Y.; Kubota, S.; Tominaga, A. Inhibitory effects of edible marine algae extracts on degranulation of RBL-2H3 cells and mouse eosinophils. Fish. Sci. 2009, 74, 1157–1165. [Google Scholar] [CrossRef]
- Syarina, P.N.; Karthivashan, G.; Abas, F.; Arulselvan, P.; Fakurazi, S. Wound healing potential of Spirulina platensis extracts on human dermal fibroblast cells. Excli J. 2015, 14, 385–393. [Google Scholar] [CrossRef]
- Morone, J.; Alfeus, A.; Vasconcelos, V.; Martins, R. Revealing the potential of cyanobacteria in cosmetics and cosmeceuticals—A new bioactive approach. Algal Res. 2019, 41, 101541. [Google Scholar] [CrossRef]
- Silva, T.H.; Alves, A.; Ferreira, B.M.; Oliveira, J.M.; Reys, L.L.; Ferreira, R.J.F.; Sousa, R.A.; Silva, S.S.; Mano, J.F.; Reis, R.L. Materials of marine origin: A review on polymers and ceramics of biomedical interest. Int. Mater. Rev. 2012, 57, 276–306. [Google Scholar] [CrossRef] [Green Version]
- Shi, C.-H.; Lee, T.-M. Field survey and culture studies of Caulerpa in Taiwan. Ph.D. Dissertation, National Sun Yat-sen University, Kaohsiung City, Taiwan, 2008. [Google Scholar]
- Lin, H.-C.; Chou, S.; Chuang, M.-Y.; Liao, T.-Y.; Tsai, W.-S.; Chiu, T.-H. The effects of Caulerpa microphysa enzyme-digested extracts on ACE-inhibitory activity and in vitro anti-tumour properties. Food Chem. 2012, 134, 2235–2241. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.S.S.P.; Costa, L.S.; Cordeiro, S.L.; Almeida-Lima, J.; Dantas-Santos, N.; Magalhães, K.D.; Sabry, D.A.; Albuquerque, I.R.L.; Pereira, M.R.; Leite, E.L.; et al. Evaluating the possible anticoagulant and antioxidant effects of sulfated polysaccharides from the tropical green alga Caulerpa cupressoides var. flabellata. J. Appl. Phycol. 2012, 24, 1159–1167. [Google Scholar] [CrossRef]
- Barbier, P.; Guise, S.; Huitorel, P.; Amade, P.; Pesando, D.; Briand, C.; Peyrot, V. Caulerpenyne from Caulerpa taxifolia has an antiproliferative activity on tumor cell line SK-N-SH and modifies the microtubule network. Life Sci. 2001, 70, 415–429. [Google Scholar] [CrossRef]
- Cavas, L.; Basbinar, Y.; Yurdakoc, K.; Olgun, N. Antiproliferative and newly attributed apoptotic activities from an invasive marine alga: Caulerpa racemosa var. cylindracea. J. Exp. Mar. Biol. Ecol. 2006, 339, 111–119. [Google Scholar] [CrossRef]
- Guldhe, A.; Singh, B.; Rawat, I.; Ramluckan, K.; Bux, F. Efficacy of drying and cell disruption techniques on lipid recovery from microalgae for biodiesel production. Fuel 2014, 128, 46–52. [Google Scholar] [CrossRef]
- Prabakaran, P.; Ravindran, A.D. A comparative study on effective cell disruption methods for lipid extraction from microalgae. Lett. Appl. Microbiol. 2011, 53, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.-Y.; Huang, X.; Cheong, K.-L. Recent Advances in Marine Algae Polysaccharides: Isolation, Structure, and Activities. Mar. Drugs 2017, 15, 388. [Google Scholar] [CrossRef] [Green Version]
- Sasaki, M.; Takagi, A.; Sasaki, D.; Nakamura, A.; Asayama, M. Characteristics and function of an extracellular polysaccharide from a green alga Parachlorella. Carbohydr. Polym. 2021, 254, 117252. [Google Scholar] [CrossRef]
- He, X.; Yamauchi, A.; Nakano, T.; Yamaguchi, T.; Ochiai, Y. The composition and anti-inflammatory effect of polysaccharides from the red alga Chondrus verrucosus. Fish. Sci. 2019, 85, 859–865. [Google Scholar] [CrossRef]
- Guo, M.; Li, Z.; Huang, Y.; Shi, M. Polysaccharides from Nostoc commune Vaucher activate macrophages via NF-κB and AKT/JNK1/2 pathways to suppress colorectal cancer growth in vivo. Food Funct. 2019, 10, 4269–4279. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Shao, H.; Zhang, C.; Hong, P.; Xiong, H. Separation of the polysaccharides in Caulerpa racemosa and their chemical composition and antitumor activity. J. Appl. Polym. Sci. 2008, 110, 1435–1440. [Google Scholar] [CrossRef]
- Chattopadhyay, K.; Adhikari, U.; Lerouge, P.; Ray, B. Polysaccharides from Caulerpa racemosa: Purification and structural features. Carbohydr. Polym. 2007, 68, 407–415. [Google Scholar] [CrossRef]
- Ciancia, M.; Fernández, P.V.; Leliaert, F. Diversity of Sulfated Polysaccharides From Cell Walls of Coenocytic Green Algae and Their Structural Relationships in View of Green Algal Evolution. Front. Plant Sci. 2020, 11, 554585. [Google Scholar] [CrossRef]
- Meltzer, E.O.; Grant, J.A. Impact of cetirizine on the burden of allergic rhinitis. Ann. Allergy Asthma Immunol. 1999, 83, 455–463. [Google Scholar] [CrossRef]
- Sicherer, S.H.; Muñoz-Furlong, A.; Sampson, H.A. Prevalence of peanut and tree nut allergy in the United States determined by means of a random digit dial telephone survey: A 5-year follow-up study. J. Allergy Clin. Immunol. 2003, 112, 1203–1207. [Google Scholar] [CrossRef]
- Ishizaka, T.J.J.o.A.; Immunology, C. Analysis of triggering events in mast cells for immunoglobulin E-mediated histamine release. J. Allergy Clin. Immunol. 1981, 67, 90–96. [Google Scholar] [CrossRef]
- Metzger, H.; Alcaraz, G.; Hohman, R.; Kinet, J.P.; Pribluda, V.; Quarto, R. The receptor with high affinity for immunoglobulin E. Annu. Rev. Immunol. 1986, 4, 419–470. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.-n.; Park, D.K.; Park, H.-J. The inhibitory activity of atractylenolide Ⅲ, a sesquiterpenoid, on IgE-mediated mast cell activation and passive cutaneous anaphylaxis (PCA). J. Ethnopharmacol. 2013, 145, 278–285. [Google Scholar] [CrossRef]
- Maruyama, H.; Tamauchi, H.; Hashimoto, M.; Nakano, T. Suppression of Th2 immune responses by mekabu fucoidan from Undaria pinnatifida sporophylls. Int. Arch. Allergy Immunol. 2005, 137, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Kurahashi, T.; Fujii, J. Roles of Antioxidative Enzymes in Wound Healing. J. Dev. Biol. 2015, 3, 57–70. [Google Scholar] [CrossRef] [Green Version]
- Kubota, K.; Okazaki, J.; Louie, O.; Kent, K.C.; Liu, B. TGF-beta stimulates collagen (I) in vascular smooth muscle cells via a short element in the proximal collagen promoter. J. Surg. Res. 2003, 109, 43–50. [Google Scholar] [CrossRef]
- Muhammad, A.A.; Pauzi, N.A.S.; Arulselvan, P.; Abas, F.; Fakurazi, S. In Vitro Wound Healing Potential and Identification of Bioactive Compounds from Moringa oleifera Lam. BioMed Res. Int. 2013, 2013, 974580. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [Green Version]
- Melrose, J. Glycosaminoglycans in Wound Healing. Bone Tissue Regen. Insights 2016, 7, BTRI.S38670. [Google Scholar] [CrossRef] [Green Version]
- Mora Huertas, A.C.; Schmelzer, C.E.H.; Hoehenwarter, W.; Heyroth, F.; Heinz, A. Molecular-level insights into aging processes of skin elastin. Biochimie 2016, 128–129, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Ender, F.; Hallmann, A.; Amon, P.; Sumper, M. Response to the Sexual Pheromone and Wounding in the Green Alga Volvox: Induction of an Extracellular Glycoprotein Consisting Almost Exclusively of Hydroxyproline. J. Biol. Chem. 1999, 274, 35023–35028. [Google Scholar] [CrossRef] [Green Version]
- Holmgaard, R.; Nielsen, J. Dermal Absorption of Pesticides: Evaluation of Variability and Prevention; Danish Environmental Protection Agency: Haraldsgade, Denmark, 2008. [Google Scholar]
- Bos, J.D.; Meinardi, M.M. The 500 Dalton rule for the skin penetration of chemical compounds and drugs. Exp. Dermatol. 2000, 9, 165–169. [Google Scholar] [CrossRef]
- Schaefer, H.; Lademann, J. The role of follicular penetration. A differential view. Skin Pharmacol. Appl. Skin Physiol. 2001, 14 (Suppl. 1), 23–27. [Google Scholar] [CrossRef]
- Wang, L.; Jayawardena, T.U.; Yang, H.-W.; Lee, H.-G.; Jeon, Y.-J. The Potential of Sulfated Polysaccharides Isolated from the Brown Seaweed Ecklonia maxima in Cosmetics: Antioxidant, Anti-melanogenesis, and Photoprotective Activities. Antioxidants 2020, 9, 724. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Su, L.; Chen, S.; Zhao, L.; Wang, H.; Ding, F.; Chen, H.; Shi, R.; Wang, Y.; Huang, Z. Physicochemical Characterization and Functional Analysis of the Polysaccharide from the Edible Microalga Nostoc sphaeroides. Molecules 2018, 23, 508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Chi, Z.; Yu, L.; Jiang, F.; Liu, C. Sulfated modification, characterization, and antioxidant and moisture absorption/retention activities of a soluble neutral polysaccharide from Enteromorpha prolifera. Int. J. Biol. Macromol. 2017, 105, 1544–1553. [Google Scholar] [CrossRef]
- Shao, P.; Chen, X.; Sun, P. Improvement of antioxidant and moisture-preserving activities of Sargassum horneri polysaccharide enzymatic hydrolyzates. Int. J. Biol. Macromol. 2015, 74, 420–427. [Google Scholar] [CrossRef]
- Wang, J.; Jin, W.; Hou, Y.; Niu, X.; Zhang, H.; Zhang, Q. Chemical composition and moisture-absorption/retention ability of polysaccharides extracted from five algae. Int. J. Biol. Macromol. 2013, 57, 26–29. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Lin, C.; Hung, W.-T.; Kuo, C.-Y.; Liao, K.-S.; Liu, Y.-C.; Yang, W.-B. I2-Catalyzed Oxidative Condensation of Aldoses with Diamines: Synthesis of Aldo-Naphthimidazoles for Carbohydrate Analysis. Molecules 2010, 15, 1340–1353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuda, T.; Tsunekawa, M.; Goto, H.; Araki, Y. Antioxidant properties of four edible algae harvested in the Noto Peninsula. J. Food Compos. Anal. 2005, 18, 625–633. [Google Scholar] [CrossRef]
- Mlcek, J.; Jurikova, T.; Skrovankova, S.; Sochor, J. Quercetin and Its Anti-Allergic Immune Response. Molecules 2016, 21, 623. [Google Scholar] [CrossRef] [PubMed]
- Quah, Y.; Lee, S.-J.; Lee, E.-B.; Birhanu, B.T.; Ali, M.S.; Abbas, M.A.; Boby, N.; Im, Z.-E.; Park, S.-C. Cornus officinalis Ethanolic Extract with Potential Anti-Allergic, Anti-Inflammatory, and Antioxidant Activities. Nutrients. 2020, 12, 3317. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Xie, W.; Zhao, Y.; Lv, X.; Yang, H.; Zeng, Q.; Zheng, Z.; Yang, X. Synthesis, Antimicrobial, Moisture Absorption and Retention Activities of Kojic Acid-Grafted Konjac Glucomannan Oligosaccharides. Polymers 2019, 11, 1979. [Google Scholar] [CrossRef] [Green Version]









| Total Saccharides % (w/w) | Monosaccharide Composition mol% | ||
|---|---|---|---|
| Mannose | Glucose | Galactose | |
| 73.4 | 59.36 | 27.16 | 13.48 |
| Polysaccharides (mg g−1) | Polyphenols (mg g−1) |
|---|---|
| 1457.17 ± 48.25 | 16.62 ± 5.39 |
| Species | Analysis Systems | Monosaccharides Composition | References |
|---|---|---|---|
| Caulerpa microphysa | HCl hydrolysis, Sugar-NAIM* derivatization, 1H NMR* and HPLC*-UV analysis | Total sugar (w/w): 73.4% Mannose 59.36%, Glucose 27.16%, Galactose 13.48% | This study |
| C. brachypus | H2SO4 hydrolysis, GLC* analysis | Rhamnose, Xylose, Glucose | Lee et al., 2004 [11] |
| C. racemosa | HCl hydrolysis, GC* analysis | Total sugar (w/w): 36–53.7% Uronic acid (w/w):3.9–7.9% Neutral sugar (Glucose 56.8%, Galactose 31.8%, Mannose 11.4%) | Ji et al., 2008 [29] |
| C. cupressoides | HCl hydrolysis, HPLC-RI* analysis | Total sugar (w/w): 52.38–59.60% Galactose, Glucose, Mannose, Xylose, Rhamnose, Fucose | Costa et al., 2012 [20] |
| Relative Humidity (%) | Sample | % Moisture Absorption at Given Time Point (h) | |||
|---|---|---|---|---|---|
| 8 | 12 | 24 | 48 | ||
| 32 | CME | 0 | 0 | 2 | 6 |
| urea | 0 | 2 | 4 | 10 | |
| hyaluronic acid | 0 | 0 | 2 | 2 | |
| collagen | 0 | 0 | 0 | 0 | |
| 75 | CME | 2 | 17 | 42 | 72 |
| urea | 0 | 22 | 68 | 87 | |
| hyaluronic acid | 0 | 20 | 39 | 59 | |
| collagen | 0 | 6 | 6 | 7 | |
| 84 | CME | 9 | 18 | 60 | 78 |
| urea | 20 | 56 | 99 | 119 | |
| hyaluronic acid | 2 | 29 | 78 | 95 | |
| collagen | 2 | 3 | 9 | 19 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, M.-C.; Yeh, H.-Y.; Shih, W.-L. Extraction Procedure, Characteristics, and Feasibility of Caulerpa microphysa (Chlorophyta) Polysaccharide Extract as a Cosmetic Ingredient. Mar. Drugs 2021, 19, 524. https://doi.org/10.3390/md19090524
Lee M-C, Yeh H-Y, Shih W-L. Extraction Procedure, Characteristics, and Feasibility of Caulerpa microphysa (Chlorophyta) Polysaccharide Extract as a Cosmetic Ingredient. Marine Drugs. 2021; 19(9):524. https://doi.org/10.3390/md19090524
Chicago/Turabian StyleLee, Meng-Chou, Han-Yang Yeh, and Wen-Ling Shih. 2021. "Extraction Procedure, Characteristics, and Feasibility of Caulerpa microphysa (Chlorophyta) Polysaccharide Extract as a Cosmetic Ingredient" Marine Drugs 19, no. 9: 524. https://doi.org/10.3390/md19090524
APA StyleLee, M. -C., Yeh, H. -Y., & Shih, W. -L. (2021). Extraction Procedure, Characteristics, and Feasibility of Caulerpa microphysa (Chlorophyta) Polysaccharide Extract as a Cosmetic Ingredient. Marine Drugs, 19(9), 524. https://doi.org/10.3390/md19090524