Formulation of New Media from Dairy and Brewery Wastes for a Sustainable Production of DHA-Rich Oil by Aurantiochytrium mangrovei
Abstract
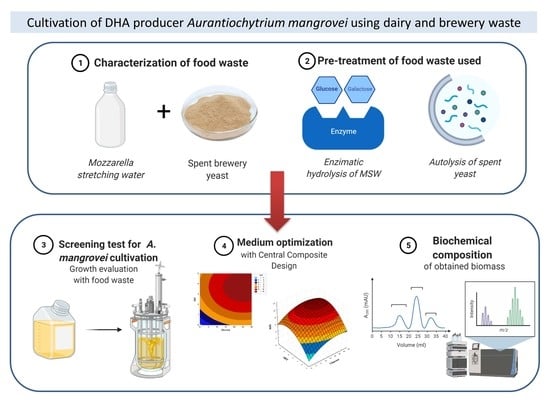
:1. Introduction
2. Results and Discussion
2.1. Characterization of MSW
2.2. Screening Tests Results
2.2.1. Evaluation of Organic Carbon Sources
2.2.2. Effect of MSW Hydrolysed and SBY on the Growth of A. mangrovei
2.3. Optimization of New MSW Hydrolysed Media
2.4. Model Confirmation and Characterization of Biomass Obtained with New MSW Optimized Medium
2.5. Economic Considerations
3. Materials and Methods
3.1. Food Waste Samples and Chemical Characterization
3.2. Organism and Cultivation
3.3. Experimental Design
Screening Tests
3.4. Hydrolysis of Dairy Wastewater
3.5. Response Surface Analysis and Formulation of Optimized Media
3.6. Analytical Methods
3.6.1. Measurement of Dry Cell Weight
3.6.2. Chemical Characterization of Food Waste
3.6.3. Lipid Extraction and Fatty Acid Methyl Esters (FAMEs)
3.6.4. Determination of Carotenoids in Microalgal Extracts by HPLC/MS Analysis
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gharami, K.; Das, M.; Das, S. Essential role of docosahexaenoic acid towards development of a smarter brain. Neurochem. Int. 2015, 89, 51–62. [Google Scholar] [CrossRef]
- Birch, E.E.; Garfield, S.; Castañeda, Y.; Hughbanks-Wheaton, D.; Uauy, R.; Hoffman, D. Visual acuity and cognitive outcomes at 4 years of age in a double-blind, randomized trial of long-chain polyunsaturated fatty acid-supplemented infant formula. Early Hum. Dev. 2007, 83, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Ambigaipalan, P. Omega-3 Polyunsaturated Fatty Acids and Their Health Benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 345–381. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhou, P.; Zhu, Y.; Xie, C.; Ma, L.; Wang, X.; Bao, Z.; Yu, L. Improvement in the docosahexaenoic acid production of Schizochytrium sp. S056 by replacement of sea salt. Bioprocess Biosyst. Eng. 2016, 39, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Graham, I.A.; Larson, T.; Napier, J.A. Rational metabolic engineering of transgenic plants for biosynthesis of omega-3 polyunsaturates. Curr. Opin. Biotechnol. 2007, 18, 142–147. [Google Scholar] [CrossRef]
- Lewis, T.E.; Nichols, P.D.; McMeekin, T.A. The Biotechnological Potential of Thraustochytrids. Mar. Biotechnol. 1999, 1, 580–587. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Song, X.; Feng, Y.; Li, W.; Cui, Q. Isolation and characterization of Aurantiochytrium species: High docosahexaenoic acid (DHA) production by the newly isolated microalga, Aurantiochytrium sp. SD116. J. Oleo Sci. 2013, 62, 143–151. [Google Scholar] [CrossRef] [Green Version]
- Humhal, T.; Kastanek, P.; Jezkova, Z.; Cadkova, A.; Kohoutkova, J.; Branyik, T. Use of saline waste water from demineralization of cheese whey for cultivation of Schizochytrium limacinum PA-968 and Japonochytrium marinum AN-4. Bioprocess Biosyst. Eng. 2017, 40, 395–402. [Google Scholar] [CrossRef]
- Ende, S.S.W.; Noke, A. Heterotrophic microalgae production on food waste and by-products. J. Appl. Phycol. 2019, 31, 1565–1571. [Google Scholar] [CrossRef]
- Russo, G.L.; Langellotti, A.L.; Blasco, T.; Oliviero, M.; Sacchi, R.; Masi, P. Production of Omega-3 Oil by Aurantiochytrium mangrovei Using Spent Osmotic Solution from Candied Fruit Industry as Sole Organic Carbon Source. Processes 2021, 9, 1834. [Google Scholar] [CrossRef]
- Ryu, B.-G.; Kim, K.; Kim, J.; Han, J.-I.; Yang, J.-W. Use of organic waste from the brewery industry for high-density cultivation of the docosahexaenoic acid-rich microalga, Aurantiochytrium sp. KRS101. Bioresour. Technol. 2013, 129, 351–359. [Google Scholar] [CrossRef]
- Castrica, M.; Ventura, V.; Panseri, S.; Ferrazzi, G.; Tedesco, D.; Balzaretti, C.M. The sustainability of urban food systems: The case of mozzarella production in the city of Milan. Sustainability 2020, 12, 682. [Google Scholar] [CrossRef] [Green Version]
- Kasmi, M. Biological Processes as Promoting Way for Both Treatment and Valorization of Dairy Industry Effluents. Waste Biomass Valorization 2018, 9, 195–209. [Google Scholar] [CrossRef]
- Zotta, T.; Solieri, L.; Iacumin, L.; Picozzi, C.; Gullo, M. Valorization of cheese whey using microbial fermentations. Appl. Microbiol. Biotechnol. 2020, 104, 2749–2764. [Google Scholar] [CrossRef]
- Yuan, X.; Liang, L.; Liu, K.; Xie, L.; Huang, L.; He, W.; Chen, Y.; Xue, T. Spent yeast as an efficient medium supplement for fucoxanthin and eicosapentaenoic acid (EPA) production by Phaeodactylum tricornutum. J. Appl. Phycol. 2020, 32, 59–69. [Google Scholar] [CrossRef]
- Pleissner, D.; Lam, W.C.; Sun, Z.; Lin, C.S.K. Food waste as nutrient source in heterotrophic microalgae cultivation. Bioresour. Technol. 2013, 137, 139–146. [Google Scholar] [CrossRef]
- Massa, M.; Buono, S.; Langellotti, A.L.; Martello, A.; Russo, G.L.; Troise, D.A.; Sacchi, R.; Vitaglione, P.; Fogliano, V. Biochemical composition and in vitro digestibility of Galdieria sulphuraria grown on spent cherry-brine liquid. New Biotechnol. 2019, 53, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Ratledge, C. Fatty acid biosynthesis in microorganisms being used for Single Cell Oil production. Biochimie 2004, 86, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Panesar, P.; Kennedy, J.; Gandhi, D.; Bunko, K. Bioutilisation of whey for lactic acid production. Food Chem. 2007, 105, 1–14. [Google Scholar] [CrossRef]
- Chokshi, K.; Pancha, I.; Ghosh, A.; Mishra, S. Microalgal biomass generation by phycoremediation of dairy industry wastewater: An integrated approach towards sustainable biofuel production. Bioresour. Technol. 2016, 221, 455–460. [Google Scholar] [CrossRef]
- Humhal, T.; Kronusová, O.; Kaštánek, P.; Potočár, T.; Kohoutková, J.; Brányik, T. Influence of nitrogen sources on growth of thraustochytrids in waste water from the demineralization of cheese whey. Czech J. Food Sci. 2019, 37, 383–390. [Google Scholar] [CrossRef]
- Gernigon, G.; Piot, M.; Beaucher, E.; Jeantet, R.; Schuck, P. Physicochemical characterization of Mozzarella cheese wheys and stretchwaters in comparison with several other sweet wheys. J. Dairy Sci. 2009, 92, 5371–5377. [Google Scholar] [CrossRef]
- Pahlavanyali, M.; Jalili, H.; Noroozi, M.; Moradi, Y.; Hallajisani, A. The effect of temperature and different carbon and nitrogen sources on the growth and fatty acid profile of a newly isolated microorganism Aurantiochytrium sp. strain SHY. Iran. J. Fish. Sci. 2020, 19, 3112–3126. [Google Scholar] [CrossRef]
- Unagul, P.; Assantachai, C.; Phadungruengluij, S.; Suphantharika, M.; Verduyn, C. Properties of the docosahexaenoic acid-producer Schizochytrium mangrovei Sk-02: Effects of glucose, temperature and salinity and their interaction. Bot. Mar. 2005, 48, 387–394. [Google Scholar] [CrossRef]
- Ju, J.-H.; Ko, D.-J.; Heo, S.-Y.; Lee, J.-J.; Kim, Y.-M.; Lee, B.-S.; Kim, M.-S.; Kim, C.-H.; Seo, J.-W.; Oh, B.-R. Regulation of lipid accumulation using nitrogen for microalgae lipid production in Schizochytrium sp. ABC101. Renew. Energy 2020, 153, 580–587. [Google Scholar] [CrossRef]
- Song, X.; Ma, Z.; Tan, Y.; Zhang, H.; Cui, Q. Wastewater recycling technology for fermentation in polyunsaturated fatty acid production. Bioresour. Technol. 2017, 235, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.K.M.; Tsui, C.K.M.; Au, D.W.T.; Vrijmoed, L.L.P. Docosahexaenoic acid production and ultrastructure of the thraustochytrid Aurantiochytrium mangrovei MP2 under high glucose concentrations. Mycoscience 2008, 49, 266–270. [Google Scholar] [CrossRef]
- Yokochi, T.; Honda, D.; Higashihara, T.; Nakahara, T. Optimization of docosahexaenoic acid production by Schizochytrium limacinum SR21. Appl. Microbiol. Biotechnol. 1998, 49, 72–76. [Google Scholar] [CrossRef]
- Yu, X.-J.; Yu, Z.-Q.; Liu, Y.-L.; Sun, J.; Zheng, J.-Y.; Wang, Z. Utilization of High-Fructose Corn Syrup for Biomass Production Containing High Levels of Docosahexaenoic Acid by a Newly Isolated Aurantiochytrium sp. YLH70. Appl. Biochem. Biotechnol. 2015, 177, 1229–1240. [Google Scholar] [CrossRef]
- Ju, J.-H.; Oh, B.-R.; Ryu, S.-K.; Heo, S.-Y.; Kim, S.-Y.; Hong, W.-K.; Kim, C.H.; Seo, J.-W. Production of Lipid Containing High Levels of Docosahexaenoic Acid by Cultivation of Aurantiochytrium sp. KRS101 Using Jerusalem Artichoke Extract. Biotechnol. Bioprocess Eng. 2018, 23, 726–732. [Google Scholar] [CrossRef]
- Wu, S.-T.; Yu, S.-T.; Lin, L.-P. Effect of culture conditions on docosahexaenoic acid production by Schizochytrium sp. S31. Process Biochem. 2005, 40, 3103–3108. [Google Scholar] [CrossRef]
- Wang, S.-K.; Wang, X.; Tian, Y.-T.; Cui, Y.-H. Nutrient recovery from tofu whey wastewater for the economical production of docosahexaenoic acid by Schizochytrium sp. S31. Sci. Total Environ. 2020, 710, 136448. [Google Scholar] [CrossRef] [PubMed]
- Park, W.-K.; Moon, M.; Shin, S.-E.; Cho, J.M.; Suh, W.I.; Chang, Y.K.; Lee, B. Economical DHA (Docosahexaenoic acid) production from Aurantiochytrium sp. KRS101 using orange peel extract and low cost nitrogen sources. Algal Res. 2018, 29, 71–79. [Google Scholar] [CrossRef]
- Patel, A.; Rova, U.; Christakopoulos, P.; Matsakas, L. Mining of squalene as a value-added byproduct from DHA producing marine thraustochytrid cultivated on food waste hydrolysate. Sci. Total Environ. 2020, 736, 139691. [Google Scholar] [CrossRef]
- Des Rosiers, C.; Labarthe, F.; Lloyd, S.G.; Chatham, J.C. Cardiac anaplerosis in health and disease: Food for thought. Cardiovasc. Res. 2011, 90, 210–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaya, K.; Kazama, Y.; Abe, T.; Shiraishi, F. Influence of medium components and pH on the production of odd-carbon fatty acids by Aurantiochytrium sp. SA-96. J. Appl. Phycol. 2020, 32, 1597–1606. [Google Scholar] [CrossRef]
- Aasen, I.M.; Ertesvåg, H.; Heggeset, T.M.B.; Liu, B.; Brautaset, T.; Vadstein, O.; Ellingsen, T.E. Thraustochytrids as production organisms for docosahexaenoic acid (DHA), squalene, and carotenoids. Appl. Microbiol. Biotechnol. 2016, 100, 4309–4321. [Google Scholar] [CrossRef]
- Galasso, C.; Corinaldesi, C.; Sansone, C. Carotenoids from Marine Organisms: Biological Functions and Industrial Applications. Antioxidants 2017, 6, 96. [Google Scholar] [CrossRef] [Green Version]
- Ye, J.; Liu, M.; He, M.; Ye, Y.; Huang, J. Illustrating and Enhancing the Biosynthesis of Astaxanthin and Docosahexaenoic Acid in Aurantiochytrium sp. SK4. Mar. Drugs 2019, 17, 45. [Google Scholar] [CrossRef] [Green Version]
- Kubo, Y.; Shiroi, M.; Higashine, T.; Mori, Y.; Morimoto, D.; Nakagawa, S.; Sawayama, S. Enhanced Production of Astaxanthin without Decrease of DHA Content in Aurantiochytrium limacinum by Overexpressing Multifunctional Carotenoid Synthase Gene. Appl. Biochem. Biotechnol. 2021, 193, 52–64. [Google Scholar] [CrossRef]
- Russo, G.L.; Langellotti, A.L.; Oliviero, M.; Sacchi, R.; Masi, P. Sustainable production of food grade omega-3 oil using aquatic protists: Reliability and future horizons. New Biotechnol. 2021, 62, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Fei, Q.; Chang, H.N.; Shang, L.; Choi, J.; Kim, N.; Kang, J. The effect of volatile fatty acids as a sole carbon source on lipid accumulation by Cryptococcus albidus for biodiesel production. Bioresour. Technol. 2011, 102, 2695–2701. [Google Scholar] [CrossRef] [PubMed]
- Maddipati, P.; Atiyeh, H.K.; Bellmer, D.D.; Huhnke, R.L. Ethanol production from syngas by Clostridium strain P11 using corn steep liquor as a nutrient replacement to yeast extract. Bioresour. Technol. 2011, 102, 6494–6501. [Google Scholar] [CrossRef] [PubMed]
- Jacob, F.F.; Striegel, L.; Rychlik, M.; Hutzler, M.; Methner, F.-J. Spent Yeast from Brewing Processes: A Biodiverse Starting Material for Yeast Extract Production. Fermentation 2019, 5, 51. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, B.C.; Prasad, L.N.; Saha, N.P. Enzymatic hydrolysis of whey and its analysis. J. Food Sci. Technol. 2017, 54, 1476–1483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, H.; Kwak, M.; Seo, J.; Ju, J.; Heo, S.; Park, S.; Hong, W. Enhanced production of carotenoids using a Thraustochytrid microalgal strain containing high levels of docosahexaenoic acid-rich oil. Bioprocess Biosyst. Eng. 2018, 41, 1355–1370. [Google Scholar] [CrossRef] [PubMed]
- Nazir, Y.; Shuib, S.; Kalil, M.S.; Song, Y.; Hamid, A.A. Optimization of Culture Conditions for Enhanced Growth, Lipid and Docosahexaenoic Acid (DHA) Production of Aurantiochytrium SW1 by Response Surface Methodology. Sci. Rep. 2018, 8, 8909. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Thiex, N. Evaluation of Analytical Methods for the Determination of Moisture, Crude Protein, Crude Fat, and Crude Fiber in Distillers Dried Grains with Solubles. J. AOAC Int. 2009, 92, 61–73. [Google Scholar] [CrossRef] [Green Version]
- ISO. NF EN ISO 17294-2 Water Quality—Application of Inductively Coupled Plasma Mass Spectrometry (ICP-MS)—Part 2: Determination of Selected Elements Including Uranium Isotopes; ISO: Geneva, Switzerland, 2016. [Google Scholar]
- Lie, S. The Ebc-Ninhydrin Method for Determination of Free Alpha Amino Nitrogen. J. Inst. Brew. 1973, 79, 37–41. [Google Scholar] [CrossRef]
- Cha, T.S.; Chen, J.W.; Goh, E.G.; Aziz, A.; Loh, S.H. Differential regulation of fatty acid biosynthesis in two Chlorella species in response to nitrate treatments and the potential of binary blending microalgae oils for biodiesel application. Bioresour. Technol. 2011, 102, 10633–10640. [Google Scholar] [CrossRef] [PubMed]
- Aued-Pimentel, S.; Lago, J.H.G.; Chaves, M.H.; Kumagai, E.E. Evaluation of a methylation procedure to determine cyclopropenoids fatty acids from Sterculia striata St. Hil. Et Nauds seed oil. J. Chromatogr. A 2004, 1054, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Conde, T.A.; Couto, D.; Melo, T.; Costa, M.; Silva, J.; Domingues, M.R.; Domingues, P. Polar lipidomic profile shows Chlorococcum amblystomatis as a promising source of value-added lipids. Sci. Rep. 2021, 11, 4355. [Google Scholar] [CrossRef]
- Castro-Puyana, M.; Herrero, M.; Urreta, I.; Mendiola, J.A.; Cifuentes, A.; Ibáñez, E.; Suárez-Alvarez, S. Optimization of clean extraction methods to isolate carotenoids from the microalga Neochloris oleoabundans and subsequent chemical characterization using liquid chromatography tandem mass spectrometry. Anal. Bioanal. Chem. 2013, 405, 4607–4616. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Parameters | Value |
|---|---|
| pH | 3.55 ± 0.2 |
| Ash (g L−1) | 26.2 ± 0.9 |
| Dry weight (g L−1) | 68.5 ± 1.1 |
| N total (g L−1) | 0.91 ± 0.09 |
| Protein content (g L−1) | 3.6 ± 0.2 |
| Lactic acid (g L−1) | 6.08 ± 0.79 |
| Citric acid (g L−1) | 1.03 ± 0.19 |
| Free amino nitrogen (mg L−1) | 0.174 ± 0.03 |
| Reducing sugars (g L−1) | 23.26 ± 0.4 |
| Lactose (g L−1) | 22.48 ± 0.7 |
| Total sugars (g L−1) | 24.12 ± 0.6 |
| COD (mg L−1) | 33506 ± 21.1 |
| Cl- (g L−1) | 14.61 ± 1 |
| Ca2+ (g L−1) | 0.69 ± 0.05 |
| Total P (mg L−1) | 87.3 ± 1.25 |
| Na2+ (g L−1) | 7.66 ± 0.8 |
| Mg2+ (mg L−1) | 95.76 ± 3.7 |
| Run | Factor Assignment | Biomass Dry Weight (Y) | ||
|---|---|---|---|---|
| X1 (Glucose) | X2 (SBY) | Experimental Value (g L−1) | Predicted Value (g L−1) | |
| 1 | −1 | 0 | 7.28 | 7.75 |
| 2 | +1 | 0 | 10.24 | 10.06 |
| 3 | +1 | +1 | 9.79 | 9.99 |
| 4 | 0 | +1 | 9.87 | 9.73 |
| 5 | 0 | 0 | 9.81 | 9.77 |
| 6 | 0 | 0 | 9.72 | 9.77 |
| 7 | +1 | −1 | 7.13 | 7.09 |
| 8 | −1 | +1 | 7.85 | 7.73 |
| 9 | 0 | 0 | 9.97 | 9.77 |
| 10 | 0 | −1 | 6.38 | 6.78 |
| 11 | 0 | 0 | 9.57 | 9.77 |
| 12 | 0 | 0 | 10.14 | 9.77 |
| 13 | −1 | −1 | 5.10 | 4.73 |
| Source | DF a | Adj SS b | Adj MS c | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 5 | 16.45 | 3.29 | 23.35 | 0.003 |
| Glucose (X1) | 1 | 4.59 | 4.59 | 32.60 | 0.005 |
| SBY (X2) | 1 | 1.27 | 1.27 | 9.07 | 0.011 |
| Linear | 2 | 5.87 | 2.93 | 20.84 | 0.001 |
| Square | 2 | 10.52 | 5.26 | 37.35 | 0.000 |
| X1 * X1 | 1 | 3.22 | 3.22 | 22.91 | 0.004 |
| X2 * X2 | 1 | 3.28 | 3.28 | 23.34 | 0.002 |
| X1 * X2 | 1 | 0.05 | 0.05 | 0.36 | 0.868 |
| Error | 7 | 0.98 | 0.14 | ||
| Lack of Fit | 3 | 0.87 | 0.29 | 9.19 | 0.129 |
| Pure Error | 4 | 0.11 | 0.02 | ||
| Total | 12 | 17.4376 |
| Parameter | Control | MSW Optimized Media |
|---|---|---|
| Biomass DW (g L−1) | 9.44 ± 0.12 | 10.07 ± 0.23 |
| Biomass productivity (g L−1 day−1) | 3.14 ± 0.06 | 3.35 ± 0.08 |
| Total lipids (%DW) | 41.1 ± 1.2 | 38.9 ± 0.88 |
| FAN consumption (%) | 80.06 | 87.24 |
| Sugar consumption (%) | 92.61 | 94.59 |
| Sample | β-Carotene | Canthaxanthin | Astaxanthin | Violaxanthin |
|---|---|---|---|---|
| Control | 0.34 ± 0.06 a | 0.62 ± 0.05 b | Trace | n.d. |
| MSW media | 2.93 ± 0.05 b | 0.29 ± 0.04 a | Trace | n.d. |
| MSW media + light | 1.85 ± 0.02 c | 0.27 ± 0.01 a | 0.28 ± 0.01 | 3.23 ± 0.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Russo, G.L.; Langellotti, A.L.; Verardo, V.; Martín-García, B.; Di Pierro, P.; Sorrentino, A.; Baselice, M.; Oliviero, M.; Sacchi, R.; Masi, P. Formulation of New Media from Dairy and Brewery Wastes for a Sustainable Production of DHA-Rich Oil by Aurantiochytrium mangrovei. Mar. Drugs 2022, 20, 39. https://doi.org/10.3390/md20010039
Russo GL, Langellotti AL, Verardo V, Martín-García B, Di Pierro P, Sorrentino A, Baselice M, Oliviero M, Sacchi R, Masi P. Formulation of New Media from Dairy and Brewery Wastes for a Sustainable Production of DHA-Rich Oil by Aurantiochytrium mangrovei. Marine Drugs. 2022; 20(1):39. https://doi.org/10.3390/md20010039
Chicago/Turabian StyleRusso, Giovanni L., Antonio L. Langellotti, Vito Verardo, Beatriz Martín-García, Prospero Di Pierro, Angela Sorrentino, Marco Baselice, Maria Oliviero, Raffaele Sacchi, and Paolo Masi. 2022. "Formulation of New Media from Dairy and Brewery Wastes for a Sustainable Production of DHA-Rich Oil by Aurantiochytrium mangrovei" Marine Drugs 20, no. 1: 39. https://doi.org/10.3390/md20010039
APA StyleRusso, G. L., Langellotti, A. L., Verardo, V., Martín-García, B., Di Pierro, P., Sorrentino, A., Baselice, M., Oliviero, M., Sacchi, R., & Masi, P. (2022). Formulation of New Media from Dairy and Brewery Wastes for a Sustainable Production of DHA-Rich Oil by Aurantiochytrium mangrovei. Marine Drugs, 20(1), 39. https://doi.org/10.3390/md20010039