Variations in the Composition, Antioxidant and Antimicrobial Activities of Cystoseira compressa during Seasonal Growth
Abstract
:1. Introduction
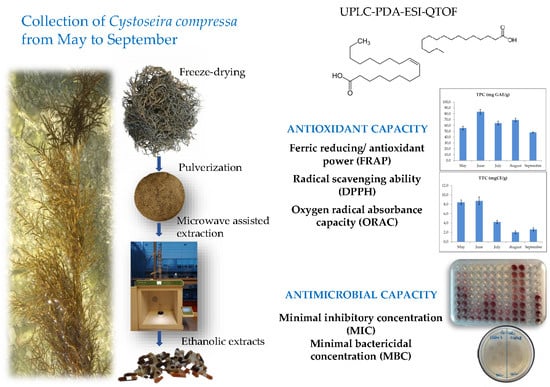
2. Results and Discussion
2.1. Total Phenolic Content, Total Tannin Content and Antioxidant Activity
2.2. Antimicrobial Activity
2.3. Chemical Analysis by UPLC-PDA-ESI-QTOF
3. Materials and Methods
3.1. Sample Collection
3.2. Pre-Treatment and Extraction
3.3. Determination of Total Phenolics, Total Tannins and Antioxidant Activity
3.4. Determination of the Antimicrobial Activity
3.5. Compound Analysis by UPLC-PDA-ESI-QTOF
3.6. Statistical Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Generalić Mekinić, I.; Skroza, D.; Šimat, V.; Hamed, I.; Čagalj, M.; Perković, Z.P. Phenolic Content of Brown Algae (Pheophyceae) Species: Extraction, Identification, and Quantification. Biomolecules 2019, 9, 244. [Google Scholar] [CrossRef] [Green Version]
- Mannino, A.M.; Micheli, C. Ecological Function of Phenolic Compounds from Mediterranean Fucoid Algae and Seagrasses: An Overview on the Genus Cystoseira Sensu Lato and Posidonia Oceanica (L.) Delile. J. Mar. Sci. Eng. 2020, 8, 19. [Google Scholar] [CrossRef] [Green Version]
- Stiger-Pouvreau, V.; Jégou, C.; Cérantola, S.; Guérard, F.; Le Lann, K. Phlorotannins in Sargassaceae Species from Brittany (France). In Advances in Botanical Research; Bourgougnon, N., Ed.; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2014; pp. 379–411. [Google Scholar] [CrossRef]
- Messina, C.; Renda, G.; Laudicella, V.; Trepos, R.; Fauchon, M.; Hellio, C.; Santulli, A. From Ecology to Biotechnology, Study of the Defense Strategies of Algae and Halophytes (from Trapani Saltworks, NW Sicily) with a Focus on Antioxidants and Antimicrobial Properties. Int. J. Mol. Sci. 2019, 20, 881. [Google Scholar] [CrossRef] [Green Version]
- Abdelhamid, A.; Jouini, M.; Bel Haj Amor, H.; Mzoughi, Z.; Dridi, M.; Ben Said, R.; Bouraoui, A. Phytochemical Analysis and Evaluation of the Antioxidant, Anti-Inflammatory, and Antinociceptive Potential of Phlorotannin-Rich Fractions from Three Mediterranean Brown Seaweeds. Mar. Biotechnol. 2018, 20, 60–74. [Google Scholar] [CrossRef] [PubMed]
- Šimat, V.; Elabed, N.; Kulawik, P.; Ceylan, Z.; Jamroz, E.; Yazgan, H.; Čagalj, M.; Regenstein, J.M.; Özogul, F. Recent Advances in Marine-Based Nutraceuticals and Their Health Benefits. Mar. Drugs 2020, 18, 627. [Google Scholar] [CrossRef] [PubMed]
- Bruno de Sousa, C.; Gangadhar, K.N.; Macridachis, J.; Pavão, M.; Morais, T.R.; Campino, L.; Varela, J.; Lago, J.H.G. Cystoseira Algae (Fucaceae): Update on Their Chemical Entities and Biological Activities. Tetrahedron Asymmetry 2017, 28, 1486–1505. [Google Scholar] [CrossRef]
- Orellana, S.; Hernández, M.; Sansón, M. Diversity of Cystoseira Sensu Lato (Fucales, Phaeophyceae) in the Eastern Atlantic and Mediterranean Based on Morphological and DNA Evidence, Including Carpodesmia Gen. Emend. and Treptacantha Gen. Emend. Eur. J. Phycol. 2019, 54, 447–465. [Google Scholar] [CrossRef]
- Kumar, S.; Sahoo, D.; Levine, I. Assessment of Nutritional Value in a Brown Seaweed Sargassum Wightii and Their Seasonal Variations. Algal Res. 2015, 9, 117–125. [Google Scholar] [CrossRef]
- Praiboon, J.; Palakas, S.; Noiraksa, T.; Miyashita, K. Seasonal Variation in Nutritional Composition and Anti-Proliferative Activity of Brown Seaweed, Sargassum Oligocystum. J. Appl. Phycol. 2018, 30, 101–111. [Google Scholar] [CrossRef]
- Gosch, B.J.; Paul, N.A.; de Nys, R.; Magnusson, M. Seasonal and Within-Plant Variation in Fatty Acid Content and Composition in the Brown Seaweed Spatoglossum Macrodontum (Dictyotales, Phaeophyceae). J. Appl. Phycol. 2015, 27, 387–398. [Google Scholar] [CrossRef]
- Mancuso, F.P.; Messina, C.M.; Santulli, A.; Laudicella, V.A.; Giommi, C.; Sarà, G.; Airoldi, L. Influence of Ambient Temperature on the Photosynthetic Activity and Phenolic Content of the Intertidal Cystoseira Compressa along the Italian Coastline. J. Appl. Phycol. 2019, 31, 3069–3076. [Google Scholar] [CrossRef]
- Britton, D.; Schmid, M.; Revill, A.T.; Virtue, P.; Nichols, P.D.; Hurd, C.L.; Mundy, C.N. Seasonal and Site-Specific Variation in the Nutritional Quality of Temperate Seaweed Assemblages: Implications for Grazing Invertebrates and the Commercial Exploitation of Seaweeds. J. Appl. Phycol. 2021, 33, 603–616. [Google Scholar] [CrossRef]
- Karkhaneh Yousefi, M.; Seyed Hashtroudi, M.; Mashinchian Moradi, A.; Ghassempour, A.R. Seasonal Variation of Fucoxanthin Content in Four Species of Brown Seaweeds from Qeshm Island, Persian Gulf and Evaluation of Their Antibacterial and Antioxidant Activities. Iran. J. Fish. Sci. 2020, 19, 2394–2408. [Google Scholar] [CrossRef]
- Marinho, G.S.; Sørensen, A.D.M.; Safafar, H.; Pedersen, A.H.; Holdt, S.L. Antioxidant Content and Activity of the Seaweed Saccharina Latissima: A Seasonal Perspective. J. Appl. Phycol. 2019, 31, 1343–1354. [Google Scholar] [CrossRef] [Green Version]
- Vizetto-Duarte, C.; Pereira, H.; De Sousa, C.B.; Rauter, A.P.; Albericio, F.; Custódio, L.; Barreira, L.; Varela, J. Fatty Acid Profile of Different Species of Algae of the Cystoseira Genus: A Nutraceutical Perspective. Nat. Prod. Res. 2015, 29, 1264–1270. [Google Scholar] [CrossRef]
- Custódio, L.; Silvestre, L.; Rocha, M.I.; Rodrigues, M.J.; Vizetto-Duarte, C.; Pereira, H.; Barreira, L.; Varela, J. Methanol Extracts from Cystoseira Tamariscifolia and Cystoseira Nodicaulis Are Able to Inhibit Cholinesterases and Protect a Human Dopaminergic Cell Line from Hydrogen Peroxide-Induced Cytotoxicity. Pharm. Biol. 2016, 54, 1687–1696. [Google Scholar] [CrossRef] [Green Version]
- Abu-Khudir, R.; Ismail, G.A.; Diab, T. Antimicrobial, Antioxidant, and Anti-Tumor Activities of Sargassum Linearifolium and Cystoseira Crinita from Egyptian Mediterranean Coast. Nutr. Cancer 2021, 73, 829–844. [Google Scholar] [CrossRef] [PubMed]
- Generalić Mekinić, I.; Čagalj, M.; Tabanelli, G.; Montanari, C.; Barbieri, F.; Skroza, D.; Šimat, V. Seasonal Changes in Essential Oil Constituents of Cystoseira Compressa: First Report. Molecules 2021, 26, 6649. [Google Scholar] [CrossRef]
- Oucif, H.; Adjout, R.; Sebahi, R.; Boukortt, F.O.; Ali-Mehidi, S.; El, S.-M.; Abi-Ayad, A. Comparison of In Vitro Antioxidant Activity of Some Selected Seaweeds from Algerian West Coast. Afr. J. Biotechnol. 2017, 16, 1474–1480. [Google Scholar] [CrossRef]
- Dulger, G.; Dulger, B. Antibacterial Activity of Two Brown Algae (Cystoseira Compressa and Padina Pavonica) against Methicillin-Resistant Staphylococcus Aureus. Br. Microbiol. Res. J. 2014, 4, 918–923. [Google Scholar] [CrossRef]
- Mhadhebi, L.; Dellai, A.; Clary-Laroche, A.; Said, R.B.; Robert, J.; Bouraoui, A. Anti-Inflammatory and Antiproliferative Activities of C. Compressa. Drug Dev. Res. 2012, 73, 82–89. [Google Scholar] [CrossRef]
- Kosanić, M.; Ranković, B.; Stanojković, T. Biological Potential of Marine Macroalgae of the Genus Cystoseira. Acta Biol. Hung. 2015, 66, 374–384. [Google Scholar] [CrossRef] [Green Version]
- Mhadhebi, L.; Mhadhebi, A.; Robert, J.; Bouraoui, A. Antioxidant, Anti-Inflammatory and Antiproliferative Effects of Aqueous Extracts of Three Mediterranean Brown Seaweeds of the Genus Cystoseira. Iran. J. Pharm. Res. 2014, 13, 207–220. [Google Scholar] [PubMed]
- Hermund, D.B. Antioxidant Properties of Seaweed-Derived Substances. In Bioactive Seaweeds for Food Applications; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 201–221. [Google Scholar] [CrossRef]
- Jacobsen, C.; Sørensen, A.D.M.; Holdt, S.L.; Akoh, C.C.; Hermund, D.B. Source, Extraction, Characterization, and Applications of Novel Antioxidants from Seaweed. Annu. Rev. Food Sci. Technol. 2019, 10, 541–568. [Google Scholar] [CrossRef] [PubMed]
- Polat, S.; Trif, M.; Rusu, A.; Šimat, V.; Čagalj, M.; Alak, G.; Meral, R.; Özogul, Y.; Polat, A.; Özogul, F. Recent Advances in Industrial Applications of Seaweeds. Crit. Rev. Food Sci. Nutr. 2021, 1–30. [Google Scholar] [CrossRef]
- Maggio, A.; Alduina, R.; Oddo, E.; Piccionello, A.P.; Mannino, A.M. Antibacterial Activity and HPLC Analysis of Extracts from Mediterranean Brown Algae. Plant Biosyst. 2020, 1–17. [Google Scholar] [CrossRef]
- Falace, A.; Zanelli, E.; Bressan, G. Morphological and Reproductive Phenology of Cystoseira Compressa (Esper) Gerloff & Nizamuddin (Fucales, Fucophyceae) in the Gulf of Trieste (North Adriatic). Ann. Ser. Hist. Nat. 2005, 5, 5–12. [Google Scholar]
- Mannino, A.M.; Vaglica, V.; Cammarata, M.; Oddo, E. Effects of Temperature on Total Phenolic Compounds in Cystoseira Amentacea (C. Agardh) Bory (Fucales, Phaeophyceae) from Southern Mediterranean Sea. Plant Biosyst.—Int. J. Deal. Asp. Plant Biol. 2016, 150, 152–160. [Google Scholar] [CrossRef]
- Güner, A.; Köksal, Ç.; Erel, Ş.B.; Kayalar, H.; Nalbantsoy, A.; Sukatar, A.; Karabay Yavaşoğlu, N.Ü. Antimicrobial and Antioxidant Activities with Acute Toxicity, Cytotoxicity and Mutagenicity of Cystoseira Compressa (Esper) Gerloff & Nizamuddin from the Coast of Urla (Izmir, Turkey). Cytotechnology 2015, 67, 135–143. [Google Scholar] [CrossRef] [Green Version]
- De La Fuente, G.; Fontana, M.; Asnaghi, V.; Chiantore, M.; Mirata, S.; Salis, A.; Damonte, G.; Scarfì, S. The Remarkable Antioxidant and Anti-Inflammatory Potential of the Extracts of the Brown Alga Cystoseira Amentacea Var. Stricta. Mar. Drugs 2021, 19, 2. [Google Scholar] [CrossRef] [PubMed]
- Alghazeer, R.; Elmansori, A.; Sidati, M.; Gammoudi, F.; Azwai, S.; Naas, H.; Garbaj, A.; Eldaghayes, I. In Vitro Antibacterial Activity of Flavonoid Extracts of Two Selected Libyan Algae against Multi-Drug Resistant Bacteria Isolated from Food Products. J. Biosci. Med. 2017, 5, 26–48. [Google Scholar] [CrossRef] [Green Version]
- Abdeldjebbar, F.Z.; Bennabi, F.; Ayache, A.; Berrayah, M.; Tassadiat, S. Synergistic Effect of Padina Pavonica and Cystoseira Compressa in Antibacterial Activity and Retention of Heavy Metals. Ukr. J. Ecol. 2021, 11, 36–40. [Google Scholar] [CrossRef]
- Bouafif, C.; Messaoud, C.; Boussaid, M.; Langar, H. Fatty Acid Profile of Cystoseira C. Agardh (Phaeophyceae, Fucales) Species from the Tunisian Coast: Taxonomic and Nutritional Assessments. Ciencias Mar. 2018, 44, 169–183. [Google Scholar] [CrossRef] [Green Version]
- Zhong, B.; Robinson, N.A.; Warner, R.D.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A.R. LC-ESI-QTOF-MS/MS Characterization of Seaweed Phenolics and Their Antioxidant Potential. Mar. Drugs 2020, 18, 331. [Google Scholar] [CrossRef] [PubMed]
- Jerković, I.; Cikoš, A.-M.; Babić, S.; Čižmek, L.; Bojanić, K.; Aladić, K.; Ul’yanovskii, N.V.; Kosyakov, D.S.; Lebedev, A.T.; Čož-Rakovac, R.; et al. Bioprospecting of Less-Polar Constituents from Endemic Brown Macroalga Fucus Virsoides J. Agardh from the Adriatic Sea and Targeted Antioxidant Effects In Vitro and In Vivo (Zebrafish Model). Mar. Drugs 2021, 19, 235. [Google Scholar] [CrossRef]
- Ristivojević, P.; Jovanović, V.; Opsenica, D.M.; Park, J.; Rollinger, J.M.; Velicković, T.Ć. Rapid Analytical Approach for Bioprofiling Compounds with Radical Scavenging and Antimicrobial Activities from Seaweeds. Food Chem. 2021, 334, 127562. [Google Scholar] [CrossRef]
- Das, U.N. Arachidonic Acid and Other Unsaturated Fatty Acids and Some of Their Metabolites Function as Endogenous Antimicrobial Molecules: A Review. J. Adv. Res. 2018, 11, 57–66. [Google Scholar] [CrossRef]
- Chanda, W.; Joseph, T.P.; Guo, X.F.; Wang, W.D.; Liu, M.; Vuai, M.S.; Padhiar, A.A.; Zhong, M.T. Effectiveness of Omega-3 Polyunsaturated Fatty Acids against Microbial Pathogens. J. Zhejiang Univ. Sci. B 2018, 19, 253–262. [Google Scholar] [CrossRef]
- Shin, S.Y.; Bajpai, V.K.; Kim, H.R.; Kang, S.C. Antibacterial Activity of Bioconverted Eicosapentaenoic (EPA) and Docosahexaenoic Acid (DHA) against Foodborne Pathogenic Bacteria. Int. J. Food Microbiol. 2007, 113, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Cvitković, D.; Dragović-Uzelac, V.; Dobrinčić, A.; Čož-Rakovac, R.; Balbino, S. The Effect of Solvent and Extraction Method on the Recovery of Lipid Fraction from Adriatic Sea Macroalgae. Algal Res. 2021, 56, 102291. [Google Scholar] [CrossRef]
- Lacey, R.W.; Lord, V.L. Sensitivity of Staphylococci to Fatty Acids: Novel Inactivation of Linolenic Acid by Serum. J. Med. Microbiol. 1981, 14, 41–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le, P.; Desbois, A. Antibacterial Effect of Eicosapentaenoic Acid against Bacillus Cereus and Staphylococcus Aureus: Killing Kinetics, Selection for Resistance, and Potential Cellular Target. Mar. Drugs 2017, 15, 334. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.-H.; Wang, Y.; Nakatsuji, T.; Liu, Y.-T.; Zouboulis, C.C.; Gallo, R.L.; Zhang, L.; Hsieh, M.-F.; Huang, C.-M. An Innate Bactericidal Oleic Acid Effective against Skin Infection of Methicillin-Resistant Staphylococcus Aureus: A Therapy Concordant with Evolutionary Medicine. J. Microbiol. Biotechnol. 2011, 21, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Čagalj, M.; Skroza, D.; Tabanelli, G.; Özogul, F.; Šimat, V. Maximizing the Antioxidant Capacity of Padina Pavonica by Choosing the Right Drying and Extraction Methods. Processes 2021, 9, 587. [Google Scholar] [CrossRef]
- Amerine, M.A.; Ough, C.S. Methods for Analysis of Musts and Wines; Wiley: Hoboken, NJ, USA, 1980. [Google Scholar]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milat, A.M.; Boban, M.; Teissedre, P.L.; Šešelja-Perišin, A.; Jurić, D.; Skroza, D.; Generalić-Mekinić, I.; Ljubenkov, I.; Volarević, J.; Rasines-Perea, Z.; et al. Effects of Oxidation and Browning of Macerated White Wine on Its Antioxidant and Direct Vasodilatory Activity. J. Funct. Foods 2019, 59, 138–147. [Google Scholar] [CrossRef]
- Prior, R.L.; Hoang, H.; Gu, L.; Wu, X.; Bacchiocca, M.; Howard, L.; Hampsch-Woodill, M.; Huang, D.; Ou, B.; Jacob, R. Assays for Hydrophilic and Lipophilic Antioxidant Capacity (Oxygen Radical Absorbance Capacity (ORACFL)) of Plasma and Other Biological and Food Samples. J. Agric. Food Chem. 2003, 51, 3273–3279. [Google Scholar] [CrossRef]
- Burčul, F.; Generalić Mekinić, I.; Radan, M.; Rollin, P.; Blažević, I. Isothiocyanates: Cholinesterase Inhibiting, Antioxidant, and Anti-Inflammatory Activity. J. Enzym. Inhib. Med. Chem. 2018, 33, 577–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skroza, D.; Šimat, V.; Smole Možina, S.; Katalinić, V.; Boban, N.; Generalić Mekinić, I. Interactions of Resveratrol with Other Phenolics and Activity against Food-Borne Pathogens. Food Sci. Nutr. 2019, 7, 2312–2318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elez Garofulić, I.; Malin, V.; Repajić, M.; Zorić, Z.; Pedisić, S.; Sterniša, M.; Smole Možina, S.; Dragović-Uzelac, V. Phenolic Profile, Antioxidant Capacity and Antimicrobial Activity of Nettle Leaves Extracts Obtained by Advanced Extraction Techniques. Molecules 2021, 26, 6153. [Google Scholar] [CrossRef]
- Verni, M.; Pontonio, E.; Krona, A.; Jacob, S.; Pinto, D.; Rinaldi, F.; Verardo, V.; Díaz-de-Cerio, E.; Coda, R.; Rizzello, C.G. Bioprocessing of Brewers’ Spent Grain Enhances Its Antioxidant Activity: Characterization of Phenolic Compounds and Bioactive Peptides. Front. Microbiol. 2020, 11, 1831. [Google Scholar] [CrossRef] [PubMed]
- Šimat, V.; Vlahović, J.; Soldo, B.; Generalić Mekinić, I.; Čagalj, M.; Hamed, I.; Skroza, D. Production and Characterization of Crude Oils from Seafood Processing by-Products. Food Biosci. 2020, 33, 100484. [Google Scholar] [CrossRef]



| May | June | July | August | September | |
|---|---|---|---|---|---|
| Temperature (°C) | 18.3 | 21.8 | 22.4 | 26.9 | 24.7 |
| Salinity (PSU) | 37.4 | 38.1 | 38.3 | 38.3 | 38.3 |
| May | June | July | August | September | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| Escherichia coli | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | >10 |
| Salmonella enteritidis | 10 | 10 | 5 | 10 | 5 | 10 | 5 | 10 | 10 | >10 |
| Enterococcus faecalis | 10 | 10 | 10 | 10 | 10 | 10 | 5 | 10 | 10 | 10 |
| Listeria monocytogenes | 10 | >10 | 2.5 | 2.5 | 2.5 | 5 | 2.5 | 5 | 5 | >10 |
| Staphylococcus aureus | 10 | >10 | 5 | 5 | 2.5 | 2.5 | 2.5 | 2.5 | 10 | 10 |
| Bacillus cereus | >10 | n.d. | 10 | >10 | 10 | >10 | 10 | >10 | >10 | n.d. |
| N° | RT (min) | Observed (m/z) | Theorical (m/z) | Error (ppm) | Score (%) | Molecular Formula | In Source Fragments | Tentative Compound | May (%) | June (%) | July (%) | August (%) | September (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.28 | 343.0367 | 343.0368 | −0.3 | 94.18 | C20H4N6O | - | 1a,9b-Dihydrophenanthro [9,10-b]oxirene-2,3,4,7,8,9-hexacarbonitrile | 4.76 | 4.50 | 4.54 | 4.96 | 6.51 |
| 2 | 0.29 | 201.0244 | 201.0247 | −1.5 | 98.89 | C4H10O9 | - | 2-(1,2,2,2-Tetrahydroxyethoxy)ethane-1,1,1,2-tetrol | 6.67 | 6.17 | 6.58 | 6.72 | 7.90 |
| 3 | 0.32 | 141.0162 | 141.0161 | 0.7 | 91.01 | C2H2N6O2 | - | Diazidoacetic acid | 1.97 | 2.02 | 2.40 | 2.01 | 1.74 |
| 4 | 0.35 | 181.0707 | 181.0712 | −2.8 | 100 | C6H14O6 | 101.0230; 89.0227; 71.0137; 59.0121 | d-Sorbitol | 3.11 | 3.57 | 1.34 | 2.34 | 1.83 |
| 5 | 0.40 | 317.0506 | 317.0509 | −0.9 | 90.44 | C12H14O10 | 209.0890 | d-glucaric acid derivate | 1.13 | 0.88 | 0.57 | 0.67 | 0.80 |
| 6 | 0.42 | 384.1510 | 384.1519 | −2.3 | 92.29 | C15H23N5O7 | - | Threonyl-histidyl-glutamic acid | 0.09 | 0.11 | 0.06 | 0.07 | 0.10 |
| 7 | 16.56 | 287.2211 | 287.2222 | −3.8 | 95.91 | C16H32O4 | - | 10,11-Dihydroxy-9,12-dioxooctadecanoic acid | 0.19 | 0.19 | 0.20 | 0.17 | 0.20 |
| 8 | 16.90 | 275.1999 | 275.2011 | −4.4 | 96.48 | C18H28O2 | 231.2098; 253.0915 | Stearidonic acid (C18:4n-3) isomer a | 0.12 | 0.42 | 0.49 | 0.32 | 0.06 |
| 9 | 16.97 | 275.2007 | 275.2011 | −1.5 | 97.68 | C18H28O2 | 231.2092; 177.0854; 255.2322; | Stearidonic acid (C18:4n-3) isomer b | 0.21 | 0.28 | 0.56 | 0.32 | 0.08 |
| 10 | 16.97 | 293.2112 | 293.2117 | −1.7 | 92.64 | C18H30O3 | 249.1835; 275.1652 | 13-ketooctadecadienoic acid isomer a | 0.07 | 0.09 | 0.24 | 0.31 | 0.13 |
| 11 | 17.08 | 287.2211 | 287.2222 | −3.8 | 95.91 | C16H32O4 | 271.2083; 253.2157 | 10,16-Dihydroxyhexadecanoic acid isomer a | 0.01 | 0.01 | 0.02 | 0.03 | 0.03 |
| 12 | 17.13 | 309.2056 | 309.2066 | −3.2 | 96.09 | C18H30O4 | 279.2287 | 6,9-Octadecadienedioic acid | 0.02 | 0.04 | 0.01 | 0.08 | 0.00 |
| 13 | 17.18 | 295.2276 | 295.2273 | 1.0 | 100 | C18H32O3 | 279.2300; 275.2019; 255.2325 | 9,10-Epoxyoctadecenoic acid (vernolic acid) | 0.02 | 0.13 | 0.07 | 0.13 | 0.08 |
| 14 | 17.20 | 277.2159 | 277.2168 | −3.2 | 91.36 | C18H30O2 | 255.2321; 239.2030; 227.2013 | gamma-Linolenic acid isomer a (C18:3n-6) | 0.10 | 0.22 | 0.29 | 0.22 | 0.08 |
| 15 | 17.22 | 429.3009 | 429.3005 | 0.9 | 91.64 | C27H42O4 | 273.1859; 135.0447 | 24-Keto-1,25-dihydroxyvitamin D3 | 0.01 | 0.57 | 0.58 | 0.05 | 0.02 |
| 16 | 17.26 | 247.1689 | 247.1698 | −3.6 | 94.96 | C16H24O2 | 233.0985 | 2,4,6-Triisopropyl benzoic acid | 0.05 | 0.02 | 0.02 | 0.24 | 0.26 |
| 17 | 17.35 | 287.2212 | 287.2222 | −3.5 | 90.62 | C16H32O4 | 271.2082; 253.2158 | 10,16-Dihydroxyhexadecanoic acid isomer b | 0.01 | 0.01 | 0.13 | 0.15 | 0.09 |
| 18 | 17.37 | 199.1694 | 199.1698 | −2.0 | 90.11 | C12H24O2 | 181.1062; 155.0336 | Lauric acid | 0.90 | 0.85 | 0.92 | 0.81 | 0.83 |
| 19 | 17.38 | 297.2426 | 297.2430 | −1.3 | 98.84 | C18H34O3 | 279.2367; 255.2334 | 10-Oxooctadecanoic acid isomer a | 0.35 | 0.39 | 0.34 | 0.37 | 0.40 |
| 20 | 17.40 | 243.1952 | 243.1960 | −3.3 | 90.78 | C14H28O3 | 197.1907 | 3-hydroxymyristic acid | 0.08 | 0.07 | 0.07 | 0.10 | 0.09 |
| 21 | 17.42 | 293.2112 | 293.2117 | −1.7 | 94.2 | C18H30O3 | 249.1833; 275.1649 | 13-ketooctadecadienoic acid isomer b | 0.04 | 0.10 | 0.11 | 0.29 | 0.04 |
| 22 | 17.43 | 427.2827 | 427.2848 | −4.9 | 90.28 | C27H40O4 | 271.1716; 188.0842; 135.0441 | Hydroxyprogesterone caproate | 0.00 | 0.17 | 0.24 | 0.02 | 0.01 |
| 23 | 17.46 | 429.3009 | 429.3005 | 0.9 | 91.64 | C27H42O4 | 273.1843; 135.0445 | 24-Keto-1,25-dihydroxyvitamin D3 isomer b | 0.00 | 0.04 | 0.08 | 0.01 | n.d. |
| 24 | 17.48 | 295.2262 | 295.2273 | −3.7 | 94.13 | C18H32O3 | 279.2295; 275.2023; 255.2321 | 9,10-Epoxyoctadecenoic acid isomer b (vernolic acid) | 0.34 | 0.43 | 0.39 | 0.40 | 0.41 |
| 25 | 17.51 | 269.2110 | 269.2117 | −2.6 | 98.63 | C16H30O3 | 251.2336 | 3-Oxohexadecanoic acid | 0.07 | 0.16 | 0.01 | 0.26 | 0.11 |
| 26 | 17.51 | 225.1857 | 225.1855 | −0.9 | 95.99 | C14H26O2 | 188.0832; 213.1870; 175.0757 | Myristoleic acid | 2.35 | 2.14 | 2.26 | 2.15 | 2.22 |
| 27 | 17.53 | 255.2319 | 255.2324 | −2.0 | 91.41 | C16H32O2 | 225.1861; 213.1845 | Hexadecanoic acid (palmitic acid) isomer a (C16:0) | 0.10 | 0.05 | 0.04 | 0.03 | 0.04 |
| 28 | 17.57 | 275.2007 | 275.2011 | −1.5 | 97.68 | C18H28O2 | 231.2093; 255.2326 | Stearidonic acid (C18:4n-3) isomer c | 0.25 | 0.35 | 0.67 | 0.69 | 0.33 |
| 29 | 17.58 | 277.2152 | 277.2168 | −5.8 | 99.51 | C18H30O2 | 255.2289; 239.2001; 227.1989 | gamma-Linolenic acid isomer b (C18:3n-6) | 0.00 | 0.01 | 0.01 | 0.01 | 0.00 |
| 30 | 17.59 | 213.18458 | 213.1855 | −3.6 | 92.41 | C13H26O2 | - | Tridecanoic acid | 1.20 | 1.19 | 1.15 | 1.12 | 1.15 |
| 31 | 17.62 | 427.2839 | 427.2848 | −2.1 | 96.07 | C27H40O4 | 271.1659; 188.0827; 135.0442 | Hydroxyprogesterone caproate isomer b | n.d. | 0.13 | 0.21 | 0.02 | 0.01 |
| 32 | 17.62 | 257.2108 | 257.2117 | −3.5 | 95.16 | C15H30O3 | 227.2037; 211.2072 | 11-Hydroxypentadecanoic acid | 0.10 | 0.09 | 0.09 | 0.07 | 0.11 |
| 33 | 17.63 | 251.2010 | 251.2011 | −0.4 | 100 | C16H28O2 | 233.9910; 207.0983 | 7,10-hexadecadienoic acid | 0.77 | 0.84 | 0.80 | 0.80 | 0.91 |
| 34 | 17.64 | 297.2429 | 297.2430 | −0.3 | 97.33 | C18H34O3 | 279.2364; 255.2332 | 10-Oxooctadecanoic acid isomer b | 0.56 | 0.63 | 0.58 | 0.53 | 0.56 |
| 35 | 17.66 | 239.2001 | 239.2011 | −4.2 | 97.7 | C15H28O2 | 227.2002; 159.8926 | Myristoleic acid methyl ester | 5.22 | 5.23 | 5.00 | 4.89 | 4.92 |
| 36 | 17.74 | 277.2162 | 277.2168 | −2.2 | 99.51 | C18H30O2 | 255.2318; 239.1991; 227.2015 | gamma-Linolenic acid isomer c (C18:3n-6) | 1.08 | 1.41 | 1.92 | 2.47 | 2.17 |
| 37 | 17.71 | 301.2158 | 301.2168 | −3.3 | 99.56 | C20H30O2 | 283.2283; 275.1972 | Eicosapentanoic acid isomer a (C20:5n-3) | 0.65 | 0.65 | 1.09 | 0.81 | 0.75 |
| 38 | 17.73 | 301.2156 | 301.2168 | −4.0 | 98.12 | C20H30O2 | 283.2287; 275.1957 | Eicosapentanoic acid isomer b (C20:5n-3) | 0.62 | 0.63 | 1.06 | 0.80 | 0.74 |
| 39 | 17.77 | 227.2001 | 227.2011 | −4.4 | 93.6 | C14H28O2 | - | Tetradecanoic acid (C14:0) | 5.04 | 5.19 | 5.34 | 5.17 | 5.22 |
| 40 | 17.81 | 271.2266 | 271.2273 | −2.6 | 97.75 | C16H32O3 | 253.0954; 225.2211 | Hydroxy-palmitic acid | 0.47 | 0.41 | 0.45 | 0.56 | 0.66 |
| 41 | 17.85 | 253.2156 | 253.2168 | −4.7 | 96.47 | C16H30O2 | - | Palmitoleic acid isomer a (C16:1n-7) | 12.65 | 11.93 | 11.45 | 11.57 | 11.74 |
| 42 | 17.94 | 241.2170 | 241.2168 | 0.8 | 100 | C15H30O2 | 223.2081 | Pentadecanoic acid (C15:0) | 3.68 | 3.89 | 3.87 | 3.72 | 3.67 |
| 43 | 17.97 | 279.2314 | 279.2324 | −3.6 | 98.25 | C18H32O2 | 267.2340; 275.2037 | Octadeca-10,12-dienoic acid (C18:2n-6) | 1.07 | 1.14 | 1.29 | 1.31 | 1.27 |
| 44 | 18.01 | 267.2318 | 267.2324 | −2.2 | 99.96 | C17H32O2 | 249.0437; 223.0291 | 9-Heptadecenoic acid (C17:1n-8) | 3.73 | 3.99 | 3.67 | 3.78 | 3.60 |
| 45 | 18.08 | 255.2321 | 255.2324 | −1.2 | 99.9 | C16H32O2 | 227.2015 | Hexadecanoic acid (palmitic acid)(C16:0) | 10.46 | 10.46 | 10.24 | 9.92 | 9.83 |
| 46 | 18.12 | 281.2486 | 281.2481 | 1.8 | 96.88 | C18H34O2 | - | Oleic acid (C18:1n-9) | 15.87 | 15.06 | 15.39 | 15.33 | 15.12 |
| 47 | 18.22 | 269.2476 | 269.2481 | −5.6 | 99.96 | C17H34O2 | 255.2325 | Heptadecanoic acid (C17:0) | 5.30 | 5.23 | 5.18 | 5.08 | 4.95 |
| 48 | 18.33 | 283.2618 | 283.2637 | −1.9 | 99.21 | C18H36O2 | - | Octadecanoic acid (stearic acid) C18:0 | 5.44 | 4.92 | 5.01 | 5.11 | 5.24 |
| 49 | 18.54 | 311.2944 | 311.2950 | −2.0 | 90.87 | C20H40O2 | 255.2307; 225.0060 | Arachidic acid | 0.68 | 0.62 | 0.67 | 0.66 | 0.68 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Čagalj, M.; Skroza, D.; Razola-Díaz, M.d.C.; Verardo, V.; Bassi, D.; Frleta, R.; Generalić Mekinić, I.; Tabanelli, G.; Šimat, V. Variations in the Composition, Antioxidant and Antimicrobial Activities of Cystoseira compressa during Seasonal Growth. Mar. Drugs 2022, 20, 64. https://doi.org/10.3390/md20010064
Čagalj M, Skroza D, Razola-Díaz MdC, Verardo V, Bassi D, Frleta R, Generalić Mekinić I, Tabanelli G, Šimat V. Variations in the Composition, Antioxidant and Antimicrobial Activities of Cystoseira compressa during Seasonal Growth. Marine Drugs. 2022; 20(1):64. https://doi.org/10.3390/md20010064
Chicago/Turabian StyleČagalj, Martina, Danijela Skroza, María del Carmen Razola-Díaz, Vito Verardo, Daniela Bassi, Roberta Frleta, Ivana Generalić Mekinić, Giulia Tabanelli, and Vida Šimat. 2022. "Variations in the Composition, Antioxidant and Antimicrobial Activities of Cystoseira compressa during Seasonal Growth" Marine Drugs 20, no. 1: 64. https://doi.org/10.3390/md20010064
APA StyleČagalj, M., Skroza, D., Razola-Díaz, M. d. C., Verardo, V., Bassi, D., Frleta, R., Generalić Mekinić, I., Tabanelli, G., & Šimat, V. (2022). Variations in the Composition, Antioxidant and Antimicrobial Activities of Cystoseira compressa during Seasonal Growth. Marine Drugs, 20(1), 64. https://doi.org/10.3390/md20010064