New Polyketides from a Hydrothermal Vent Sediment Fungus Trichoderma sp. JWM29-10-1 and Their Antimicrobial Effects
Abstract
:1. Introduction
2. Results
2.1. Structure Elucidation
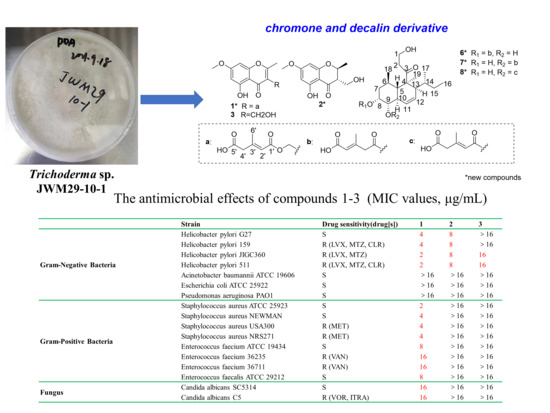
2.2. Antimicrobial Effects of Compounds 1–12
3. Conclusions and Discussion
4. Materials and Methods
4.1. General Experimental Procedure
4.2. Fungal Material
4.3. Fermentation and Extraction
4.4. Compound Isolation
4.5. Spectroscopic Data of New Compounds
4.6. Absolute Configuration Determination of Compounds 6–8
4.7. Antimicrobial Assays
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Banerjee, P.; Mandhare, A.; Bagalkote, V. Marine natural products as source of new drugs: An updated patent review (July 2018–July 2021). Expert Opin. Ther. Pat. 2022, 32, 317–363. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Frank, M.; Yu, X.Q.; Yu, H.Q.; Tran-Cong, N.; Gao, Y.; Proksch, P. Secondary metabolites from marine-derived fungi from China. Prog. Chem. Org. Nat. Prod. 2020, 111, 81–153. [Google Scholar] [PubMed]
- Sood, M.; Kapoor, D.; Kumar, V.; Sheteiwy, M.; Ramakrishnan, M.; Landi, M.; Araniti, F.; Sharma, A. Trichoderma: The “secrets” of a multitalented biocontrol agent. Plants 2020, 9, 762. [Google Scholar] [CrossRef] [PubMed]
- Su, D.; Ding, L.J.; He, S. Marine-derived Trichoderma species as a promising source of bioactive secondary metabolites. Mini Rev. Med. Chem. 2018, 18, 1702–1713. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tang, W.; Huang, Q.; Li, Y.; Wei, M.; Jiang, L.; Liu, C.; Yu, X.; Zhu, H. Trichoderma: A treasure house of structurally diverse secondary metabolites with medicinal importance. Front. Microbiol. 2021, 12, 2037. [Google Scholar] [CrossRef] [PubMed]
- Khamthong, N.; Rukachaisirikul, V.; Tadpetch, K.; Kaewpet, M.; Phongpaichit, S.; Preedanon, S.; Sakayaroj, J. Tetrahydroanthraquinone and xanthone derivatives from the marine-derived fungus Trichoderma aureoviride PSU-F95. Arch. Pharm. Res. 2012, 35, 461–468. [Google Scholar] [CrossRef]
- Gou, X.S.; Jia, J.; Xue, Y.X.; Ding, W.J.; Dong, Z.T.; Tian, D.M.; Chen, M.; Bi, H.K.; Hong, K.; Tang, J.S. New pyrones and their analogs from the marine mangrove-derived Aspergillus sp. DM94 with antibacterial activity against Helicobacter pylori. Appl. Microbiol. Biot. 2020, 104, 7971–7978. [Google Scholar] [CrossRef]
- De, J.; Kumla, D.; Dethoup, T.; Kijjoa, A. Bioactive compounds from terrestrial and marine-derived fungi of the genus Neosartorya. Molecules 2022, 27, 2351. [Google Scholar]
- Tanahashi, T.; Takenaka, Y.; Nagakura, N.; Hamada, N. 2,3-Dialkylchromones from Mycobiont Cultures of the Lichen Graphis scripta. Heterocycles 2000, 53, 1589–1593. [Google Scholar] [CrossRef]
- Wang, X.; Xu, T.; Sun, L.; Ceng, R.; Su, S.; Yang, X.; Yang, Y.; Ding, Z. The chemical diversity, the attractant, anti-acetylcholinesterase, and antifungal activities of metabolites from biocontrol Trichoderma harzianum uncovered by OSMAC strategy. Bioorg. Chem. 2021, 114, 105148. [Google Scholar] [CrossRef]
- Yamada, T.; Umebayashi, Y.; Kawashima, M.; Kikuchi, T.; Tanaka, R. Determination of the chemical structures of tandyukisins B–D, isolated from a Marine Sponge-Derived Fungus. Mar. Drugs. 2015, 13, 3231–3240. [Google Scholar] [CrossRef]
- Rahbæk, L.; Sperry, S.; Piper, J. Deoxynortrichoharzin, a new polyketide from the saltwater culture of a sponge-derived Paecilomyces fungus. J. Nat. Prod. 1998, 61, 1571–1573. [Google Scholar] [CrossRef]
- Rukachaisirikul, V.; Chinpha, S.; Phongpaichit, S.; Saikhwan, N.; Sakayaroj, J.; Preedanon, S. Sesquiterpene and monoterpene derivatives from the soil-derived fungus Trichoderma reesei PSU-SPSF013. Phytochem. Lett. 2019, 30, 124–129. [Google Scholar] [CrossRef]
- Ding, W.Y.; Cao, W.G.; Yao, Y. Synthesis of dimethyl-3-perfluoroalkyl-4-(3-oxo-2-triphenyl-phosphoranylidenbutanylidene)-pent-2-enedioate and its cyclization. Chin. J. Chem. 2010, 13, 468–474. [Google Scholar] [CrossRef]
- Mutanyatta-Comar, J.; Phale, O.J.K.; Abegaz, B.; Croft, K. Phloroglucinol derivatives and flavones from Helichrysum paronychioides. Bull. Chem. Soc. Ethiopia. 2006, 20, 61–68. [Google Scholar]
- Duh, C.Y.; Wang, S.K.; Chung, S.G.; Chou, G.C.; Dai, C.F. Cytotoxic Cembrenolides and Steroids from the Formosan Soft Coral Sarcophyton crassocaule. J. Nat. Prod. 2000, 63, 1634–1637. [Google Scholar] [CrossRef]
- Nakadate, S.; Nozawa, K.; Horie, H.; Fujii, Y.; Nagai, M.; Hosoe, T.; Kawai, K.I.; Yaguchi, T.; Fukushima, K. Eujavanicols A–C, decalin derivatives from Eupenicillium javanicum. J. Nat. Prod. 2007, 70, 1510–1512. [Google Scholar] [CrossRef]
- DiBari, L.; Pescitelli, G.; Pratelli, C.; Pini, D.; Salvadori, P. Determination of absolute configuration of acyclic 1,2-diols with Mo2(OAc)4. 1. Snatzke’s method revised. J. Org. Chem. 2001, 66, 4819–4825. [Google Scholar]
- Liu, J.; Du, D.; Si, Y.; Lu, H.; Wu, X.; Li, Y.; Liu, Y.; Yu, S. Application of dimolybdenum reagent Mo2(OAc)4 for determination of the absolute configurations of vic-diols. Chin. J. Org. Chem. 2010, 30, 1270–1278. [Google Scholar]
- Bovio, E. Marine fungi from sponges: Biodiversity, chemodiversity and biotechnological applications. Mar. Drugs 2019, 17, 220. [Google Scholar] [CrossRef] [Green Version]
- Keswani, C.; Mishra, S.; Sarma, B.; Singh, S.; Singh, H. Unraveling the efficient applications of secondary metabolites of various Trichoderma spp. Appl. Microbiol. Biot. 2014, 98, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Liu, X.H.; Li, X.N.; Ji, N.Y. Antifungal and antimicroalgal trichothecene sesquiterpenes from the marine algicolous fungus Trichoderma brevicompactum A-DL-9-2. J. Agric. Food. Chem. 2020, 68, 15440–15448. [Google Scholar] [CrossRef] [PubMed]
- Lewis, K. The science of antibiotic discovery. Cell 2020, 18, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Cooper, M.A.; Shlaes, D. Fix the antibiotics pipeline. Nature 2011, 472, 32. [Google Scholar] [CrossRef]
- Govindaraj Vaithinathan, A.; Vanitha, A. WHO global priority pathogens list on antibiotic resistance: An urgent need for action to integrate One Health data. Perspect. Public Health 2018, 138, 87–88. [Google Scholar] [CrossRef]
- Huang, Y.Q.; Hang, X.D.; Jiang, X.Q.; Zeng, L.P.; Jia, J.; Xie, Y.; Li, F.; Bi, H.K. In vivo activities of zinc linolenate, a delective antibacterial agent against Helicobacter pylori. Antimicrob. Agents Chemother. 2019, 63, e00004-19. [Google Scholar] [CrossRef] [Green Version]
- Qadi, M.; Jaradat, N. In vitro antitumor, antibacterial, and antifungal activities of phenylthio-ethyl benzoate derivatives. Arab. J. Sci. Eng. 2022, 46, 5339–5344. [Google Scholar]
- Lv, H.; Wang, K.; Xue, Y.; Chen, J.; Su, H.; Zhang, J.; Wu, Y.; Jia, J.; Bi, H.K.; Wang, H.; et al. Three new metabolites from the marine-derived fungus Aspergillus sp. WHUF03110. Nat. Prod. Commun. 2021, 16, 10936–10940. [Google Scholar] [CrossRef]





| NO. | 1 a | 2 b | ||
|---|---|---|---|---|
| δC | δH (J in Hz) | δC | δH (J in Hz) | |
| 2 | 167.6 | 76.6 | 4.66 (1H, m) | |
| 3 | 114.8 | 54.3 | 2.59 (1H, dt, 9.6, 4.0) | |
| 4 | 181.0 | 198.2 | ||
| 4a | 104.9 | 104.1 | ||
| 5 | 162.3 | 165.3 | ||
| 6 | 98.2 | 6.34 (1H, brs) | 95.5 | 6.02 (1H, d, 2.4) |
| 7 | 165.9 | 169.5 | ||
| 8 | 92.6 | 6.34 (1H, brs) | 94.7 | 6.00 (1H, d, 2.4) |
| 8a | 157.6 | 164.0 | ||
| 9 | 18.6 | 2.50 (3H, s) | 19.3 | 1.54 (3H, d, 6.4) |
| 10 | 56.0 | 5.11 (2H, s) | 58.1 | 4.26 (1H, dd, 11.2, 4.0) 3.79 (1H, dd, 11.2, 4.0) |
| 5-OH | 12.63 (1H, s) | |||
| 7-OMe | 55.9 | 3.85 (3H, s) | 56.2 | 3.82 (3H, s) |
| 1′ | 165.7 | |||
| 2′ | 119.6 | 5.81 (1H, s) | ||
| 3′ | 151.5 | |||
| 4′ | 45.5 | 3.15 (2H, s) | ||
| 5′ | 174.6 | |||
| 6′ | 19.2 | 2.25 (3H, s) | ||
| NO. | 6 | 7 | 8 | |||
|---|---|---|---|---|---|---|
| δC | δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) | |
| 1 | 58.1 | 3.90 (1H, m, Ha) | 58.1 | 3.90 (1H, m, Ha) | 58.1 | 3.93 (1H, m, Ha) |
| 3.82 (1H, m, Hb) | 3.85 (1H, m, HB) | 3.85 (1H, m, Hb) | ||||
| 2 | 41.3 | 2.85 (1H, brd,18.8, Ha) | 41.3 | 2.88 (1H, brd, 18.8, Ha) | 41.2 | 2.89 (1H, ddd, 18.8, 6.0, 3.6, Ha) |
| 2.66 (1H, brd, 18.8, Hb) | 2.69 (1H, brd, 18.4, Ha) | 2.70 (1H, ddd, 18.8, 7.2, 4.0, Ha) | ||||
| 3 | 215.5 | 215.3 | 215.5 | |||
| 4 | 52.6 | 52.6 | 52.7 | |||
| 5 | 43.2 | 1.96 (1H, m) | 43.8 | 2.04 (1H, t, m) | 43.7 | 2.05 (1H, m) |
| 6 | 31.6 | 1.57 (1H, m) | 30.4 | 1.80 (1H, m) | 30.4 | 1.83 (1H, m) |
| 7 | 39.0 | 1.82 (1H, brd,12.0, Hα) | 41.0 | 1.84 (1H, m, Hα) | 40.8 | 1.86 (1H, m, Hα) |
| 1.55 (1H, m, Hβ) | 1.58 (1H, brd, 13.6, Hβ) | 1.58 (1H, m, Hβ) | ||||
| 8 | 74.0 | 5.24 (1H, d, 3.2) | 67.6 | 4.15 (1H, brs) | 67.7 | 4.15 (1H, q, 3.2) |
| 9 | 74.3 | 3.56 (1H, dd, 10.8, 3.2) | 79.1 | 4.75 (1H, d,11.2) | 77.5 | 4.77 (1H, dd, 11.6, 2.8) |
| 10 | 40.1 | 2.10 (1H, m) | 36.3 | 2.46 (1H, t, 11.2) | 36.3 | 2.50 (1H, m) |
| 11 | 125.7 | 6.01 (1H, brd,10.4) | 125.1 | 5.54 (1H, d, 10.8) | 125.2 | 5.61 (1H, m) |
| 12 | 124.1 | 5.70 (1H, brd,10.4) | 124.6 | 5.69 (1H, d,10.8) | 124.6 | 5.68 (1H, ddd, 10.4, 4.4,2.8) |
| 13 | 52.4 | 1.93 (1H, m) | 52.5 | 1.94 (1H, m) | 52.5 | 1.94 (1H, m) |
| 14 | 37.3 | 1.10 (1H, m) | 37.3 | 1.11 (1H, m) | 37.3 | 1.13 (1H, m) |
| 15 | 24.6 | 0.74 (1H, m, a) | 24.5 | 0.74 (1H, m) | 24.5 | 0.73 (1H, m) |
| 1.45 (1H, m, b) | 1.48 (1H, m) | 1.49 (1H, m) | ||||
| 16 | 12.7 | 0.76 (3H, m) | 12.6 | 0.77 (3H, t, 4.4) | 12.7 | 0.76 (3H, t, 6.4) |
| 17 | 19.4 | 0.92 (3H, d, 6.4) | 19.3 | 0.92 (3H, d, 6.4) | 19.3 | 0.91 (3H, d, 6.8) |
| 18 | 22.4 | 0.58 (3H, d, 5.6) | 22.4 | 0.60 (3H, d, 7.2) | 22.4 | 0.60 (3H, d, 6.8) |
| 19 | 19.5 | 1.23 (3H, s) | 19.5 | 1.25 (3H, s) | 19.4 | 1.25 (3H, s) |
| 1′ | 170.2 | 169.0 | 165.1 | |||
| 2′ | 46.4 | 3.23 (2H, s) | 46.3 | 3.27 (2H, s) | 119.4 | 5.89 (1H, s) |
| 3′ | 153.7 | 153.4 | 152.5 | |||
| 4′ | 119.3 | 5.82 (1H, s) | 119.5 | 5.85 (1H, s) | 45.7 | 3.19 (2H, s) |
| 5′ | 170.4 | 170.3 | 174.3 | |||
| 6′ | 19.4 | 2.24(3H, s) | 19.5 | 2.27 (3H, s) | 19.5 | 2.28 (3H, s) |
| MIC (µg/mL) for: | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Strain | Drug Sensitivity(Drug [s]) a | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
| Gram-Negative Bacteria | Helicobacter pylori G27 | S | 4 | 8 | 32 | >64 | 32 | >64 | >64 | >64 | >64 | >64 | >64 | 64 |
| Helicobacter pylori 159 | R (LVX, MTZ, CLR) | 4 | 8 | 32 | >64 | 32 | >64 | >64 | >64 | >64 | >64 | >64 | 64 | |
| Helicobacter pylori JIGC360 | R (LVX, MTZ) | 2 | 8 | 16 | >64 | 32 | >64 | >64 | >64 | >64 | >64 | >64 | 64 | |
| Helicobacter pylori 511 | R (LVX, MTZ, CLR) | 2 | 8 | 16 | >64 | 32 | >64 | >64 | >64 | >64 | >64 | >64 | 64 | |
| Acinetobacter baumannii ATCC 19,606 | S | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | |
| Escherichia coli ATCC 25,922 | S | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | |
| Pseudomonas aeruginosa PAO1 | S | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | |
| Gram-Positive Bacteria | Staphylococcus aureus ATCC 25,923 | S | 2 | 32 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 |
| Staphylococcus aureus NEWMAN | S | 4 | 32 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | |
| Staphylococcus aureus USA300 | R (MET) | 4 | 32 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | |
| Staphylococcus aureus NRS271 | R (MET) | 4 | 32 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | |
| Enterococcus faecium ATCC 19,434 | S | 8 | 32 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | |
| Enterococcus faecium 36,235 | R (VAN) | 16 | 32 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | |
| Enterococcus faecium 36,711 | R (VAN) | 16 | 32 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | |
| Enterococcus faecalis ATCC 29,212 | S | 8 | 32 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | |
| Fungus | Candida albicans SC5314 | S | 16 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 |
| Candida albicans C5 | R (VOR, ITRA) | 16 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | |
| Aspergillus fumigatus Af293 | S | 64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, C.; Chen, J.; Liu, J.; Tian, D.; Lan, D.; Liu, T.; Wu, B.; Bi, H.; Tang, J. New Polyketides from a Hydrothermal Vent Sediment Fungus Trichoderma sp. JWM29-10-1 and Their Antimicrobial Effects. Mar. Drugs 2022, 20, 720. https://doi.org/10.3390/md20110720
Lai C, Chen J, Liu J, Tian D, Lan D, Liu T, Wu B, Bi H, Tang J. New Polyketides from a Hydrothermal Vent Sediment Fungus Trichoderma sp. JWM29-10-1 and Their Antimicrobial Effects. Marine Drugs. 2022; 20(11):720. https://doi.org/10.3390/md20110720
Chicago/Turabian StyleLai, Changrong, Jiayi Chen, Jing Liu, Danmei Tian, Donghe Lan, Tongzheng Liu, Bin Wu, Hongkai Bi, and Jinshan Tang. 2022. "New Polyketides from a Hydrothermal Vent Sediment Fungus Trichoderma sp. JWM29-10-1 and Their Antimicrobial Effects" Marine Drugs 20, no. 11: 720. https://doi.org/10.3390/md20110720
APA StyleLai, C., Chen, J., Liu, J., Tian, D., Lan, D., Liu, T., Wu, B., Bi, H., & Tang, J. (2022). New Polyketides from a Hydrothermal Vent Sediment Fungus Trichoderma sp. JWM29-10-1 and Their Antimicrobial Effects. Marine Drugs, 20(11), 720. https://doi.org/10.3390/md20110720