Metabolites Produced by a New Lactiplantibacillus plantarum Strain BF1-13 Isolated from Deep Seawater of Izu-Akazawa Protect the Intestinal Epithelial Barrier from the Dysfunction Induced by Hydrogen Peroxide
Abstract
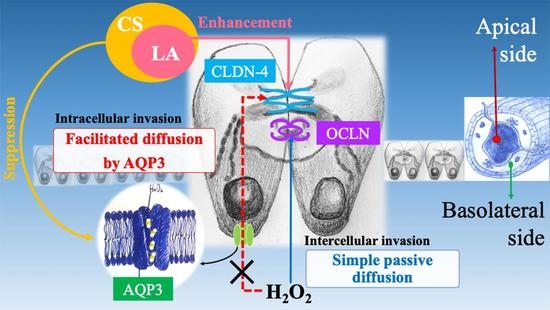
:1. Introduction
2. Results
2.1. Characteristics of Three Lactiplantibacillus Strains
2.2. Protection on the Intestinal Epithelial Barrier by the Metabolites Produced by Strain BF1-13 against the Dysfunction Caused by H2O2 Treatment in Caco-2 Cells
2.3. Enhancement on the TJs-Related Proteins by the Metabolites Produced by Strain BF1-13 from the Suppression Induced by H2O2
2.3.1. Enhancement on CLDN-4 by the Metabolites Produced by Strain BF1-13 through Immunofluorescent Microscopy
2.3.2. Inducement on CLDN-4 mRNA by the Metabolites Produced by Strain BF1-13 through Quantitative RT-PCR
2.4. Suppression on AQP3 by the Metabolites Produced by Strain BF1-13
2.4.1. Suppression on AQP3 by the Metabolites Produced by Strain BF1-13 through Immunofluorescent Microscopy
2.4.2. Suppression on AQP3 mRNA by the Metabolites Produced by Strain BF1-13 through Quantitative RT-PCR
2.5. Effect by LA Contained in the Metabolites Produced by Strain BF1-13 on the Intestinal Epithelial Barrier with H2O2 Treatment
2.5.1. Protection on the Intestinal Epithelial Barrier by LA against the Dysfunction Caused by H2O2 Treatment
2.5.2. Enhancement on TJs-Related Proteins by LA Contained in the Metabolites Produced by Strain BF1-13
2.5.3. Effect on AQP3 by LA Contained in the Metabolites Produced by Strain BF1-13
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Bacterial Strains and 16S rRNA Sequencing
4.3. Cell Culture
4.4. Bacteria Incubation and the Preparation of Metabolites Containing CSs from the Isolated Strains
4.5. Measurement of the Intestinal TJs Barrier Function
4.6. Immunofluorescence Staining
4.7. RNA Extraction and Quantitative RT-PCR
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- König, J.; Wells, J.; Cani, P.; García-Ródenas, C.; MacDonald, T.; Mercenier, A.; Whyte, J.; Troost, F.; Brummer, R. Human Intestinal Barrier Function in Health and Disease. Clin. Transl. Gastroenterol. 2016, 7, e196. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M.; Madsen, K.; Spiller, R.; Van Meerveld, B.; Verne, G. Intestinal Barrier Function in Health and Gastrointestinal Disease. Neurogastroenterol. Motil. 2012, 24, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Natsuga, K. Epidermal Barriers. Cold Spring Harb. Perspect. Med. 2014, 4, a018218. [Google Scholar] [CrossRef] [PubMed]
- Liévin-Le Moal, V.; Servin, A. Pathogenesis of Human Enterovirulent Bacteria: Lessons from Cultured, Fully Differentiated Human Colon Cancer Cell Lines. Microbiol. Mol. Biol. Rev. 2013, 77, 380–439. [Google Scholar] [CrossRef] [Green Version]
- Srinivasan, B.; Kolli, A.; Esch, M.; Abaci, H.; Shuler, M.; Hickman, J. TEER Measurement Techniques For In Vitro Barrier Model Systems. J. Lab. Autom. 2015, 20, 107–126. [Google Scholar] [CrossRef] [Green Version]
- Birben, E.; Sahiner, U.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative Stress and Antioxidant Defense. WAO J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [Green Version]
- Saeidnia, S.; Abdollahi, M. Toxicological and Pharmacological Concerns on Oxidative Stress and Related Diseases. Toxicol. Appl. Pharm. 2013, 273, 442–455. [Google Scholar] [CrossRef]
- Sies, H. Role of metabolic H2O2 generation: Redox signaling and oxidative stress. J. Biol. Chem. 2014, 289, 8735–8741. [Google Scholar] [CrossRef] [Green Version]
- Rao, R. Oxidative Stress-Induced Disruption of Epithelial and Endothelial Tight Junctions. Front. Biosci. 2008, 13, 7210–7226. [Google Scholar] [CrossRef] [Green Version]
- Bienert, G.; Chaumont, F. Aquaporin-Facilitated Transmembrane Diffusion of Hydrogen Peroxide. Biochim. Biophys. Acta 2014, 1840, 1596–1604. [Google Scholar] [CrossRef]
- Jandhyala, S.M.; Talukdar, R.; Subramanyamet, C.; Vuyyuru, H.; Sasikala, M.; Reddy, D.N. Role of the normal gut microbiota. World J. Gastroenterol. 2015, 21, 87887–88803. [Google Scholar] [CrossRef] [PubMed]
- Ghaffar, T.; Irshad, M.; Anwar, Z.; Aqil, T.; Zulifqar, Z.; Tariq, A.; Kamran, M.; Ehsan, N.; Mehmood, S. Recent Trends in Lactic Acid Biotechnology: A Brief Review on Production to Purification. J. Radiat. Res. Appl. Sci. 2014, 7, 222–229. [Google Scholar] [CrossRef]
- Alves de Oliveira, R.; Komesu, A.; Vaz Rossell, C.; Maciel Filho, R. Challenges and Opportunities in Lactic Acid Bioprocess Design—From Economic to Production Aspects. Biochem. Eng. J. 2018, 133, 219–239. [Google Scholar] [CrossRef]
- Grishina, A.; Kulikova, I.; Alieva, L.; Dodson, A.; Rowland, I.; Jin, J. Antigenotoxic Effect of Kefir and Ayran Supernatants on Fecal Water-Induced DNA Damage in Human Colon Cells. Nutr. Cancer. 2011, 63, 73–79. [Google Scholar] [CrossRef]
- Bengoa, A.A.; Iraporda, C.; Garrote, G.L.; Abraham, A.G. Kefir micro-organisms: Their role in grain assembly and health properties of fermented milk. J. Appl. Microbiol. 2018, 126, 686–700. [Google Scholar] [CrossRef] [Green Version]
- Forsyth, G.; Kapitany, R.; Hamilton, D. Organic Acid Proton Donors Decrease Intestinal Secretion Caused by Enterotoxins. Am. J. Physiol. Gastrointest. Liver Physiol. 1981, 241, G227–G234. [Google Scholar] [CrossRef]
- Seth, A.; Yan, F.; Polk, D.; Rao, R. Probiotics Ameliorate the Hydrogen Peroxide-Induced Epithelial Barrier Disruption by A PKC- And MAP Kinase-Dependent Mechanism. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 294, G1060–G1069. [Google Scholar] [CrossRef] [Green Version]
- Miyauchi, E.; O’Callaghan, J.; Buttó, L.; Hurley, G.; Melgar, S.; Tanabe, S.; Shanahan, F.; Nally, K.; O’Toole, P. Mechanism of Protection of Transepithelial Barrier Function By Lactobacillus salivarius: Strain Dependence and Attenuation by Bacteriocin Production. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G1029–G1041. [Google Scholar] [CrossRef] [Green Version]
- Yoda, K.; Miyazawa, K.; Hosoda, M.; Hiramatsu, M.; Yan, F.; He, F. Lactobacillus GG-Fermented Milk Prevents DSS-Induced Colitis and Regulates Intestinal Epithelial Homeostasis through Activation of Epidermal Growth Factor Receptor. Eur. J. Nutr. 2013, 53, 105–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pohanka, M. D-Lactic Acid as A Metabolite: Toxicology, Diagnosis, And Detection. BioMed. Res. Int. 2020, 2020, 3419034. [Google Scholar] [CrossRef] [PubMed]
- Kostinek, M.; Pukall, R.; Rooney, A.; Schillinger, U.; Hertel, C.; Holzapfel, W.; Franz, C. Lactobacillus arizonensis Is a Later Heterotypic Synonym of Lactobacillus plantarum. Int. J. Syst. Evol. Microbiol. 2005, 55, 2485–2489. [Google Scholar] [CrossRef] [Green Version]
- Swezey, J.; Nakamura, L.; Abbott, T.; Peterson, R. Lactobacillus arizonensis Sp. Nov., Isolated from Jojoba Meal. Int. J. Syst. Evol. Microbiol. 2000, 50, 1803–1809. [Google Scholar] [CrossRef] [Green Version]
- Bollag, W.; Aitkens, L.; White, J.; Hyndman, K. Aquaporin-3 in the Epidermis: More Than Skin Deep. Am. J. Physiol. Cell Physiol. 2020, 318, C1144–C1153. [Google Scholar] [CrossRef] [PubMed]
- Rai, T.; Sasaki, S.; Uchida, S. Polarized Trafficking of the Aquaporin-3 Water Channel Is Mediated by An NH2-Terminal Sorting Signal. Am. J. Physiol. Cell Physiol. 2006, 290, C298–C304. [Google Scholar] [CrossRef] [PubMed]
- Blume, L.F.; Denker, M.; Gieseler, F.; Kunze, T. Temperature Corrected Transepithelial Electrical Resistance (TEER) Measurement to Quantify Rapid Changes in Paracellular Permeability. Pharmazie 2010, 65, 19–24. [Google Scholar] [PubMed]
- Landy, J.; Ronde, E.; English, N.; Clark, S.; Hart, A.; Knight, S.; Ciclitira, P.; Al-Hassi, H. Tight Junctions In Inflammatory Bowel Diseases And Inflammatory Bowel Disease Associated Colorectal Cancer. World J. Gastroenterol. 2016, 22, 3117–3126. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, M.S.; Kumar, V.; Al-Abbasi, F.A.; Kamal, M.A.; Anwar, F. Risk of Colorectal Cancer in Inflammatory Bowel Diseases. Semin. Cancer Biol. 2020, 64, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Gu, P.; Feagins, L. Dining With Inflammatory Bowel Disease: A Review of the Literature on Diet in the Pathogenesis and Management of IBD. Inflamm. Bowel Diseases. 2019, 26, 181–191. [Google Scholar] [CrossRef] [Green Version]











| Strain BF1-13 | Strain JCM11125 | Strain H-6 | ||
|---|---|---|---|---|
| Temperature (°C) | 5 | − | − | − |
| 10 | − | − | − | |
| 20 | − | + | + | |
| 27 | + | + | + | |
| 37 | + | + | + | |
| 40 | + | + | + | |
| 45 | − | − | − | |
| pH | 2 | + | + | - |
| 3 | + | + | + | |
| 4 | + | + | + | |
| 6.5 | + | + | + | |
| 9 | + | + | + | |
| 10 | − | − | − | |
| 11 | − | − | − | |
| NaCl (%, m/v) | 0 | + | + | + |
| 1 | + | + | + | |
| 2 | + | + | + | |
| 3 | + | + | + | |
| 5 | + | + | + | |
| 10 | − | − | − | |
| Fermentation substrate | Glucose | + | + | + |
| Sorbitol | + | + | + | |
| Trehalose | + | + | + | |
| Xylose | + | + | + | |
| Arabinose | + | + | + | |
| Mannitol | + | + | + | |
| Lactose | + | + | + |
| Strain | BF1-13 | JCM11125 | H-6 |
|---|---|---|---|
| CFU/mL | 1.67 × 109 | 7.44 × 108 | 8.71 × 108 |
| pH | 4.6 | 4.9 | 4.9 |
| Latic acid (mM) | 26.3 | 19.8 | 20.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diao, X.; Yamada, K.; Shibata, Y.; Imada, C. Metabolites Produced by a New Lactiplantibacillus plantarum Strain BF1-13 Isolated from Deep Seawater of Izu-Akazawa Protect the Intestinal Epithelial Barrier from the Dysfunction Induced by Hydrogen Peroxide. Mar. Drugs 2022, 20, 87. https://doi.org/10.3390/md20020087
Diao X, Yamada K, Shibata Y, Imada C. Metabolites Produced by a New Lactiplantibacillus plantarum Strain BF1-13 Isolated from Deep Seawater of Izu-Akazawa Protect the Intestinal Epithelial Barrier from the Dysfunction Induced by Hydrogen Peroxide. Marine Drugs. 2022; 20(2):87. https://doi.org/10.3390/md20020087
Chicago/Turabian StyleDiao, Xiaozhen, Katsuhisa Yamada, Yuji Shibata, and Chiaki Imada. 2022. "Metabolites Produced by a New Lactiplantibacillus plantarum Strain BF1-13 Isolated from Deep Seawater of Izu-Akazawa Protect the Intestinal Epithelial Barrier from the Dysfunction Induced by Hydrogen Peroxide" Marine Drugs 20, no. 2: 87. https://doi.org/10.3390/md20020087
APA StyleDiao, X., Yamada, K., Shibata, Y., & Imada, C. (2022). Metabolites Produced by a New Lactiplantibacillus plantarum Strain BF1-13 Isolated from Deep Seawater of Izu-Akazawa Protect the Intestinal Epithelial Barrier from the Dysfunction Induced by Hydrogen Peroxide. Marine Drugs, 20(2), 87. https://doi.org/10.3390/md20020087