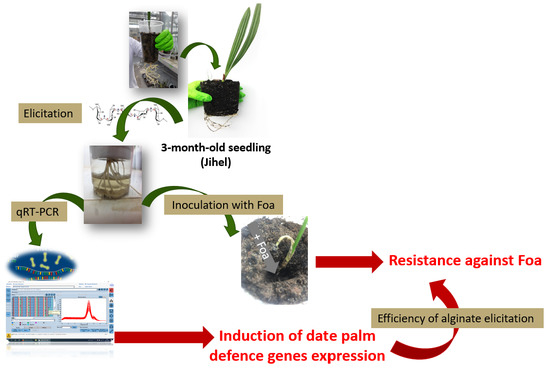
Induction of Defense Gene Expression and the Resistance of Date Palm to Fusarium oxysporum f. sp. Albedinis in Response to Alginate Extracted from Bifurcaria bifurcata
Abstract
:1. Introduction
2. Results
2.1. Date Palm Gene Expression in Response to Alginate Treatment
2.1.1. PAL Activity and Gene Expression
2.1.2. Expression Profile of Genes Involved in Oxidative Burst
2.1.3. PR-Protein Gene Expression
2.2. Effect of Alginate on Fusarium Wilt Disease
2.2.1. Disease Severity
2.2.2. Disease Incidence
2.2.3. Effect of Alginate on Date Palm Death Rate
3. Discussion
4. Materials and Methods
4.1. Extraction and Purification of Alginate
4.2. Elicitation of Date Palm Natural Defenses
4.3. Extraction and Determination of PAL Activity
4.4. Extraction of Total RNA and Synthesis of cDNA
4.5. Primer Design and Efficiency
4.6. Quantitative Real-Time PCR
4.7. Fungal Material
4.8. Inoculation Test
4.9. Disease Evaluation
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- El Modafar, C. Mechanisms of date palm resistance to Bayoud disease: Current state of knowledge and research prospects. Physiol. Mol. Plant Pathol. 2010, 74, 287–294. [Google Scholar] [CrossRef]
- Malençon, G. Le “Bayoud”, maladie fusarienne du palmier-dattier en Afrique du nord. Fruits 1950, 5, 279–289. [Google Scholar]
- Fernandez, D.; Lourd, M.; Ouinten, M.; Geiger, J.P. Le bayoud du palmier dattier Une maladie qui menace la phoeiniciculture. Phytoma Défense Végétaux 1995, 36–39. [Google Scholar]
- Tantaoui, A.; Boisson, C. Compatibilité végétative d’isolats du Fusarium oxysporum f. sp. albedinis et de Fusarium oxysporum de la rhizosphère du Palmier dattier et des sols de palmeraies. Phytopathol. Mediterr. 1991, 30, 155–163. Available online: http://www.jstor.org/stable/42685783 (accessed on 14 January 2022).
- Tantaoui, A.; Fernandez, D. Comparaison entre Fusarium oxysporum f. sp. albedinis et Fusarium oxysporum des sols de palmeraies par l’étude du polymorphisme de longueur des fragments de restriction (RFLP). Phytopathol. Mediterr. 1993, 32, 235–244. Available online: http://www.jstor.org/stable/42685902 (accessed on 14 January 2022).
- Fernandez, D.; Ouinten, M.; Tantaoui, A.; Geiger, J.-P. Molecular records of micro-evolution within the Algerian population of Fusarium oxysporum f. sp. albedinis during its spread to new oases. Eur. J. Plant Pathol. 1997, 103, 485–490. [Google Scholar] [CrossRef]
- Fernandez, D.; Tantaoui, A. Random amplified polymorphic DNA (RAPD) analysis: A tool for rapid characterization of Fusarium oxysporum f. sp. albedinis isolates? Phytopathol. Mediterr. 1994, 33, 223–229. Available online: http://www.jstor.org/stable/42685953 (accessed on 14 January 2022).
- Tantaoui, A.; Ouinten, M.; Geiger, J.-P.; Fernandez, D. Characterization of a single clonal lineage of Fusarium oxysporum f. sp. albedinis causing Bayoud disease of date palm in Morocco. Phytopathology 1996, 86, 787–792. [Google Scholar] [CrossRef] [Green Version]
- Fernandez, D.; Ouinten, M.; Tantaoui, A.; Geiger, J.-P.; Daboussi, M.-J.; Langin, T. Fot 1 insertions in the Fusarium oxysporum f. sp. albedinis genome provide diagnostic PCR targets for detection of the date palm pathogen. Appl. Environ. Microbiol. 1998, 64, 633–636. [Google Scholar] [CrossRef] [Green Version]
- Essarioui, A.; Ben-Amar, H.; Khoulassa, S.; Meziani, R.; Amamou, A.; Mokrini, F. Gestion du Bayoud du palmier dattier dans les oasis marocaines. Rev. Maroc. Sci. Agron. Vétérinaires 2018, 6, 537–543. [Google Scholar]
- Saaidi, M. Comportement au champ de 32 cultivars de palmier dattier vis-à-vis du bayoud: 25 années d’observations. Agronomie 1992, 12, 359–370. [Google Scholar] [CrossRef] [Green Version]
- Saaidi, M. Amelioration genetique du palmier dattier: Criteres de selection, techniques et resultats. Options Méditerranéennes 1990, 11, 133–134. [Google Scholar]
- El Modafar, C.; Tantaoui, A.; El Boustani, E. Time course accumulation and fungitoxicity of date palm phytoalexins towards Fusarium oxysporum f. sp. albedinis. J. Phytopathol. 1999, 147, 477–484. [Google Scholar] [CrossRef]
- Ziouti, A.; El Modafar, C.; El Mandili, A.; El Boustani, E.; Macheix, J.J. Identification des acides caféoylshikimiques des racines du palmier dattier, principaux composés fongitoxiques vis-à-vis de Fusarium oxysporum f. sp. albedinis. J. Phytopathol. 1996, 144, 197–202. [Google Scholar] [CrossRef]
- El Modafar, C.; Tantaoui, A.; El Boustani, E. Effect of caffeoylshikimic acid of date palm roots on activity and production of Fusarium oxysporum f. sp. albedinis cell wall-degrading enzymes. J. Phytopathol. 2000, 148, 101–108. [Google Scholar] [CrossRef]
- El Modafar, C.; Tantaoui, A.; El Boustani, E. Changes in cell wall-bound phenolic compounds and lignin in roots of date palm cultivars differing in susceptibility to Fusarium oxysporum f. sp. albedinis. J. Phytopathol. 2000, 148, 405–411. [Google Scholar] [CrossRef]
- El Modafar, C.; El Boustani, E. Relationship between cell wall susceptibility to cellulases and pectinases of Fusarium oxysporum and susceptibility of date palm cultivars. Biol. Plant. 2000, 43, 571–576. [Google Scholar] [CrossRef]
- El Modafar, C.; El Boustani, E. Cell wall-bound phenolic acid and lignin contents in date palm as related to its resistance to Fusarium oxysporum. Biol. Plant. 2001, 44, 125–130. [Google Scholar] [CrossRef]
- El Modafar, C.; Tantaoui, A.; El Boustani, E.-S. Differential induction of phenylalanine ammonia-lyase activity in date palm roots in response to inoculation with Fusarium oxysporum f. sp. albedinis and to elicitation with fungal wall elicitor. J. Plant Physiol. 2001, 158, 715–722. [Google Scholar] [CrossRef]
- Amraoui, H.; Sedra, M.H.; Hamdaoui, A. Mise en évidence d’enzymes à activité antifongique chez le palmier dattier: Dosages des activités chitinases et β-glucanases, comme réaction au Fusarium oxysporum f. sp. albedinis, agent causal du bayoud. Al Awamia 2005, 116, 18–34. [Google Scholar]
- Renard-Merlier, D.; Randoux, B.; Nowak, E.; Farcy, F.; Durand, R.; Reignault, P. Iodus 40, salicylic acid, heptanoyl salicylic acid and trehalose exhibit different efficacies and defence targets during a wheat/powdery mildew interaction. Phytochemistry 2007, 68, 1156–1164. [Google Scholar] [CrossRef] [PubMed]
- Walters, D.R.; Fountaine, J.M. Practical application of induced resistance to plant diseases: An appraisal of effectiveness under field conditions. J. Agric. Sci. 2009, 147, 523–535. [Google Scholar] [CrossRef]
- Reza, M.; Moghaddam, B.; Van Den Ende, W. Sugars and plant innate immunity. J. Exp. Bot. 2012, 63, 3989–3998. [Google Scholar] [CrossRef] [Green Version]
- Trouvelot, S.; Héloir, M.; Poinssot, B.; Gauthier, A.; Paris, F.; Guillier, C.; Combier, M.; Trdá, L.; Daire, X.; Adrian, M. Carbohydrates in plant immunity and plant protection: Roles and potential application as foliar sprays. Front. Plant Sci. 2014, 5, 592. [Google Scholar] [CrossRef] [Green Version]
- Jamiołkowska, A. Natural Compounds as Elicitors of Plant Resistance Against Diseases and New Biocontrol Strategies. Agronomy 2020, 10, 173. [Google Scholar] [CrossRef] [Green Version]
- Zheng, F.; Chen, L.; Zhang, P.; Zhou, J.; Lu, X.; Tian, W. Carbohydrate polymers exhibit great potential as effective elicitors in organic agriculture: A review. Carbohydr. Polym. 2019, 230, 115637. [Google Scholar] [CrossRef]
- Cook, J.; Zhang, J.; Norrie, J.; Blal, B.; Cheng, Z. Seaweed extract (Stella Maris®) activates innate immune responses in Arabidopsis thaliana and protects host against bacterial pathogens. Mar. Drugs 2018, 16, 221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Somai-Jemmali, L.; Siah, A.; Randoux, B.; Magnin-Robert, M.; Halama, P.; Hamada, W.; Reignault, P. Brown alga Ascophyllum nodosum extract-based product, Dalgin Active®, triggers defense mechanisms and confers protection in both bread and durum wheat against Zymoseptoria tritici. J. Appl. Phycol. 2020, 32, 3387–3399. [Google Scholar] [CrossRef]
- Bouissil, S.; El Alaoui-Talibi, Z.; Pierre, G.; Michaud, P.; El Modafar, C.; Delattre, C. Use of alginate extracted from Moroccan brown algae to stimulate natural defense in date palm roots. Molecules 2020, 25, 720. [Google Scholar] [CrossRef] [Green Version]
- Heil, M.; Bostock, R.M. Induced systemic resistance (ISR) against pathogens in the context of induced plant defences. Ann. Bot. 2002, 89, 503–512. [Google Scholar] [CrossRef] [Green Version]
- El Modafar, C.; Elgadda, M.; El Boutachfaiti, R.; Abouraicha, E.; Zehhar, N.; Petit, E.; El Alaoui-Talibi, Z.; Courtois, B.; Courtois, J. Induction of natural defence accompanied by salicylic acid-dependant systemic acquired resistance in tomato seedlings in response to bioelicitors isolated from green algae. Sci. Hortic. (Amst.) 2012, 138, 55–63. [Google Scholar] [CrossRef]
- Zhang, C.; Howlader, P.; Liu, T.; Sun, X.; Jia, X.; Zhao, X.; Shen, P.; Qin, Y.; Wang, W.; Yin, H. Alginate Oligosaccharide (AOS) induced resistance to Pst DC3000 via salicylic acid-mediated signaling pathway in Arabidopsis thaliana. Carbohydr. Polym. 2019, 225, 115221. [Google Scholar] [CrossRef]
- Chaliha, C.; Rugen, M.D.; Field, R.A.; Kalita, E. Glycans as modulators of plant defense against filamentous pathogens. Front. Plant Sci. 2018, 9, 928. [Google Scholar] [CrossRef]
- Ben Salah, I.; Aghrouss, S.; Douira, A.; Aissam, S.; El Alaoui-Talibi, Z.; Filali-Maltouf, A.; El Modafar, C. Seaweed polysaccharides as bio-elicitors of natural defenses in olive trees against verticillium wilt of olive. J. Plant Interact. 2018, 13, 248–255. [Google Scholar] [CrossRef]
- Abouraïcha, E.; El Alaoui-Talibi, Z.; El Boutachfaiti, R.; Petit, E.; Courtois, B.; Courtois, J.; El Modafar, C. Induction of natural defense and protection against Penicillium expansum and Botrytis cinerea in apple fruit in response to bioelicitors isolated from green algae. Sci. Hortic. (Amst.) 2015, 181, 121–128. [Google Scholar] [CrossRef]
- Abouraïcha, E.F.; El Alaoui-talibi, Z.; Tadlaoui-ouafi, A.; El Boutachfaiti, R.; Petit, E.; Douira, A.; Courtois, B.; Courtois, J.; El Modafar, C. Glucuronan and oligoglucuronans isolated from green algae activate natural defense responses in apple fruit and reduce postharvest blue and gray mold decay. J. Appl. Phycol. 2016, 29, 471–480. [Google Scholar] [CrossRef]
- Aitouguinane, M.; Bouissil, S.; Mouhoub, A.; Rchid, H.; Fendri, I.; Abdelkafi, S.; El-hadj, M.D.O.; Boual, Z.; Dubessay, P.; Gardarin, C.; et al. Induction of Natural Defenses in Tomato Seedlings by Using Alginate and Oligoalginates Derivatives Extracted from Moroccan Brown Algae. Mar. Drugs 2020, 18, 521. [Google Scholar] [CrossRef]
- El Modafar, C.; El Boustani, E. The role of phenolics in plant defense mechanisms. In Biopesticides of Plant Origin; Roger, R., Ed.; Lavoisier Publishing: Paris, France, 2005; pp. 157–172. [Google Scholar]
- Benhamou, N.; Rey, P. Stimulateurs des défenses naturelles des plantes: Une nouvelle stratégie phytosanitaire dans un contexte d’écoproduction durable. I. Principes de la résistance induite. Phytoprotection 2012, 92, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Lehman, S.; Serrano, M.; L’Haridon, F.; Tjiamos, S.E.; Metraux, J.P. Reactive oxygen species and plant resistance to fungal pathogen. Phytochemistry 2015, 112, 54–62. [Google Scholar] [CrossRef] [Green Version]
- Dey, N.; Roy, U.K.; Aditya, M.; Bhattacharjee, S. Defensive strategies of ROS in Programmed Cell Death associated with hypertensive response in plant pathogenesis. Ann. Syst. Biol. 2020, 3, 1–9. [Google Scholar]
- Camejo, D.; Guzmán-Cedeño, A.; Vera-Macias, L.; Jiménez, A. Oxidative post-translational modifications controlling plant-pathogen interaction. Plant Physiol. Biochem. 2019, 144, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Orozco-Cárdenas, M.L.; Narváez-Vásquez, J.; Ryan, C.A. Hydrogen peroxide acts as a second messenger for the induction of defense genes in tomato plants in response to wounding, systemin, and methyl jasmonate. Plant Cell 2001, 13, 179–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khedia, J.; Agarwal, P.; Agarwal, P.K. Deciphering hydrogen peroxide-induced signalling towards stress tolerance in plants. 3 Biotech 2019, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, Q.; Jiang, H.; Wang, B.; Bi, Y.; Li, Y.; Prusky, D. Reactive oxygen species-mediated the accumulation of suberin polyphenolics and lignin at wound sites on muskmelons elicited by benzo (1, 2, 3)-thiadiazole-7-carbothioic acid S-methyl ester. Postharvest Biol. Technol. 2020, 170, 111325. [Google Scholar] [CrossRef]
- Deboever, E.; Deleu, M.; Mongrand, S.; Lins, L.; Fauconnier, M.-L. Plant–pathogen interactions: Underestimated roles of phyto-oxylipins. Trends Plant Sci. 2020, 25, 22–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camejo, D.; Guzmán-Cedeño, Á.; Moreno, A. Reactive oxygen species, essential molecules, during plant–pathogen interactions. Plant Physiol. Biochem. 2016, 103, 10–23. [Google Scholar] [CrossRef]
- Vidhyasekaran, P. Plant Innate Immunity Signals and Signaling Systems: Bioengineering and Molecular Manipulation for Crop Disease Management; Springer Nature: Basingstoke, UK, 2020; ISBN 9402419403. [Google Scholar] [CrossRef]
- Davies, K.J.A. Oxidative stress, antioxidant defenses, and damage removal, repair, and replacement systems. IUBMB Life 2000, 50, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, S.; Singh, S.P.; Upadhyay, S.K. Role of superoxide dismutases (SODs) in stress tolerance in plants. In Molecular Approaches in Plant Biology and Environmental Challenges; Springer: Berlin/Heidelberg, Germany, 2019; pp. 51–77. [Google Scholar] [CrossRef]
- Kidwai, M.; Ahmad, I.Z.; Chakrabarty, D. Class III peroxidase: An indispensable enzyme for biotic/abiotic stress tolerance and a potent candidate for crop improvement. Plant Cell Rep. 2020, 39, 1381–1393. [Google Scholar] [CrossRef] [PubMed]
- Thakur, M.; Udayashankar, A.C. Lipoxygenases and their function in plant innate mechanism. In Bioactive Molecules in Plant Defense; Springer: Cham, Switzerland, 2019; pp. 133–143. [Google Scholar] [CrossRef]
- Viswanath, K.K.; Varakumar, P.; Pamuru, R.R.; Basha, S.J.; Mehta, S.; Rao, A.D. Plant lipoxygenases and their role in plant physiology. J. Plant Biol. 2020, 63, 83–95. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, F.; Melotto, M.; Yao, J.; He, S.Y. Jasmonate signaling and manipulation by pathogens and insects. J. Exp. Bot. 2017, 68, 1371–1385. [Google Scholar] [CrossRef]
- Jain, D.; Khurana, J.P. Role of pathogenesis-related (PR) proteins in plant defense mechanism. In Molecular Aspects of Plant-Pathogen Interaction; Springer: Berlin/Heidelberg, Germany, 2018; pp. 265–281. [Google Scholar] [CrossRef]
- Huang, L.; Shen, M.; Morris, G.A.; Xie, J. Sulfated polysaccharides: Immunomodulation and signaling mechanisms. Trends Food Sci. Technol. 2019, 92, 1–11. [Google Scholar] [CrossRef]
- Andryukov, B.G.; Besednova, N.N.; Kuznetsova, T.A.; Zaporozhets, T.S.; Ermakova, S.P.; Zvyagintseva, T.N.; Chingizova, E.A.; Gazha, A.K.; Smolina, T.P. Sulfated polysaccharides from marine algae as a basis of modern biotechnologies for creating wound dressings: Current achievements and future prospects. Biomedicines 2020, 8, 301. [Google Scholar] [CrossRef]
- Muthukumar, J.; Chidambaram, R.; Sukumaran, S. Sulfated polysaccharides and its commercial applications in food industries—A review. J. Food Sci. Technol. 2021, 58, 2453–2466. [Google Scholar] [CrossRef]
- Aziz, A.; Poinssot, B.; Daire, X.; Adrian, M.; Bézier, A.; Lambert, B.; Joubert, J.-M.; Pugin, A. Laminarin Elicits Defense Responses in Grapevine and Induces Protection Against Botrytis cinerea and Plasmopara viticola. Mol. Plant-Microbe Interact. 2003, 16, 1118–1128. [Google Scholar] [CrossRef] [Green Version]
- Gauthier, A.; Trouvelot, S.; Kelloniemi, J.; Frettinger, P.; Wendehenne, D.; Daire, X.; Joubert, J.-M.; Ferrarini, A.; Delledonne, M.; Flors, V. The sulfated laminarin triggers a stress transcriptome before priming the SA-and ROS-dependent defenses during grapevine’s induced resistance against Plasmopara viticola. PLoS ONE 2014, 9, e88145. [Google Scholar] [CrossRef]
- Shine, M.B.; Xiao, X.; Kachroo, P.; Kachroo, A. Signaling mechanisms underlying systemic acquired resistance to microbial pathogens. Plant Sci. 2019, 279, 81–86. [Google Scholar] [CrossRef]
- Panpatte, D.G.; Jhala, Y.K.; Vyas, R.V. Signaling pathway of induced systemic resistance. In Molecular Aspects of Plant Beneficial Microbes in Agriculture; Elsevier: Amsterdam, The Netherlands, 2020; pp. 133–141. [Google Scholar] [CrossRef]
- Chadha, S. Plant–Microbe Interaction: Gene-to-Metabolite Network. In Bioactive Molecules in Plant Defense; Springer: Berlin/Heidelberg, Germany, 2019; pp. 75–100. [Google Scholar] [CrossRef]
- Abdul Malik, N.A.; Kumar, I.S.; Nadarajah, K. Elicitor and receptor molecules: Orchestrators of plant defense and immunity. Int. J. Mol. Sci. 2020, 21, 963. [Google Scholar] [CrossRef] [Green Version]
- Zebelo, S.A. Decrypting Early Perception of Biotic Stress on Plants. In Co-Evolution of Secondary Metabolites; Springer Nature: Basingstoke, UK, 2020; pp. 577–592. [Google Scholar] [CrossRef]
- Bentham, A.R.; De la Concepcion, J.C.; Mukhi, N.; Zdrzałek, R.; Draeger, M.; Gorenkin, D.; Hughes, R.K.; Banfield, M.J. A molecular roadmap to the plant immune system. J. Biol. Chem. 2020, 295, 14916–14935. [Google Scholar] [CrossRef] [PubMed]
- Nishad, R.; Ahmed, T.; Rahman, V.J.; Kareem, A. Modulation of plant defense system in response to microbial interactions. Front. Microbiol. 2020, 11, 1298. [Google Scholar] [CrossRef]
- Peng, Y.; van Wersch, R.; Zhang, Y. Convergent and divergent signaling in PAMP-triggered immunity and effector-triggered immunity. Mol. Plant-Microbe Interact. 2018, 31, 403–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dey, P.; Ramanujam, R.; Venkatesan, G.; Nagarathnam, R. Sodium alginate potentiates antioxidant defense and PR proteins against early blight disease caused by Alternaria solani in Solanum. PLoS ONE 2019, 14, e0223216. [Google Scholar] [CrossRef] [Green Version]
- Soukaina, B.; Zainab, E.A.-T.; Guillaume, P.; Halima, R.; Philippe, M.; Cherkaoui, E.M.; Cédric, D. Radical depolymerization of alginate extracted from moroccan brown seaweed bifurcaria bifurcata. Appl. Sci. 2020, 10, 4166. [Google Scholar] [CrossRef]
- Yaish, M.W.; Patankar, H.V.; Assaha, D.V.M.; Zheng, Y.; Al-Yahyai, R.; Sunkar, R. Genome-wide expression profiling in leaves and roots of date palm (Phoenix dactylifera L.) exposed to salinity. BMC Genom. 2017, 18, 246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Sedra, M.H.; Besri, M. Évaluation de la résistance du palmier dattier au bayoud causé par Fusarium oxysporum f sp albedinis. Recherche d’une méthode de discrimination des vitroplants acclimatés en serre. Agronomie 1994, 14, 467–472. [Google Scholar] [CrossRef]
- Boutaj, H.; Chakhchar, A.; Meddich, A.; Wahbi, S.; El, Z.; Talibi, A. Bioprotection of olive tree from Verticillium wilt by autochthonous endomycorrhizal fungi. J. Plant Dis. Prot. 2020, 127, 349–357. [Google Scholar] [CrossRef]
- Campbell, C.L.; Madden, L.V. Introduction to Plant Disease Epidemiology; John Wiley & Sons.: Hoboken, NJ, USA, 1990; ISBN 0471832367. [Google Scholar]







| Disease Incidence (%) | PDP 1 (%) | FMS 2 | AUDPC 3 (%) | |
|---|---|---|---|---|
| Foa | 65 a | 50 a | 2.11 a | 40.35 a |
| Laminarin | 52.5 ab | 47.5 a | 1.71 b | 32.11 b |
| Alginate | 47.5 b | 12.5 b | 0.68 c | 13.86 c |
| Genes | Primers |
|---|---|
| PAL785 | F:5′-GGGATTGGAAAAGTCTGCAG-3′ R:5′-ACCACATGTACCCATAGCC-3′ |
| LOX655 | F: 5′-AGGCCTCCAACCAATACAG-3′ R: 5′-TCGTGGAAGGCCTTGAAGT-3′ |
| POD304/305 | F: 5′-TTCTCTCAGGTGGGCATACAA-3′ R: 5′-AAAGCTCCCAGGATCCATTT-3′ |
| SOD751 | F: 5′-TCCATGCCGCCCAGGTCT-3′ R: 5′-CATCTAACCTATTCGCCTTG-3′ |
| GLUC438 | F: 5′-CGGCCCATCAGACTCCAA-3′ R: 5′-GCCTCAATTACCAATTTTGCA-3′ |
| CHIT070 | F: 5′-CCATGAAACAACTGGTGGG-3′ R: 5′-TTTACCAGCCGGTCCATAG-3′ |
| eEF1a [71] | F: 5′-GATCCCTTCCTACACTCGAATCC-3′ R: 5′-TCCTTTCCCATTGGTATTTGCT-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouissil, S.; Guérin, C.; Roche, J.; Dubessay, P.; El Alaoui-Talibi, Z.; Pierre, G.; Michaud, P.; Mouzeyar, S.; Delattre, C.; El Modafar, C. Induction of Defense Gene Expression and the Resistance of Date Palm to Fusarium oxysporum f. sp. Albedinis in Response to Alginate Extracted from Bifurcaria bifurcata. Mar. Drugs 2022, 20, 88. https://doi.org/10.3390/md20020088
Bouissil S, Guérin C, Roche J, Dubessay P, El Alaoui-Talibi Z, Pierre G, Michaud P, Mouzeyar S, Delattre C, El Modafar C. Induction of Defense Gene Expression and the Resistance of Date Palm to Fusarium oxysporum f. sp. Albedinis in Response to Alginate Extracted from Bifurcaria bifurcata. Marine Drugs. 2022; 20(2):88. https://doi.org/10.3390/md20020088
Chicago/Turabian StyleBouissil, Soukaina, Claire Guérin, Jane Roche, Pascal Dubessay, Zainab El Alaoui-Talibi, Guillaume Pierre, Philippe Michaud, Said Mouzeyar, Cédric Delattre, and Cherkaoui El Modafar. 2022. "Induction of Defense Gene Expression and the Resistance of Date Palm to Fusarium oxysporum f. sp. Albedinis in Response to Alginate Extracted from Bifurcaria bifurcata" Marine Drugs 20, no. 2: 88. https://doi.org/10.3390/md20020088
APA StyleBouissil, S., Guérin, C., Roche, J., Dubessay, P., El Alaoui-Talibi, Z., Pierre, G., Michaud, P., Mouzeyar, S., Delattre, C., & El Modafar, C. (2022). Induction of Defense Gene Expression and the Resistance of Date Palm to Fusarium oxysporum f. sp. Albedinis in Response to Alginate Extracted from Bifurcaria bifurcata. Marine Drugs, 20(2), 88. https://doi.org/10.3390/md20020088