Disulfated Ophiuroid Type Steroids from the Far Eastern Starfish Pteraster marsippus and Their Cytotoxic Activity on the Models of 2D and 3D Cultures
Abstract
:1. Introduction
2. Results
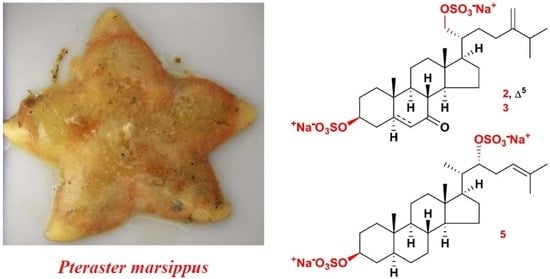
2.1. The Isolation and Structure Elucidation of Compounds 1–5 from P. marsippus
2.2. In Vitro Anticancer Activity of Compounds 1–5
3. Materials and Methods
3.1. General Procedures
3.2. Animal Material
3.3. Extraction and Isolation
3.4. Compound Characterization Data
3.5. Solvolysis of the Mixture of 2 and 3
3.6. Bioactivity Assay
3.6.1. Cell Lines
3.6.2. Cell Culture Conditions
3.6.3. Preparation of Compounds for the Determination of Cytotoxic Activity
3.6.4. Formation of 3D Spheroids by Liquid Overlay Technique (LOT)
3.6.5. Cytotoxic Activity Assay (MTS)
2D Cell Culture (Monolayer)
3D Cell Culture (Spheroids)
3.6.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kornprobst, J.M.; Sallenave, C.; Barnathan, G. Sulfated compounds from marine organisms. Comp. Biochem. Physiol. 1998, 119B, 1–51. [Google Scholar] [CrossRef]
- Stonik, V.A. Marine polar steroids. Russ. Chem. Rev. 2001, 70, 673–715. [Google Scholar] [CrossRef]
- Carvalhal, F.; Correia-da-Silva, M.; Sousa, E.; Pinto, M.; Kijjoa, A. SULFATION PATHWAYS: Sources and biological activities of marine sulfated steroids. J. Mol. Endocrinol. 2018, 61, T211–T231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minale, L.; Riccio, R.; Zollo, F. Steroidal oligoglycosides and polyhydroxysteroids from Echinoderms. Fortschr. Chem. Org. Naturst. 1993, 62, 75–308. [Google Scholar] [CrossRef]
- Stonik, V.A.; Ivanchina, N.V.; Kicha, A.A. New polar steroids from starfish. Nat. Prod. Commun. 2008, 3, 1587–1610. [Google Scholar] [CrossRef] [Green Version]
- Ivanchina, N.V.; Kicha, A.A.; Stonik, V.A. Steroid glycosides from marine organisms. Steroids 2011, 76, 425–454. [Google Scholar] [CrossRef] [PubMed]
- Dong, G.; Xu, T.H.; Yang, B.; Lin, X.P.; Zhou, X.F.; Yang, X.W.; Liu, Y.H. Chemical constituents and bioactivities of starfish. Chem. Biodivers. 2011, 8, 740–791. [Google Scholar] [CrossRef]
- Ivanchina, N.V.; Kicha, A.A.; Malyarenko, T.V.; Stonik, V.A. Advances in Natural Products Discovery; Gomes, A.R., Rocha-Santos, T., Duarte, A., Eds.; Nova Science Publishers: Hauppauge, NY, USA, 2017; Volume 6, pp. 191–224. [Google Scholar]
- Xia, J.M.; Miao, Z.; Xie, C.L.; Zhang, J.W.; Yang, X.W. Chemical constituents and bioactivities of starfishes: An update. Chem. Biodivers. 2020, 17, e1900638. [Google Scholar] [CrossRef] [Green Version]
- Stonik, V.A.; Kicha, A.A.; Malyarenko, T.V.; Ivanchina, N.V. Asterosaponins: Structures, taxonomic distribution, biogenesis and biological activities. Mar. Drugs 2020, 18, 584. [Google Scholar] [CrossRef]
- D’Auria, M.V.; Finamore, E.; Minale, L.; Pizza, C.; Riccio, R.; Zollo, F.; Pusset, M.; Tirard, P. Steroids from the starfish Euretaster insignis: A novel group of sulphated 3β,21-dihydroxysteroids. J. Chem. Soc. Perkin Trans. 1984, 1, 2277–2282. [Google Scholar] [CrossRef]
- Levina, E.V.; Andriyaschenko, P.V.; Stonik, V.A.; Kalinovsky, A.I. Ophiuroid-type steroids in starfish of the genus Pteraster. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1996, 114B, 49–52. [Google Scholar] [CrossRef]
- Levina, E.V.; Andriyaschenko, P.V.; Kalinovsky, A.I.; Stonik, V.A. New ophiuroid-type steroids from the starfish Pteraster tesselatus. J. Nat. Prod. 1998, 61, 1423–1426. [Google Scholar] [CrossRef] [PubMed]
- Levina, E.V.; Andriyashchenko, P.V.; Kalinovsky, A.I.; Dmitrenok, P.S.; Stonik, V.A. Steroid compounds from the Far Eastern starfish Diplopteraster multipes. Russ. J. Bioorgan. Chem. 2002, 28, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Ivanchina, N.V.; Kicha, A.A.; Kalinovsky, A.I.; Dmitrenok, P.S.; Stonik, V.A. Hemolytic steroid disulfates from the Far Eastern starfish Pteraster pulvillus. J. Nat. Prod. 2003, 66, 298–301. [Google Scholar] [CrossRef] [PubMed]
- Levina, E.V.; Kalinovsky, A.I.; Dmitrenok, P.S. Steroid compounds from the Far East starfish Pteraster obscurus and the ophiura Asteronyx loveni. Russ. J. Bioorg. Chem. 2007, 33, 341–346. [Google Scholar] [CrossRef]
- Fu, X.; Schmitz, F.J.; Lee, R.H.; Papkoff, J.S.; Slate, D.L. Inhibitors of protein tyrosine kinase pp60v-src: Sterol sulfates from the brittle star Ophiarachna incrassata. J. Nat. Prod. 1994, 57, 1591–1594. [Google Scholar] [CrossRef]
- McKee, T.C.; Cardellina, J.H.; Riccio, R.; D’Auria, M.V.; Iorizzi, M.; Minale, L.; Moran, R.A.; Gulakowski, R.J.; McMahon, J.B.; Buckheit, R.W.; et al. HIV-inhibitory natural products. 11. Comparative studies of sulfated sterols from marine invertebrate. J. Med. Chem. 1994, 37, 793–797. [Google Scholar] [CrossRef]
- Sepe, V.; Bifulco, G.; Renga, B.; D’Amore, C.; Fiorucci, S.; Zampella, A. Discovery of sulfated sterols from marine invertebrates as a new class of marine natural antagonists of farnesoid-X-receptor. J. Med. Chem. 2011, 54, 1314–1320. [Google Scholar] [CrossRef]
- Gazha, A.K.; Ivanushko, L.A.; Levina, E.V.; Fedorov, S.N.; Zaporozets, T.S.; Stonik, V.A.; Besednova, N.N. Steroid sulfates from ophiuroids (brittle stars): Action on some factors of innate and adaptive immunity. Nat. Prod. Commun. 2016, 11, 749–752. [Google Scholar] [CrossRef] [Green Version]
- Kicha, A.A.; Kalinovsky, A.I.; Antonov, A.S.; Radchenko, O.S.; Ivanchina, N.V.; Malyarenko, T.V.; Savchenko, A.M.; Stonik, V.A. Determination of C-23 configuration in (20R)-23-hydroxycholestane side chain of steroid compounds by 1H and 13C NMR spectroscopy. Nat. Prod. Commun. 2013, 8, 1219–1222. [Google Scholar] [CrossRef] [Green Version]
- D’Auria, M.V.; Riccio, R.; Minale, L.; La Barre, S.; Pusset, J. Novel marine steroid sulfates from Pacific ophiuroids. J. Org. Chem. 1987, 52, 3947–3952. [Google Scholar] [CrossRef]
- Hamdy, A.-H.A.; Aboutabl, E.A.; Sameer, S.; Hussein, A.A.; Díaz-Marrero, A.R.; Darias, J.; Cueto, M. 3-Keto-22-epi-28-nor-cathasterone, a brassinosteroid-related metabolite from Cystoseira myrica. Steroids 2009, 74, 927–930. [Google Scholar] [CrossRef] [PubMed]
- Nes, W.R.; Varkey, T.E.; Krevitz, K. The stereochemistry of sterols at C-20 and its biosynthetic implications. J. Am. Chem. Soc. 1977, 99, 260–262. [Google Scholar] [CrossRef] [PubMed]
- Vanderach, D.J.; Djerassi, C. Marine natural products. Synthesis of four naturally occurring 20.beta.-H cholanic acid derivatives. J. Org. Chem. 1978, 43, 1442–1448. [Google Scholar] [CrossRef]
- Amann, A.; Ourisson, G.; Luu, B. A novel stereospecific synthesis of 22-hydroxylated triterpenes and steroids: Syntheses of 22R-hydroxylanosterol and 22R-hydroxydesmosterol. Synthesis 1987, 1987, 696–700. [Google Scholar] [CrossRef]
- Hietter, H.; Trifilieff, E.; Richert, L.; Beck, J.-P.; Luu, B.; Ourisson, G. Antagonistic action of cholesterol towards the toxicity of hydroxysterols on cultured hepatoma cells. Biochem. Biophys. Res. Commun. 1984, 120, 657–664. [Google Scholar] [CrossRef]
- Ivanchina, N.V.; Kicha, A.A.; Malyarenko, T.V.; Kalinovsky, A.I.; Dmitrenok, P.S.; Stonik, V.A. Biosynthesis of polar steroids from the Far Eastern starfish Patiria (=Asterina) pectinifera. Cholesterol and cholesterol sulfate are converted into polyhydroxylated sterols and monoglycoside asterosaponin P1 in feeding experiments. Steroids 2013, 78, 1183–1191. [Google Scholar] [CrossRef]
- Amelian, A.; Wasilewska, K.; Megias, D.; Winnicka, K. Application of standard cell cultures and 3D in vitro tissue models as an effective tool in drug design and development. Pharmacol. Rep. 2017, 69, 861–870. [Google Scholar] [CrossRef]
- Edmondson, R.; Broglie, J.J.; Adcock, A.F.; Yang, L. Three-dimensional cell culture systems and their applications in drug discovery and cell-based biosensors. Assay Drug Dev. Technol. 2014, 12, 207–218. [Google Scholar] [CrossRef] [Green Version]
- AAT Bioquest. Available online: https://www.aatbio.com/tools/ic50-calculator (accessed on 10 November 2020).





| Position | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| 1β α | 1.89 dt (13.8, 3.7) 1.11 m | 2.02 dt (13.9, 3.7) 1.26 td (13.9, 3.5) | 1.81 dt (13.9, 3.5) 1.07 td (13.9, 3.7) | 1.76 dt (13.8, 3.7) 0.98 td (13.8, 3.8) | 1.89 dt (13.5, 3.5) 1.11 m |
| 2α β | 2.05 m 1.63 m | 2.15 m 1.76 m | 2.06 m 1.60 m | 2.02 m 1.53 m | 2.05 m 1.62 m |
| 3 | 4.13 m (ΔW = 39.3 Hz) | 4.26 m | 4.25 m | 4.24 m | 4.13 m |
| 4α β | 2.53 ddd (13.2, 4.8, 2.2) 2.34 td (13.2, 2.0) | 2.79 ddd (14.0, 5.0, 2.3) 2.54 ddd (14.0, 11.8, 1.9) | 1.86 m 1.57 m | 1.81 m 1.43 m | 2.53 ddd (13.4, 4.8, 2.2) 2.33 m |
| 5 | – | – | 1.52 m | 1.22 m | – |
| 6β α | 5.38 m | 5.68 br d (1.6) | 2.46 t (12.3) 1.94 dd (12.3, 3.2) | 1.33 t (12.8) 1.55 m | 5.38 m |
| 7β α | 1.97 m 1.56 m | – | – | 3.25 td (10.6, 5.2) | 1.96 m 1.54 m |
| 8 | 1.48 m | 2.31 dd (12.6, 10.7) | 2.47 m | 1.40 m | 1.47 m |
| 9 | 0.97 td (11.7, 4.6) | 1.53 m | 1.09 m | 0.71 m | 0.96 m |
| 10 | – | – | – | – | – |
| 11α β | 1.57 m 1.04 m | 1.63 m | 1.62 m 1.55 m | 1.58 m 1.35 m | 1.54 m 1.05 m |
| 12β α | 2.04 m 1.25 td (13.0, 4.2) | 2.07 m 1.22 m | 2.02 m 1.16 m | 1.99 m 1.19 m | 2.01 dt (12.8, 3.5) 1.23 td (12.8, 4.2) |
| 13 | – | – | – | – | – |
| 14 | 1.06 m | 1.35 m | 1.47 m | 1.19 m | 1.09 m |
| 15α β | 1.64 m 1.13 m | 2.40 m 1.28 m | 2.20 m 1.02 m | 1.90 m 1.48 m | 1.61 m 1.09 m |
| 16α β | 1.87 m 1.37 m | 1.90 m 1.39 m | 1.88 m 1.35 m | 1.83 m 1.34 m | 2.22 m 1.16 m |
| 17 | 1.48 m | 1.48 m | 1.48 m | 1.43 m | 1.63 m |
| 18 | 0.75 s | 0.75 s | 0.72 s | 0.73 s | 0.70 s |
| 19 | 1.03 s | 1.24 s | 1.12 s | 0.86 s | 1.02 s |
| 20 | 1.72 m | 1.69 m | 1.69 m | 1.68 m | 1.58 m |
| 21 | 4.21 dd (9.8, 3.7) 3.94 dd (9.8, 6.4) | 4.18 dd (9.6, 4.0) 3.99 dd (9.6, 5.7) | 4.17 dd (9.6, 4.1) 3.96 dd (9.6, 5.7) | 4.18 dd (9.7, 3.8) 3.94 dd (9.7, 6.2) | 0.96 d (6.7) |
| 22 | 1.64 m 1.48 m | 1.64 m 1.49 m | 1.64 m 1.49 m | 1.64 m 1.48 m | 4.36 dd (10.6, 4.5) |
| 23 | 2.17 ddd (15.0, 11.1, 4.6) 2.04 m | 2.19 m 2.02 m | 2.19 m 2.02 m | 2.17 m 2.03 m | 2.66 m 2.34 m |
| 24 | – | – | – | – | 5.05 t (7.7) |
| 25 | 2.25 quin (6.7) | 2.25 m | 2.25 m | 2.25 quin | – |
| 26 | 1.02 d (6.8) | 1.02 d (6.8) | 1.02 d (6.8) | 1.02 d (6.8) | 1.69 s |
| 27 | 1.03 d (6.8) | 1.03 d (6.8) | 1.03 d (6.8) | 1.03 d (6.8) | 1.65 s |
| 28 | 4.71 br s 4.68 br d (1.3) | 4.71 br d (1.2) 4.69 br d (1.2) | 4.71 br d (1.2) 4.69 br d (1.2) | 4.71 br s 4.68 br d (1.5) |
| Position | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| 1 | 38.5 | 37.4 | 37.1 | 38.1 | 38.4 |
| 2 | 30.0 | 29.6 | 29.4 | 29.7 | 30.0 |
| 3 | 79.9 | 78.0 | 78.8 | 79.4 | 79.9 |
| 4 | 40.4 | 40.1 | 36.2 | 35.9 | 40.4 |
| 5 | 141.7 | 168.3 | 48.2 | 43.6 | 141.6 |
| 6 | 123.2 | 126.8 | 46.9 | 39.9 | 123.3 |
| 7 | 33.0 | 204.4 | 214.3 | 75.7 | 33.0 |
| 8 | 33.3 | 46.6 | 51.1 | 44.1 | 33.3 |
| 9 | 51.7 | 51.4 | 56.7 | 54.0 | 51.6 |
| 10 | 37.7 | 39.7 | 37.0 | 36.0 | 37.7 |
| 11 | 22.1 | 22.2 | 22.9 | 22.6 | 22.1 |
| 12 | 40.2 | 39.1 | 39.2 | 40.5 | 41.0 |
| 13 | 43.4 | 44.2 | 43.6 | 44.5 | 43.3 |
| 14 | 58.0 | 51.3 | 50.4 | 57.3 | 58.0 |
| 15 | 25.2 | 27.3 | 25.9 | 27.9 | 25.4 |
| 16 | 28.7 | 28.9 | 28.8 | 29.0 | 29.2 |
| 17 | 51.8 | 50.7 | 50.7 | 51.2 | 53.2 |
| 18 | 12.5 | 12.7 | 12.6 | 12.9 | 12.1 |
| 19 | 19.7 | 17.6 | 12.0 | 12.7 | 19.7 |
| 20 | 41.1 | 41.0 | 41.0 | 41.0 | 39.4 |
| 21 | 69.3 | 69.2 | 69.2 | 69.4 | 12.8 |
| 22 | 29.7 | 29.8 | 29.8 | 29.7 | 82.6 |
| 23 | 31.6 | 31.8 | 31.8 | 31.8 | 31.7 |
| 24 | 157.9 | 157.8 | 157.8 | 157.9 | 121.2 |
| 25 | 34.9 | 34.8 | 34.8 | 34.9 | 134.8 |
| 26 | 22.4 | 22.3 | 22.4 | 22.3 | 26.0 |
| 27 | 22.5 | 22.5 | 22.5 | 22.5 | 18.1 |
| 28 | 106.9 | 107.0 | 107.8 | 106.9 |
| Position | 2a | 3a | ||
|---|---|---|---|---|
| δH | δC | δH | δC | |
| 1β α | 1.98 m 1.22 m | 37.6 | 1.77 m 1.03 m | 37.3 |
| 2α β | 1.89 m 1.61 m | 31.9 | 1.80 m 1.46 m | 31.8 |
| 3 | 3.54 m | 71.2 | 3.52 m | 71.3 |
| 4α β | 2.48 ddd (13.5, 4.6, 2.1) 2.39 ddd (13.5, 11.5, 2.0) | 42.8 | 1.56 m 1.44 m | 48.4 |
| 5 | – | 169.1 | 1.48 m | 169.1 |
| 6 | 5.65 m | 126.3 | 2.45 t (13.0) 1.92 dd (13.0, 3.2) | 47.0 |
| 7 | – | 204.6 | – | 214.4 |
| 8 | 2.31 dd (12.8, 10.8) | 46.6 | 2.47 t (12.1) | 51.1 |
| 9 | 1.51 m | 51.6 | 1.08 m | 57.0 |
| 10 | – | 39.7 | – | 37.2 |
| 11 | 1.64 m | 22.3 | 1.62 m 1.56 m | 22.9 |
| 12β α | 1.97 m 1.19 m | 39.3 | 1.92 m 1.14 m | 39.4 |
| 13 | – | 44.2 | – | 43.6 |
| 14 | 1.32 m | 51.3 | 1.40 m | 50.4 |
| 15α β | 2.39 m 1.28 m | 27.3 | 2.19 m 1.01 m | 25.9 |
| 16α β | 1.87 m 1.39 m | 28.4 | 1.86 m 1.37 m | 28.4 |
| 17 | 1.45 m | 50.6 | 1.45 m | 50.9 |
| 18 | 0.73 s | 12.7 | 0.70 s | 12.8 |
| 19 | 1.23 s | 17.8 | 1.11 s | 12.1 |
| 20 | 1.51 m | 43.2 | 1.50 m | 43.2 |
| 21 | 3.69 dd (10.7, 4.2) 3.54 dd (10.7, 5.5) | 63.2 | 3.68 dd (10.9, 3.8) 3.53 dd (10.9, 5.6) | 63.2 |
| 22 | 1.63 m 1.44 m | 29.4 | 1.61 m 1.43 m | 29.3 |
| 23 | 2.15 m 1.98 m | 32.3 | 2.13 m 1.97 m | 32.3 |
| 24 | – | 157.8 | – | 157.5 |
| 25 | 2.25 quin | 34.9 | 2.25 quin | 34.9 |
| 26 | 1.03 d (6.8) | 22.5 | 1.03 d (6.7) | 22.5 |
| 27 | 1.03 d (6.8) | 22.3 | 1.03 d (6.7) | 22.3 |
| 28 | 4.73 br s 4.69 br d (1.4) | 106.9 | 4.72 br s 4.68 br d (1.4) | 106.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kicha, A.A.; Kalinovsky, A.I.; Malyarenko, T.V.; Malyarenko, O.S.; Ermakova, S.P.; Popov, R.S.; Stonik, V.A.; Ivanchina, N.V. Disulfated Ophiuroid Type Steroids from the Far Eastern Starfish Pteraster marsippus and Their Cytotoxic Activity on the Models of 2D and 3D Cultures. Mar. Drugs 2022, 20, 164. https://doi.org/10.3390/md20030164
Kicha AA, Kalinovsky AI, Malyarenko TV, Malyarenko OS, Ermakova SP, Popov RS, Stonik VA, Ivanchina NV. Disulfated Ophiuroid Type Steroids from the Far Eastern Starfish Pteraster marsippus and Their Cytotoxic Activity on the Models of 2D and 3D Cultures. Marine Drugs. 2022; 20(3):164. https://doi.org/10.3390/md20030164
Chicago/Turabian StyleKicha, Alla A., Anatoly I. Kalinovsky, Timofey V. Malyarenko, Olesya S. Malyarenko, Svetlana P. Ermakova, Roman S. Popov, Valentin A. Stonik, and Natalia V. Ivanchina. 2022. "Disulfated Ophiuroid Type Steroids from the Far Eastern Starfish Pteraster marsippus and Their Cytotoxic Activity on the Models of 2D and 3D Cultures" Marine Drugs 20, no. 3: 164. https://doi.org/10.3390/md20030164
APA StyleKicha, A. A., Kalinovsky, A. I., Malyarenko, T. V., Malyarenko, O. S., Ermakova, S. P., Popov, R. S., Stonik, V. A., & Ivanchina, N. V. (2022). Disulfated Ophiuroid Type Steroids from the Far Eastern Starfish Pteraster marsippus and Their Cytotoxic Activity on the Models of 2D and 3D Cultures. Marine Drugs, 20(3), 164. https://doi.org/10.3390/md20030164