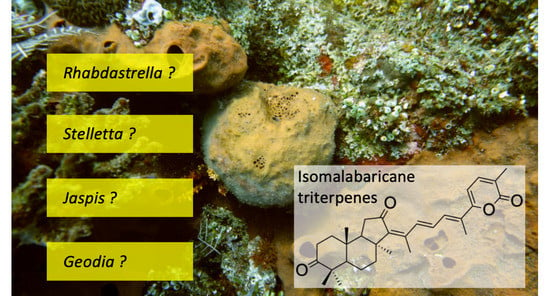
Good Practices in Sponge Natural Product Studies: Revising Vouchers with Isomalabaricane Triterpenes
Abstract
:1. Introduction
2. Results
2.1. Revision of Vouchers
2.2. NP Journal Taxonomy/Voucher Guidelines
3. Discussion
3.1. Rhabdastrella globostellata Is a Variable Species
3.2. ITTs Are Chemotaxonomy Markers
3.3. Sponge Misidentifications Have Multiple Reasons
3.4. The Accessibility of Vouchers Is Crucial
3.5. Recommended Protocol for Sponge NP Studies
- Upon collection, take pictures of the whole specimen (external and internal appearance). A picture of the specimen is priceless for a sponge taxonomist and can already give a lot of information about the species identity (shape, external/internal color). Usually, the voucher is a small subsample so one loses substantial information about the external morphology if a taxonomist only examines the voucher.
- Prepare the voucher from the start, upon collection of the specimen in the field. Just after collection, cut a small piece (1 cm × 1 cm) including the surface of the sponge. Place the piece in 10 times its volume of EtOH 96% to preserve the DNA and the morphology. Change the EtOH at least twice, after ~1 h and after ~6 h. Of course, a larger voucher is perfectly ok, but then increase the amount of EtOH 96% accordingly to properly preserve the DNA (that being said, DNA is usually preserved better in a small piece). With this mode of preservation, the voucher could be used in the future for morphology and molecular work (e.g., barcoding, population genetics, genomics, etc.) and it is easily stored at room temperature.
- A voucher should have the following metadata recorded and reported in the publication: date of collection, collector, locality name, GPS coordinates, depth and habitat (e.g., coral reef, seagrass bed, mangrove, rocky bottom) of the locality where the specimen was collected. These descriptors will be useful for several metadata analyses (e.g., [99]) as well as for the taxonomist.
- Collaborate with a sponge taxonomist for the identification. Indicate in the publication the name of the taxonomist identifier. Sponge experts can be found on the World Porifera Database (WPD, http://www.marinespecies.org/porifera, accessed 1 March 2022): WPD editors are listed on the home webpage, and additional experts are listed in the “Who is who in sponge science” list [100].
- Check the validity and spelling of the species name on the WPD before publishing. All species and their taxonomy are recorded and updated in the WPD, part of the World Register of Marine Species (WoRMS).
- With the help of the sponge taxonomist, include in the publication a short description of specimen with (i) external and internal color (the best is a picture), (ii) overall shape, and if possible, (iii) spicule repertoire with spicule measurements. It will significantly help other taxonomists to trust or not the identification. These descriptions can be in the Materials section (e.g., [33,101]) or in the Supplementary Information (e.g., [35]). This description should be mandatory if the specimen is identified to the genus level only. Additionally, including a molecular barcode of the specimen in the publication (and deposited in Genbank) can be very useful: 28S and COI (cytochrome c oxidase 1) are the two most widely used barcodes to help with sponge identification.
- Deposit the sponge voucher in a recognized national or city museum with a unique museum number to be included in the publication. The sponge taxonomist collaborator may help you with this last step.
4. Materials and Methods
4.1. Abbreviations
4.2. Comparative Material and Vouchers
4.3. Spicule Preparations
4.4. Voucher Guidelines in NP Journals
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leal, M.C.; Hilario, A.; Munro, M.H.G.; Blunt, J.W.; Calado, R. Natural products discovery needs improved taxonomic and geographic information. Nat. Prod. Rep. 2016, 33, 747–750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bennett, B.C.; Balick, M.J. Does the name really matter? The importance of botanical nomenclature and plant taxonomy in biomedical research. J. Ethnopharmacol. 2014, 152, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Rivera, D.; Allkin, R.; Obón, C.; Alcaraz, F.; Verpoorte, R.; Heinrich, M. What is in a name? The need for accurate scientific nomenclature for plants. J. Ethnopharmacol. 2014, 152, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Van Soest, R.W.M.; Braekman, J.C. Chemosystematics of Porifera: A review. Mem. Qld. Mus. 1999, 44, 569–589. [Google Scholar]
- Erpenbeck, D.; van Soest, R.M. Status and Perspective of Sponge Chemosystematics. Mar. Biotechnol. 2007, 9, 2–19. [Google Scholar] [CrossRef]
- Leal, M.C.; Puga, J.; Serôdio, J.; Gomes, N.C.M.; Calado, R. Trends in the Discovery of New Marine Natural Products from Invertebrates over the Last Two Decades—Where and What Are We Bioprospecting? PLoS ONE 2012, 7, e30580. [Google Scholar] [CrossRef] [Green Version]
- Van Soest, R.W.M.; Braekman, J.C.; Faulkner, D.J.; Hajdu, E.; Harper, M.K.; Vacelet, J. The genus Batzella: A chemosystematic problem. Bull. L’institut R. Sci. Nat. Belg. Série Biol. 1996, 66, 89–101. [Google Scholar]
- Wilkinson, M.D.; Dumontier, M.; Aalbersberg, I.J.; Appleton, G.; Axton, M.; Baak, A.; Blomberg, N.; Boiten, J.-W.; da Silva Santos, L.B.; Bourne, P.E.; et al. The FAIR Guiding Principles for scientific data management and stewardship. Sci. Data 2016, 3, 160018. [Google Scholar] [CrossRef] [Green Version]
- Ravi, B.N.; Wells, R.J.; Croft, K.D. Malabaricane triterpenes from a Fijian collection of the sponge Jaspis stellifera. J. Org. Chem. 1981, 46, 1998–2001. [Google Scholar] [CrossRef]
- Stonik, V.A.; Kolesnikova, S.A. Malabaricane and Isomalabaricane Triterpenoids, Including Their Glycoconjugated Forms. Mar. Drugs 2021, 19, 327. [Google Scholar] [CrossRef]
- Ebada, S.S.; Lin, W.; Proksch, P. Bioactive Sesterterpenes and Triterpenes from Marine Sponges: Occurrence and Pharmacological Significance. Mar. Drugs 2010, 8, 313–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, S.-A.; Zhou, Q.; Guo, W.-Z.; Qiu, Y.; Wang, R.; Jin, M.; Zhang, W.; Li, K.; Yamori, T.; Dan, S.; et al. In Vitro Antitumor Activity of Stellettin B, a Triterpene from Marine Sponge Jaspis stellifera, on Human Glioblastoma Cancer SF295 Cells. Mar. Drugs 2014, 12, 4200–4213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, R.; Zhang, Q.; Peng, X.; Zhou, C.; Zhong, Y.; Chen, X.; Qiu, Y.; Jin, M.; Gong, M.; Kong, D. Stellettin B Induces G1 Arrest, Apoptosis and Autophagy in Human Non-small Cell Lung Cancer A549 Cells via Blocking PI3K/Akt/mTOR Pathway. Sci. Rep. 2016, 6, 27071. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Zhou, Q.; Zhang, L.; Zhong, Y.; Fan, G.; Zhang, Z.; Wang, R.; Jin, M.; Qiu, Y.; Kong, D. Stellettin B induces apoptosis in human chronic myeloid leukemia cells via targeting PI3K and Stat5. Oncotarget 2017, 8, 28906–28921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, S.-Y.; Chen, N.-F.; Lin, P.-Y.; Su, J.-H.; Chen, B.-H.; Kuo, H.-M.; Sung, C.-S.; Sung, P.-J.; Wen, Z.-H.; Chen, W.-F. Anti-Invasion and Antiangiogenic Effects of Stellettin B through Inhibition of the Akt/Girdin Signaling Pathway and VEGF in Glioblastoma Cells. Cancers 2019, 11, 220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carter, H.J. Contributions to our Knowledge of the Spongida. -Pachytragida. Ann. Mag. Nat. Hist. 1883, 5, 344–369, pls XIV-XV. [Google Scholar] [CrossRef]
- De Voogd, N.J.A.B.; Boury-Esnault, N.; Carballo, J.L.; Cárdenas, P.; Díaz, M.-C.; Dohrmann, M.; Downey, R.; Hajdu, E.; Hooper, J.N.A.; Kelly, M.; et al. World Porifera Database. Available online: http://www.marinespecies.org/porifera (accessed on 13 February 2022).
- Ryu, G.; Matsunaga, S.; Fusetani, N. Globostellatic Acids A−D, New Cytotoxic Isomalabaricane Triterpenes from the Marine Sponge Stelletta globostellata. J. Nat. Prod. 1996, 59, 512–514. [Google Scholar] [CrossRef]
- Rao, Z.; Deng, S.; Wu, H.; Jiang, S. Rhabdastrellic Acid-A, a Novel Triterpenoid from the Marine Sponge Rhabdastrella globostellata. J. Nat. Prod. 1997, 60, 1163–1164. [Google Scholar] [CrossRef]
- Van Soest, R.W.M.; Boury-Esnault, N.; Vacelet, J.; Dohrmann, M.; Erpenbeck, D.; De Voogd, N.J.; Santodomingo, N.; Vanhoorne, B.; Kelly, M.; Hooper, J.N.A. Global Diversity of Sponges (Porifera). PLoS ONE 2012, 7, e35105. [Google Scholar]
- Jørgensen, C.B. On the spicule-formation of Spongilla lacustris (L.) 1. The dependence of the spicule-formation on the content of dissolved and solid silicic acid of the milieu. Biol. Medd. (Det K. Dan. Vidensk. Selsk.) 1944, 19, 1–45. [Google Scholar]
- Maldonado, M.; Carmona, M.C.; Uriz, M.J.; Cruzado, A. Decline in Mesozoic reef-building sponges explained by silicon limitation. Nature 1999, 401, 785–788. [Google Scholar] [CrossRef]
- Cárdenas, P.; Rapp, H.T. Disrupted spiculogenesis in deep-water Geodiidae (Porifera, Demospongiae) growing in shallow waters. Invertebr. Biol. 2013, 132, 173–194. [Google Scholar] [CrossRef]
- Cárdenas, P.; Xavier, J.R.; Reveillaud, J.; Schander, C.; Rapp, H.T. Molecular phylogeny of the Astrophorida (Porifera, Demospongiae) reveals an unexpected high level of spicule homoplasy. PLoS ONE 2011, 6, e18318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tasdemir, D.; Mangalindan, G.C.; Concepcion, G.P.; Verbitski, S.M.; Rabindran, S.; Miranda, M.; Greenstein, M.; Hooper, J.N.A.; Harper, M.K.; Ireland, C.M. Bioactive Isomalabaricane Triterpenes from the Marine Sponge Rhabdastrella globostellata. J. Nat. Prod. 2002, 65, 210–214. [Google Scholar] [CrossRef]
- Kennedy, J.A. Resolving the ‘Jaspis stellifera’ complex. Mem. Qld. Mus. 2000, 45, 453–476. [Google Scholar]
- Lai, K.-H.; Huang, Z.-H.; El-Shazly, M.; Peng, B.-R.; Wei, W.-C.; Su, J.-H. Isomalabaricane Triterpenes from the Marine Sponge Rhabdastrella sp. Mar. Drugs 2021, 19, 206. [Google Scholar] [CrossRef]
- Zhang, W.-H.; Che, C.-T. Isomalabaricane-Type Nortriterpenoids and Other Constituents of the Marine Sponge Geodia japonica. J. Nat. Prod. 2001, 64, 1489–1492. [Google Scholar] [CrossRef]
- Su, J.Y.; Meng, Y.H.; Zeng, L.M.; Fu, X.; Schmitz, F.J. Stellettin A, a New Triterpenoid Pigment from the Marine Sponge Stelletta tenuis. J. Nat. Prod. 1994, 57, 1450–1451. [Google Scholar] [CrossRef]
- Lin, H.-w.; Wang, Z.-l.; Wu, J.-h.; Shi, N.; Zhang, H.-j.; Chen, W.-s.; Morris-Natschke, S.L.; Lin, A.-S. Stellettins L and M, Cytotoxic Isomalabaricane-Type Triterpenes, and Sterols from the Marine Sponge Stelletta tenuis. J. Nat. Prod. 2007, 70, 1114–1117. [Google Scholar] [CrossRef]
- Tang, S.; Xu, R.; Lin, W.; Duan, H. Jaspiferin A and B: Two New Secondary Metabolites from theSouth China Sea Sponge Jaspis stellifera. Rec. Nat. Prod. 2012, 6, 398–401. [Google Scholar]
- Jin, D.-J.; Tang, S.-A.; Xing, G.-S.; Zhao, W.-J.; Zhao, C.; Duan, H.-Q.; Lin, W.-H. Jaspiferins C–F, four new isomalabaricane-type triterpenoids from the South China Sea sponge Jaspis stellifera. J. Asian Nat. Prod. Res. 2014, 16, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Fouad, M.; Edrada, R.A.; Ebel, R.; Wray, V.; Muller, W.E.G.; Lin, W.H.; Proksch, P. Cytotoxic Isomalabaricane Triterpenes from the Marine Sponge Rhabdastrella globostellata. J. Nat. Prod. 2006, 69, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, N.; Momose, R.; Shibazaki, A.; Gonoi, T.; Fromont, J.; Kobayashi, J.I. Stelliferins J–N, isomalabaricane-type triterpenoids from Okinawan marine sponge Rhabdastrella cf. globostellata. Tetrahedron 2011, 67, 6689–6696. [Google Scholar] [CrossRef]
- Kolesnikova, S.A.; Lyakhova, E.G.; Kalinovsky, A.I.; Berdyshev, D.V.; Pislyagin, E.A.; Popov, R.S.; Grebnev, B.B.; Makarieva, T.N.; Minh, C.V.; Stonik, V.A. Cyclobutastellettolides A and B, C19 Norterpenoids from a Stelletta sp. Marine Sponge. J. Nat. Prod. 2019, 82, 3196–3200. [Google Scholar] [CrossRef]
- Ravi, B.N.; Wells, R.J. Malabaricane triterpenes from a Great Barrier Reef collection of the sponge Jaspis stellifera. Aust. J. Chem. 1982, 35, 39–50. [Google Scholar] [CrossRef]
- McCabe, T.; Clardy, J.; Minale, L.; Pizza, C.; Zollo, F.; Riccio, R. A triterpenoid pigment with the isomalabaricane skeleton from the marine sponge Stelletta sp. Tetrahedron Lett. 1982, 23, 3307–3310. [Google Scholar] [CrossRef]
- Tsuda, M.; Ishibashi, M.; Agemi, K.; Sasaki, T.; Kobayashi, J.i. Stelliferins A–F, new antineoplastic isomalabaricane triterpenes from the Okinawan marine sponge Jaspis stellifera. Tetrahedron 1991, 47, 2181–2194. [Google Scholar] [CrossRef]
- Oku, N.; Matsunaga, S.; Wada, S.-i.; Watabe, S.; Fusetani, N. New Isomalabaricane Triterpenes from the Marine Sponge Stelletta globostellata That Induce Morphological Changes in Rat Fibroblasts. J. Nat. Prod. 2000, 63, 205–209. [Google Scholar] [CrossRef]
- Kobayashi, J.i.; Yuasa, K.; Kobayashi, T.; Sasaki, T.; Tsuda, M. Jaspiferals A∼G, new cytotoxic isomalabaricane-type nortriterpenoids from Okinawan marine sponge Jaspis stellifera. Tetrahedron 1996, 52, 5745–5750. [Google Scholar] [CrossRef]
- McCormick, J.L.; McKee, T.C.; Cardellina, J.H.; Leid, M.; Boyd, M.R. Cytotoxic Triterpenes from a Marine Sponge, Stelletta sp. J. Nat. Prod. 1996, 59, 1047–1050. [Google Scholar] [CrossRef]
- McKee, T.C.; Bokesch, H.R.; McCormick, J.L.; Rashid, M.A.; Spielvogel, D.; Gustafson, K.R.; Alavanja, M.M.; Cardellina, J.H.; Boyd, M.R. Isolation and Characterization of New Anti-HIV and Cytotoxic Leads from Plants, Marine, and Microbial Organisms. J. Nat. Prod. 1997, 60, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Bourguet-Kondracki, M.L.; Longeon, A.; Debitus, C.; Guyot, M. New cytotoxic isomalabaricane-type sesterterpenes from the New Caledonian marine sponge Rhabdastrella globostellata. Tetrahedron Lett. 2000, 41, 3087–3090. [Google Scholar] [CrossRef]
- Zampella, A.; D’Auria, M.V.; Debitus, C.; Menou, J.-L. New Isomalabaricane Derivatives from a New Species of Jaspis Sponge Collected at the Vanuatu Islands. J. Nat. Prod. 2000, 63, 943–946. [Google Scholar] [CrossRef] [PubMed]
- Tabudravu, J.N.; Jaspars, M. Stelliferin Riboside, a Triterpene Monosaccharide Isolated from the Fijian Sponge Geodia globostellifera. J. Nat. Prod. 2001, 64, 813–815. [Google Scholar] [CrossRef]
- Meragelman, K.M.; McKee, T.C.; Boyd, M.R. New Cytotoxic Isomalabaricane Triterpenes from the Sponge Jaspis Species. J. Nat. Prod. 2001, 64, 389–392. [Google Scholar] [CrossRef]
- Lv, F.; Deng, Z.; Li, J.; Fu, H.; van Soest, R.W.M.; Proksch, P.; Lin, W. Isomalabaricane-Type Compounds from the Marine Sponge Rhabdastrella aff. distincta. J. Nat. Prod. 2004, 67, 2033–2036. [Google Scholar] [CrossRef]
- Lv, F.; Xu, M.; Deng, Z.; de Voogd, N.J.; van Soest, R.W.M.; Proksch, P.; Lin, W. Rhabdastrellins A−F, Isomalabaricane Triterpenes from the Marine Sponge Rhabdastrella aff. distincta. J. Nat. Prod. 2008, 71, 1738–1741. [Google Scholar] [CrossRef]
- Tang, S.-A.; Deng, Z.-W.; Li, J.; Fu, H.-Z.; Pei, Y.-H.; Zhang, S.; Lin, W.-H. A New Isomalabaricane Triterpenoid from Sponge Jaspis sp. Chin. Chem. Lett. 2005, 16, 353–355. [Google Scholar]
- Tang, S.; Pei, Y.; Fu, H.; Deng, Z.; Li, J.; Proksch, P.; Lin, W. Jaspolides A–F, Six New Isomalabricane-Type Terpenoids from the Sponge Jaspis sp. Chem. Pharm. Bull. 2006, 54, 4–8. [Google Scholar] [CrossRef] [Green Version]
- Tang, S.; Deng, Z.; Proksch, P.; Lin, W. Jaspolides G and H, unique bisisomalabaricanes from the Chinese marine sponge Jaspis sp. Tetrahedron Lett. 2007, 48, 5443–5447. [Google Scholar] [CrossRef]
- Clement, J.A.; Li, M.; Hecht, S.M.; Kingston, D.G.I. Bioactive Isomalabaricane Triterpenoids from Rhabdastrella globostellata that Stabilize the Binding of DNA Polymerase β to DNA. J. Nat. Prod. 2006, 69, 373–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agrawal, M. Isolation and Structural Elucidation of Cytotoxic Agents from Marine Invertebrates and Plants Sourced from the Great Barrier Reef, Australia. Ph.D. Thesis, James Cook University, Douglas, Australia, 2007. Available online: http://researchonline.jcu.edu.au/2047/ (accessed on 13 February 2022).
- Aoki, S.; Sanagawa, M.; Watanabe, Y.; Setiawan, A.; Arai, M.; Kobayashi, M. Novel isomarabarican triterpenes, exhibiting selective anti-proliferative activity against vascular endothelial cells, from marine sponge Rhabdastrella globostellata. Bioorg. Med. Chem. 2007, 15, 4818–4828. [Google Scholar] [CrossRef] [PubMed]
- Hirashima, M.; Tsuda, K.; Hamada, T.; Okamura, H.; Furukawa, T.; Akiyama, S.-i.; Tajitsu, Y.; Ikeda, R.; Komatsu, M.; Doe, M.; et al. Cytotoxic Isomalabaricane Derivatives and a Monocyclic Triterpene Glycoside from the Sponge Rhabdastrella globostellata. J. Nat. Prod. 2010, 73, 1512–1518. [Google Scholar] [CrossRef]
- Li, J.; Xu, B.; Cui, J.; Deng, Z.; de Voogd, N.J.; Proksch, P.; Lin, W. Globostelletins A–I, cytotoxic isomalabaricane derivatives from the marine sponge Rhabdastrella globostellata. Bioorg. Med. Chem. 2010, 18, 4639–4647. [Google Scholar] [CrossRef]
- Li, J.; Zhu, H.; Ren, J.; Deng, Z.; Voogd, N.J.d.; Proksch, P.; Lin, W. Globostelletins J–S, isomalabaricanes with unusual cyclopentane sidechains from the marine sponge Rhabdastrella globostellata. Tetrahedron 2012, 68, 559–565. [Google Scholar] [CrossRef]
- Xue, D.-Q.; Mao, S.-C.; Yu, X.-Q.; Guo, Y.-W. Isomalabaricane triterpenes with potent protein-tyrosine phosphatase 1B (PTP1B) inhibition from the Hainan sponge Stelletta sp. Biochem. Syst. Ecol. 2013, 49, 101–106. [Google Scholar] [CrossRef]
- Xu, W.-g.; Wang, J.; Qiao, W.; Zhao, C.; Tang, S.-a. Jaspiferins H–J, New Isomalabaricane-Type Terpenoids from the South China Sea Marine Sponge Jaspis stellifera. Chem. Nat. Compd. 2018, 54, 84–87. [Google Scholar] [CrossRef]
- Xu, W.-G.; Wang, J.; Xing, G.-S.; Xu, J.-J.; Qiao, W.; Zhao, C.; Tang, S.-A. Jaspiferin G, a new isomalabaricane-type triterpenoid from the sponge Jaspis stellifera. Z. Nat. C 2016, 71, 111. [Google Scholar] [CrossRef]
- Li, Y.; Tang, H.; Tian, X.; Lin, H.; Wang, M.; Yao, M. Three new cytotoxic isomalabaricane triterpenes from the marine sponge Stelletta tenuis. Fitoterapia 2015, 106, 226–230. [Google Scholar] [CrossRef]
- Kiem, P.V.; Dung, D.T.; Yen, P.H.; Nhiem, N.X.; Quang, T.H.; Tai, B.H.; Minh, C.V. New isomalabaricane analogues from the sponge Rhabdastrella providentiae and their cytotoxic activities. Phytochem. Lett. 2018, 26, 199–204. [Google Scholar] [CrossRef]
- Dung, D.T.; Hang, D.T.T.; Nhiem, N.X.; Quang, T.H.; Tai, B.H.; Yen, P.H.; Hoai, N.T.; Thung, D.C.; Van Minh, C.; Van Kiem, P. Rhabdaprovidines D–G, Four New 6,6,5-Tricyclic Terpenoids from the Vietnamese Sponge Rhabdastrella providentiae. Nat. Prod. Commun. 2018, 13, 1934578X1801301004. [Google Scholar] [CrossRef]
- Dung, D.T.; Yen, P.H.; Nhiem, N.X.; Quang, T.H.; Tai, B.H.; Van Minh, C.; Kim, D.C.; Oh, H.; Kim, Y.C.; Van Kiem, P. New Acetylated Terpenoids from Sponge Rhabdastrella providentiae Inhibit NO Production in LPS Stimulated BV2 Cells. Nat. Prod. Commun. 2018, 13, 1934578X1801300602. [Google Scholar] [CrossRef] [Green Version]
- Kolesnikova, S.A.; Lyakhova, E.G.; Kozhushnaya, A.B.; Kalinovsky, A.I.; Berdyshev, D.V.; Popov, R.S.; Stonik, V.A. New Isomalabaricane-Derived Metabolites from a Stelletta sp. Marine Sponge. Molecules 2021, 26, 678. [Google Scholar] [CrossRef] [PubMed]
- Trang, D.T.; Dung, D.T.; Nhiem, N.X.; Cuc, N.T.; Yen, P.H.; Hang, D.T.T.; Linh, T.M.; Mai, N.C.; Huong, P.T.T.; Tai, B.H.; et al. New tetracyclic and pentacyclic isomalabaricanes from the marine sponge Rhabdastrella globostellata (Carter, 1883). Tetrahedron Lett. 2022, 89, 153607. [Google Scholar] [CrossRef]
- Sollas, W.J. Report on the Tetractinellida collected by H.M.S. Challenger, during the years 1873-1876. Report on the Scientific Results of the Voyage of H.M.S. Challenger, 1873–1876. Zoology 1888, 25, 1–458, pls I-XLIV. [Google Scholar]
- Thiele, J. Studien über pazifische Spongien. I. Japanische Demospongien. Zool. Orig.-Abh. Aus Dem Gesamtgeb. Der Zool. Stuttg. 1898, 24, 1–72, pls I-VIII. [Google Scholar]
- Sim, C.J. A taxonomic study on the marine sponges in Korea. 4. Choristida (Geodiidae). Korean J. Zool. 1982, 25, 1–8. [Google Scholar]
- Lehnert, H.; Stone, R.P. A comprehensive inventory of the Gulf of Alaska sponge fauna with the description of two new species and geographic range extensions. Zootaxa 2016, 4144, 18. [Google Scholar] [CrossRef]
- Theobald, N.; Wells, R.J.; Djerassi, C. Minor and trace sterols in marine invertebrates. 8. Isolation, structure elucidation, and partial synthesis of two novel sterols—stelliferasterol and isostelliferasterol. J. Am. Chem. Soc. 1978, 100, 7677–7684. [Google Scholar] [CrossRef]
- Carballeira, N.; Thompson, J.E.; Ayanoglu, E.; Djerassi, C. Biosynthetic studies of marine lipids. 5. The biosynthesis of long-chain branched fatty acids in marine sponges. J. Org. Chem. 1986, 51, 2751–2756. [Google Scholar] [CrossRef]
- Li, Z.; Peng, C.; Shen, Y.; Miao, X.; Zhang, H.; Lin, H. l,l-Diketopiperazines from Alcaligenes faecalis A72 associated with South China Sea sponge Stelletta tenuis. Biochem. Syst. Ecol. 2008, 36, 230–234. [Google Scholar] [CrossRef]
- Huyen, V.T.T.; Minh, L.T.H.; Quyen, V.T.; Anh, N.M.; Cuc, N.T.K.; Luyen, N.T.; Dat, N.T. Antimicrobial metabolites from Streptomyces sp. strain PDH23 derived from marine sponge Rhabdastrella globostellata. Bangladesh J. Pharmacol. 2020, 15, 69–70. [Google Scholar] [CrossRef]
- Khushi, S.; Salim, A.A.; Elbanna, A.H.; Nahar, L.; Capon, R.J. New from Old: Thorectandrin Alkaloids in a Southern Australian Marine Sponge, Thorectandra choanoides (CMB-01889). Mar. Drugs 2021, 19, 97. [Google Scholar] [CrossRef] [PubMed]
- Tai, C.-J.; Huang, C.-Y.; Ahmed, A.F.; Orfali, R.S.; Alarif, W.M.; Huang, Y.M.; Wang, Y.-H.; Hwang, T.-L.; Sheu, J.-H. An Anti-Inflammatory 2,4-Cyclized-3,4-Secospongian Diterpenoid and Furanoterpene-Related Metabolites of a Marine Sponge Spongia sp. from the Red Sea. Mar. Drugs 2021, 19, 38. [Google Scholar] [CrossRef]
- Yu, H.-B.; Gu, B.-B.; Iwasaki, A.; Jiang, W.-L.; Ecker, A.; Wang, S.-P.; Yang, F.; Lin, H.-W. Dactylospenes A–E, Sesterterpenes from the Marine Sponge Dactylospongia elegans. Mar. Drugs 2020, 18, 491. [Google Scholar] [CrossRef]
- Abdjul, D.B.; Yagi, A.; Yamazaki, H.; Kirikoshi, R.; Takahashi, O.; Namikoshi, M.; Uchida, R. Anti-mycobacterial haliclonadiamine alkaloids from the Okinawan marine sponge Haliclona sp. collected at Iriomote Island. Phytochem. Lett. 2018, 26, 130–133. [Google Scholar] [CrossRef]
- Kapojos, M.M.; Abdjul, D.B.; Yamazaki, H.; Kirikoshi, R.; Takahashi, O.; Rotinsulu, H.; Wewengkang, D.S.; Sumilat, D.A.; Ukai, K.; Namikoshi, M. Protein tyrosine phosphatase 1B inhibitory polybromobiphenyl ethers and monocyclofarnesol-type sesquiterpenes from the Indonesian marine sponge Lamellodysidea cf. herbacea. Phytochem. Lett. 2018, 24, 10–14. [Google Scholar] [CrossRef]
- Salib, M.N.; Jamison, M.T.; Molinski, T.F. Bromo-spiroisoxazoline Alkaloids, Including an Isoserine Peptide, from the Caribbean Marine Sponge Aplysina lacunosa. J. Nat. Prod. 2020, 83, 1532–1540. [Google Scholar] [CrossRef]
- Jiao, W.-H.; Li, J.; Wang, D.; Zhang, M.-M.; Liu, L.-Y.; Sun, F.; Li, J.-Y.; Capon, R.J.; Lin, H.-W. Cinerols, Nitrogenous Meroterpenoids from the Marine Sponge Dysidea cinerea. J. Nat. Prod. 2019, 82, 2586–2593. [Google Scholar] [CrossRef]
- Bergquist, P.R. Shallow water Demospongiae from Heron Island. Univ. Qld. Pap. Great Barrier Reef Comm. Heron Isl. Res. Stn. 1969, 1, 62–72. [Google Scholar]
- Schmitt, S.; Tsai, P.; Bell, J.; Fromont, J.; Ilan, M.; Lindquist, N.; Perez, T.; Rodrigo, A.; Schupp, P.J.; Vacelet, J.; et al. Assessing the complex sponge microbiota: Core, variable and species-specific bacterial communities in marine sponges. ISME J. 2012, 6, 564–576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinert, G.; Taylor, M.W.; Deines, P.; Simister, R.L.; de Voogd, N.J.; Hoggard, M.; Schupp, P.J. In four shallow and mesophotic tropical reef sponges from Guam the microbial community largely depends on host identity. PeerJ 2016, 4, e1936. [Google Scholar] [CrossRef] [PubMed]
- Dat, T.T.H.; Steinert, G.; Thi Kim Cuc, N.; Smidt, H.; Sipkema, D. Archaeal and bacterial diversity and community composition from 18 phylogenetically divergent sponge species in Vietnam. PeerJ 2018, 6, e4970. [Google Scholar] [CrossRef] [Green Version]
- Cho, J.H.; Djerassi, C. Minor and trace sterols from marine invertebrates. 58. Stereostructure and synthesis of new sponge sterols jaspisterol and isojaspisterol. J. Org. Chem. 1987, 52, 4517–4521. [Google Scholar] [CrossRef]
- Zumberge, J.A.; Love, G.D.; Cárdenas, P.; Sperling, E.A.; Gunasekera, S.; Rohrssen, M.; Grosjean, E.; Grotzinger, J.P.; Summons, R.E. Demosponge steroid biomarker 26-methylstigmastane provides evidence for Neoproterozoic animals. Nat. Ecol. Evol. 2018, 2, 1709–1714. [Google Scholar] [CrossRef] [PubMed]
- Cárdenas, P.; Pérez, T.; Boury-Esnault, N. Sponge Systematics facing new challenges. Adv. Mar. Biol. 2012, 61, 79–209. [Google Scholar] [PubMed]
- Morrow, C.; Cárdenas, P. Proposal for a revised classification of the Demospongiae (Porifera). Front. Zool. 2015, 12, 1–27. [Google Scholar] [CrossRef] [Green Version]
- Dohrmann, M.; Kelley, C.; Kelly, M.; Pisera, A.; Hooper, J.N.A.; Reiswig, H.M. An integrative systematic framework helps to reconstruct skeletal evolution of glass sponges (Porifera, Hexactinellida). Front. Zool. 2017, 14, 18. [Google Scholar] [CrossRef] [Green Version]
- Voigt, O.; Wülfing, E.; Wörheide, G. Molecular Phylogenetic Evaluation of Classification and Scenarios of Character Evolution in Calcareous Sponges (Porifera, Class Calcarea). PLoS ONE 2012, 7, e33417. [Google Scholar] [CrossRef] [Green Version]
- Alvizu, A.; Eilertsen, M.H.; Xavier, J.R.; Rapp, H.T. Increased taxon sampling provides new insights into the phylogeny and evolution of the subclass Calcaronea (Porifera, Calcarea). Org. Divers. Evol. 2018, 18, 279–290. [Google Scholar] [CrossRef]
- Ruiz, C.; Muricy, G.; Lage, A.; Domingos, C.; Chenesseau, S.; Pérez, T. Descriptions of new sponge species and genus, including aspiculate Plakinidae, overturn the Homoscleromorpha classification. Zool. J. Linn. Soc. 2017, 179, 707–724. [Google Scholar] [CrossRef]
- Li, Z.; He, L.; Miao, X. Cultivable Bacterial Community from South China Sea Sponge as Revealed by DGGE Fingerprinting and 16S rDNA Phylogenetic Analysis. Curr. Microbiol. 2007, 55, 465–472. [Google Scholar] [CrossRef]
- Li, Z.-Y.; He, L.-M.; Wu, J.; Jiang, Q. Bacterial community diversity associated with four marine sponges from the South China Sea based on 16S rDNA-DGGE fingerprinting. J. Exp. Mar. Biol. Ecol. 2006, 329, 75–85. [Google Scholar] [CrossRef]
- Lindgren, N.G. Beitrag zur Kenntniss der Spongienfauna des Malaiischen Archipels und der Chinesischen Meere Zool. Anz. 1897, 547, 480–487. [Google Scholar]
- García-Ruiz, C.; Sarabia, F. Chemistry and Biology of Bengamides and Bengazoles, Bioactive Natural Products from Jaspis Sponges. Mar. Drugs 2014, 12, 1580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Q.; Nay, B.; Yang, M.; Ni, Y.; Wang, H.; Yao, L.; Li, X. Marine sponges of the genus Stelletta as promising drug sources: Chemical and biological aspects. Acta Pharm. Sin. B 2019, 9, 237–257. [Google Scholar] [CrossRef]
- Principe, P.P.; Fisher, W.S. Spatial Distribution of Collections Yielding Marine Natural Products. J. Nat. Prod. 2018, 81, 2307–2320. [Google Scholar] [CrossRef] [PubMed]
- Schönberg, C.H.L. Who Is Who in Sponge Science 2021. World Porifera Database. 2021. Available online: http://www.marinespecies.org/porifera (accessed on 1 July 2021).
- Russell, F.; Harmody, D.; McCarthy, P.J.; Pomponi, S.A.; Wright, A.E. Indolo[3,2-a]carbazoles from a Deep-Water Sponge of the Genus Asteropus. J. Nat. Prod. 2013, 76, 1989–1992. [Google Scholar] [CrossRef]



| Ref. | Identifier | Description of Voucher 1. Picture 2. Color 3. Spicules 4. Barcodes | Voucher (Y/N) | Voucher in Museum (Y/N/-) | Voucher Accessed (Y/N/-) | Source Original ID | Source Revised ID | Misidentification (Y/N/?) |
|---|---|---|---|---|---|---|---|---|
| Ravi et al. (1981) [9] | - | 2 | N | - | - | J. stellifera | R. globostellata | Y |
| Ravi & Wells (1982) [36] | - | - | N | - | - | J. stellifera | R. globostellata | Y |
| McCabe et al. (1982) [37] | Bergquist | 2 | N | - | - | Stelletta sp. | Rhabdastrella ? | Y |
| Tsuda et al. (1991) [38] | Fromont | 2 | N | - | - | J. stellifera | R. globostellata | Y |
| Su et al. (1994) [29] | JH. Li | - | Y | N | N | S. tenuis | R. globostellata | Y |
| Ryu et al. (1996) [18], Oku et al. (2000) [39] | Van Soest | - | Y | Y | Y | S. globostellata | R. globostellata | N |
| Kobayashi et al. (1996) [40] | Fromont | 2 | N | - | - | J. stellifera | R. globostellata | Y |
| McCormick et al. (1996) [41], McKee et al. (1997) [42] | Pomponi | 2 | Y | Y | Y 2 | Stelletta sp. | R. globostellata | Y |
| Rao et al. (1997) [19] | Van Soest | 2 | Y | Y | Y 1 | R. globostellata | - | N |
| Bourguet-Kondracki et al. (2000) [43] | Lévi | - | Y | Y | Y | R. globostellata | - | N |
| Zampella et al. (2000) [44] | Hooper | - | Y | Y | Y 2 | Jaspis sp. | R. globostellata | Y |
| Tabudravu & Jaspars (2001) [45] | Hooper | - | Y | Y | Y | G. globostellifera | R. globostellata | Y |
| Zhang & Che (2001) [28] | Chupu | - | Y | N | N | G. japonica | Geodia sp.? | Y |
| Meragelman et al. (2001) [46] | Kelly | - | Y | Y | Y 2 | Jaspis sp. | R. globostellata | Y |
| Tasdemir et al. (2002) [25] | Harper | - | Y | N | Y | R. globostellata | Rhabdastrella cf. globostellata | N |
| Lv et al. (2004) [47], Lv et al. (2008) [48] | Van Soest | - | Y | Y | Y 1 | R. aff. distincta | R. globostellata | Y |
| Tang et al. (2005) [49] Tang et al. (2006) [50], Tang et al. (2007) [51] | Van Soest? 3 | - | Y | N | Y | Jaspis sp. | R. globostellata | Y |
| Clement et al. (2006) [52] | Kelly | 1 | Y | Y 1 | Y 2 | R. globostellata | - | N |
| Fouad et al. (2006) [33] | Van Soest | 3 | Y | Y | Y | R. globostellata | - | N |
| Agrawal (2007) [53] | - | - | N | - | - | R. globostellata | - | - |
| Aoki et al. (2007) [54] | de Voogd | - | Y | Y | Y | R. globostellata | Rhabdastrella sp. | Y |
| Lin et al. (2007) [30] | JH. Li | - | Y | N | N | S. tenuis | R. globostellata | Y |
| Hirashima et al. (2010) [55] | Van Soest | - | Y | Y | Y | R. globostellata | - | N |
| Li et al. (2010) [56] Li et al. (2012) [57] | de Voogd | 1 | Y | Y | Y | R. globostellata | - | N |
| Tanaka et al. (2011) [34] | Fromont | 2, 3 | Y | Y | Y | R. cf. globostellata | R. globostellata | N |
| Tang et al. (2012) [31] | - | - | Y | N | N | J. stellifera | Rhabdastrella sp. | Y |
| Xue et al. (2013) [58] | JH. Li | - | Y | N | Y 2 | Stelletta sp. | R. ?globostellata | Y |
| Jin et al. (2014) [32], Xu et al. (2016) [59], Xu et al. (2018) [60] | Tang | - | Y | N | N | J. stellifera | Rhabdastrella sp. | Y |
| Li et al. (2015) [61] | JH. Li | - | Y | N | Y | S. tenuis | R. globostellata | Y |
| Kiem et al. (2018) [62], Dung et al. (2018a) [63], Dung et al. (2018b) [64] | Thun | 1 | Y | N | Y | R. providentiae | R. globostellata | Y |
| Kolesnikova et al. (2019) [35] Kolesnikova et al. (2021) [65] | Grebnev | 1, 2, 3 | Y | N | Y | Stelletta sp. | R. globostellata | Y |
| Lai et al. (2021) [27] | HH. Li | 1 4 | Y 4 | - | - | Rhabdastrella sp. | - | N |
| Trang et al. (2022) [66] | - | 4 | Y | N | Y | R. globostellata | - | N |
| Species | Compounds |
|---|---|
| Rhabdastrella globostellata | Isomalabaricane triterpene groups (ITTs) Stellettins1 Stellettins A-R, W 2 (–)-Stelettin E Rhabdastrellic Acid-A (=E isomer of stellettin G) 22,23-dihydrostellettins B, D Rhabdastrellins A-F Jaspiferins C-H Stelliferins Stelliferins A-N 29-hydroxystelliferin A, D, E 3-Epi-29-hydroxystelliferin A, E 3-Epi-29-acetoxystelliferin E Stelliferin A, D, ribosides Geoditins A-B (23E) Isogeoditins A-B (23Z) 13-(E)-isogeoditin A Globostellatic acids Globostellatic acids A-M Globostelletin A-I 13,17-Globostellatic acid X methyl esters Globostellatic acid F methyl ester Globostellatic acid B methyl ester |
| ITT derivatives Aurorals 1-4 (derivatives of globostellatic acids) Cyclobutastellettolides A and B Globostelletins A-I (derivatives of stellettin E?) Globostelletins J-R (cyclopentane side chain) Jaspiferals A-G (derivatives of globostellatic acids) 3-O-Acetyl-jaspiferals B/D/E methyl ester Jaspiferins A-B, I-J (derivatives of stelliferins?) Jaspiferoic acids A–B dimethyl esters (derivatives of globostellatic acids) Jaspolides A-B Jaspolides C-F (derivatives) Jaspolides G-H (dimeric) Rhabdastins A-G (derivatives of stelliferins?) Rhabdastrellins G-K Rhabdaprovidines A-C (biochemical degradation of ITTs?) Rhabdaprovidines D-G Stellettins S-V (nor-terpenoids) Rhabdaglostelones A-C (tetra/pentacyclic) | |
Other compounds Monocyclic triterpene glycoside: Rhabdastoside A, Sterols, [71] Fatty acids, [72] gibepyrone F, gibepyrone C, p-hydroxy benzaldehyde, 3-Indole-3-aldehyde, thymine, p-hydroxy benzaldehyde, 3-indole-3-aldehyde cyclo (S-leucyl-S-prolyl), cyclo (L-Pro-L-Phe) (from bacterial symbiont [73]), cyclo (L-Pro-L-Leu) (from bacterial symbiont [73]), cyclo (D-Pro-D-Val) (from bacterial symbiont [74]) | |
| Rhabdastrella sp. 3 Taiwan | Rhabdastins H-I |
| Rhabdastrella sp. 4 Indonesia | Globostellatic acid X methyl esters, Globostellatic acid F methyl ester, 13-(E)-Globostellatic acid B methyl ester, Acetyljaspiferal E |
| Geodia sp. South China Sea | Geoditins A-B (23E) Stellettins A-B |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cárdenas, P.; Gamage, J.; Hettiarachchi, C.M.; Gunasekera, S. Good Practices in Sponge Natural Product Studies: Revising Vouchers with Isomalabaricane Triterpenes. Mar. Drugs 2022, 20, 190. https://doi.org/10.3390/md20030190
Cárdenas P, Gamage J, Hettiarachchi CM, Gunasekera S. Good Practices in Sponge Natural Product Studies: Revising Vouchers with Isomalabaricane Triterpenes. Marine Drugs. 2022; 20(3):190. https://doi.org/10.3390/md20030190
Chicago/Turabian StyleCárdenas, Paco, Jayani Gamage, Chamari M. Hettiarachchi, and Sunithi Gunasekera. 2022. "Good Practices in Sponge Natural Product Studies: Revising Vouchers with Isomalabaricane Triterpenes" Marine Drugs 20, no. 3: 190. https://doi.org/10.3390/md20030190
APA StyleCárdenas, P., Gamage, J., Hettiarachchi, C. M., & Gunasekera, S. (2022). Good Practices in Sponge Natural Product Studies: Revising Vouchers with Isomalabaricane Triterpenes. Marine Drugs, 20(3), 190. https://doi.org/10.3390/md20030190