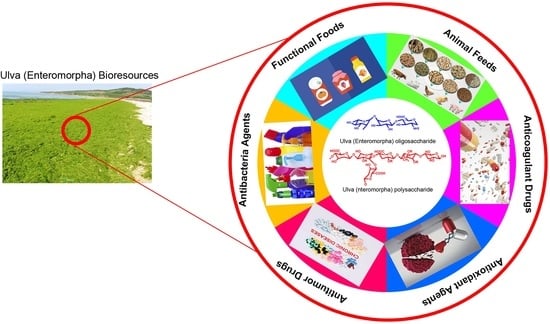
Ulva (Enteromorpha) Polysaccharides and Oligosaccharides: A Potential Functional Food Source from Green-Tide-Forming Macroalgae
Abstract
:1. Introduction
2. Ulva Polysaccharide
2.1. Chemical Composition and Structure of Ulva Polysaccharide
2.2. Extraction and Purification of Ulva Polysaccharide
2.3. Activity of Ulva Polysaccharide
2.3.1. Antioxidant Activity
2.3.2. Antitumor Activity
2.3.3. Immune Regulatory Activity
2.3.4. Anticoagulant Activity
2.3.5. Hypolipidemic Activity
3. Ulva Oligosaccharides
3.1. Preparation of Ulva Oligosaccharides
3.2. Separation and Purification of Ulva Oligosaccharides
3.3. Activity of Ulva Oligosaccharides
4. Conclusions and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blomster, J.; Bäck, S.; Fewer, D.P.; Kiirikki, M.; Lehvo, A.; Maggs, C.A.; Stanhope, M.J. Novel morphology in Enteromorpha (Ulvophyceae) forming green tides. Am. J. Bot. 2002, 89, 1756–1763. [Google Scholar] [CrossRef]
- Zhong, L.; Zhang, J.; Ding, Y. Energy Utilization of Algae Biomass Waste Enteromorpha Resulting in Green Tide in China: Pyrolysis Kinetic Parameters Estimation Based on Shuffled Complex Evolution. Sustainability 2020, 12, 2086. [Google Scholar] [CrossRef] [Green Version]
- Silveira Coelho, M.; da Silva Menezes, B.; Rivero Meza, S.L.; Lainetti Gianasi, B.; de las Mercedes Salas-Mellado, M.; Copertino, M.; da Rosa Andrade Zimmermann de Souza, M. Potential Utilization of Green Tide-Forming Macroalgae from Patos Lagoon, Rio Grande-RS, Brazil. J. Aquat. Food Prod. Technol. 2016, 25, 1096–1106. [Google Scholar] [CrossRef]
- Van Alstyne, K.L.; Nelson, T.A.; Ridgway, R.L. Environmental Chemistry and Chemical Ecology of “Green Tide” Seaweed Blooms. Integr. Comp. Biol. 2015, 55, 518–532. [Google Scholar] [CrossRef]
- Wang, Z.; Xiao, J.; Fan, S.; Li, Y.; Liu, X.; Liu, D. Who made the world’s largest green tide in China?—An integrated study on the initiation and early development of the green tide in Yellow Sea. Limnol. Oceanogr. 2015, 60, 1105–1117. [Google Scholar] [CrossRef]
- Yabe, T.; Ishii, Y.; Amano, Y.; Koga, T.; Hayashi, S.; Nohara, S.; Tatsumoto, H. Green tide formed by free-floating Ulva spp. at Yatsu tidal flat, Japan. Limnology 2009, 10, 239–245. [Google Scholar] [CrossRef]
- Zhang, W.; Oda, T.; Yu, Q.; Jin, J.-O. Fucoidan from Macrocystis pyrifera Has Powerful Immune-Modulatory Effects Compared to Three Other Fucoidans. Mar. Drugs 2015, 13, 1084–1104. [Google Scholar] [CrossRef] [Green Version]
- Xiao, J.; Wang, Z.; Liu, D.; Fu, M.; Yuan, C.; Yan, T. Harmful macroalgal blooms (HMBs) in China’s coastal water: Green and golden tides. Harmful Algae 2021, 107, 102061. [Google Scholar] [CrossRef]
- Hayden, H.S.; Blomster, J.; Maggs, C.A.; Silva, P.C.; Stanhope, M.J.; Waaland, J.R. Linnaeus was right all along: Ulva and Enteromorpha are not distinct genera. Eur. J. Phycol. 2003, 38, 277–294. [Google Scholar] [CrossRef]
- Jiao, L.; Jiang, P.; Zhang, L.; Wu, M. Antitumor and immunomodulating activity of polysaccharides from Enteromorpha intestinalis. Biotechnol. Bioprocess Eng. 2010, 15, 421–428. [Google Scholar] [CrossRef]
- Tang, Z.; Gao, H.; Wang, S.; Wen, S.; Qin, S. Hypolipidemic and antioxidant properties of a polysaccharide fraction from Enteromorpha prolifera. Int. J. Biol. Macromol. 2013, 58, 186–189. [Google Scholar] [CrossRef]
- Chattopadhyay, K.; Mandal, P.; Lerouge, P.; Driouich, A.; Ghosal, P.; Ray, B. Sulphated polysaccharides from Indian samples of Enteromorpha compressa (Ulvales, Chlorophyta): Isolation and structural features. Food Chem. 2007, 104, 928–935. [Google Scholar] [CrossRef]
- Jiang, F.; Chi, Z.; Ding, Y.; Quan, M.; Tian, Y.; Shi, J.; Liu, C. Wound Dressing Hydrogel of Enteromorpha prolifera Polysaccharide–Polyacrylamide Composite: A Facile Transformation of Marine Blooming into Biomedical Material. ACS Appl. Mater. Interfaces 2021, 13, 14530–14542. [Google Scholar] [CrossRef]
- Zhong, R.; Wan, X.; Wang, D.; Zhao, C.; Liu, D.; Gao, L.; Cao, H. Polysaccharides from Marine Enteromorpha: Structure and function. Trends Food Sci. Technol. 2020, 99, 11–20. [Google Scholar] [CrossRef]
- Tang, Z.; Yu, Z.; Zhao, W.; Guo, J.; Gao, L.; Qin, S. Ultrasonic extraction of polysaccharides from Enteromorpha. Mod. Food Sci. Technol. 2011, 27, 56–59. [Google Scholar]
- Liu, W.; Zhou, S.; Balasubramanian, B.; Zeng, F.; Sun, C.; Pang, H. Dietary seaweed (Enteromorpha) polysaccharides improves growth performance involved in regulation of immune responses, intestinal morphology and microbial community in banana shrimp Fenneropenaeus merguiensis. Fish Shellfish. Immunol. 2020, 104, 202–212. [Google Scholar] [CrossRef]
- Shang, Q.; Wang, Y.; Pan, L.; Niu, Q.; Li, C.; Jiang, H.; Cai CHao, J.; Li, G.; Yu, G. Dietary Polysaccharide from Enteromorpha Clathrata Modulates Gut Microbiota and Promotes the Growth of Akkermansia muciniphila, Bifidobacterium spp. and Lactobacillus spp. Mar. Drugs 2018, 16, 167. [Google Scholar] [CrossRef] [Green Version]
- Xie, C.; Zhang, Y.; Niu, K.; Liang, X.; Wang, H.; Shan, J.; Wu, X. Enteromorpha polysaccharide-zinc replacing prophylactic antibiotics contributes to improving gut health of weaned piglets. Anim. Nutr. 2021, 7, 641–649. [Google Scholar] [CrossRef]
- Guo, F.; Han, M.; Lin, S.; Ye, H.; Chen, J.; Zhu, H.; Lin, W. Enteromorpha prolifera polysaccharide prevents high-fat diet-induced obesity in hamsters: A NMR-based metabolomic evaluation. J. Food Sci. 2021, 86, 3672–3685. [Google Scholar] [CrossRef]
- Guo, F.; Zhuang, X.; Han, M.; Lin, W. Polysaccharides from Enteromorpha prolifera protect against carbon tetrachloride-induced acute liver injury in mice via activation of Nrf2/HO-1 signaling, and suppression of oxidative stress, inflammation and apoptosis. Food Funct. 2020, 11, 4485–4498. [Google Scholar] [CrossRef]
- Li, X.; Guozhu, Z.; Zhifei, L.; Yang, S.; Peijun, L.; Jing, L. Hypoglycemic activity of Enteromorpha intestinalis polysaccharide. Sci. Technol. Food Ind. 2021, 42, 321–326. [Google Scholar] [CrossRef]
- Zhu, B.; Ni, F.; Xiong, Q.; Yao, Z. Marine oligosaccharides originated from seaweeds: Source, preparation, structure, physiological activity and applications. Crit. Rev. Food Sci. Nutr. 2021, 61, 60–74. [Google Scholar] [CrossRef]
- Liu, X.; Liu, D.; Lin, G.; Wu, Y.; Gao, L.; Ai, C.; Zhao, C. Anti-ageing and antioxidant effects of sulfate oligosaccharides from green algae Ulva lactuca and Enteromorpha prolifera in SAMP8 mice. Int. J. Biol. Macromol. 2019, 139, 342–351. [Google Scholar] [CrossRef]
- Shao, L.; Xu, J.; Shi, M.; Wang, X.; Li, Y.; Kong, L.; Hider, R.; Zhou, T. Preparation, antioxidant and antimicrobial evaluation of hydroxamated degraded polysaccharides from Enteromorpha prolifera. Food Chem. 2017, 237, 481–487. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Xu, L.; Zhou, Q.; Hao, S.; Zhou, T.; Xie, H. Isolation, purification, and antioxidant activities of degraded polysaccharides from Enteromorpha prolifera. Int. J. Biol. Macromol. 2015, 81, 1026–1030. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, X.; Mo, X.; Qi, H. Degradation and the antioxidant activity of polysaccharide from Enteromorpha linza. Carbohydr. Polym. 2013, 92, 2084–2087. [Google Scholar] [CrossRef]
- Cho, M.; Yang, C.; Kim, S.M.; You, S. Molecular characterization and biological activities of watersoluble sulfated polysaccharides from Enteromorpha prolifera. Food Sci. Biotechnol. 2010, 19, 525–533. [Google Scholar] [CrossRef]
- Qi, X.; Mao, W.; Gao, Y.; Chen, Y.; Chen, Y.; Zhao, C.; Shan, J. Chemical characteristic of an anticoagulant-active sulfated polysaccharide from Enteromorpha clathrata. Carbohydr. Polym. 2012, 90, 1804–1810. [Google Scholar] [CrossRef]
- Ray, B. Polysaccharides from Enteromorpha compressa: Isolation, purification and structural features. Carbohydr. Polym. 2006, 66, 408–416. [Google Scholar] [CrossRef]
- Yu, Y.; Li, Y.; Du, C.; Mou, H.; Wang, P. Compositional and structural characteristics of sulfated polysaccharide from Enteromorpha prolifera. Carbohydr. Polym. 2017, 165, 221–228. [Google Scholar] [CrossRef]
- Qi, X.; Li, H.; Guo, S.; Chen, Y.; Chen, Y.; Xu, J.; Mao, W. Isolation and composition analysis of the polysaccharides from the green algae Enteromorpha prolifera collected in different seasons and sea areas. Period. Ocean. Univ. China 2010, 40, 7–12. [Google Scholar]
- Qi, X.; Mao, W.; Chen, Y.; Chen, Y.; Zhao, C.; Li, N.; Wang, C. Chemical characteristics and anticoagulant activities of two sulfated polysaccharides from Enteromorpha linza (Chlorophyta). J. Ocean. Univ. China 2013, 12, 175–182. [Google Scholar] [CrossRef]
- Shi, X. Investigation of Chemical Composition and Biological Activities of Polysaccharides from Enteromorpha; Institute of Oceanology, Chinese Academy of Sciences: Qingdao, China, 2009. [Google Scholar]
- Ji, G.; Yu, G.; Wu, J.; Zhao, X.; Yang, B.; Wang, L.; Mei, X. Extraction, isolation and physiochemical character studies of polysaccharides from Enteromorpha clathrata in outbreak period. Chin. J. Mar. Drugs 2009, 28, 7–12. [Google Scholar]
- Jiao, L.; Li, X.; Li, T.; Jiang, P.; Zhang, L.; Wu, M.; Zhang, L. Characterization and anti-tumor activity of alkali-extracted polysaccharide from Enteromorpha intestinalis. Int. Immunopharmacol. 2009, 9, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xiong, F.; Liu, Y.; Liu, F.; Hao, Z.; Chen, H. Total fractionation and characterization of the water-soluble polysaccharides isolated from Enteromorpha intestinalis. Int. J. Biol. Macromol. 2018, 111, 319–325. [Google Scholar] [CrossRef]
- Wu, M.; Jiao, L.; Sun, Y.; Li, T.; Zhang, L. Isolation purification and analysis on the EPIII of polysaccharide of Enteromorpha. J. Northeast Nor. Univ. 2007, 39, 97–100. [Google Scholar]
- Sun, S. Study on lowering blood lipid of Polysaccharide from Enteromorpha prolifera by alkali extraction. Chin. J. Mod. Drug Appl. 2010, 4, 118–119. [Google Scholar]
- Song, X.; Guo, X.; Zhou, W.; Wen, Y.; Zhu, C.; Yang, H. Composition and biological activity of water-soluble polysaccharide from Enteromorpha prolifera. Lishizhen Med. Mater. Med. Res. 2010, 21, 2448–2450. [Google Scholar]
- Lin, G.; Wu, D.; Xiao, X.; Huang, Q.; Chen, H.; Liu, D.; Zhao, C. Structural characterization and antioxidant effect of green alga Enteromorpha prolifera polysaccharide in Caenorhabditis elegans via modulation of microRNAs. Int. J. Biol. Macromol. 2020, 150, 1084–1092. [Google Scholar] [CrossRef]
- Guo, L.; Chen, Y. Optimization of ultrasonic-assisted extraction of polysaccharides from Enteromorpha prolifera by response surface methodology. Food Sci. 2010, 31, 117–121. [Google Scholar]
- Yang, B.; Zhao, M.; Jiang, Y. Anti-glycated activity of polysaccharides of longan (Dimocarpus longan Lour.) fruit pericarp treated by ultrasonication. Food Chem. 2009, 114, 629–633. [Google Scholar] [CrossRef]
- Zhou, C.; Ma, H. Ultrasonic Degradation of Polysaccharide from a Red Algae (Porphyra yezoensis). J. Agric. Food Chem. 2006, 54, 2223–2228. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Tong, G.Z.; Qu, Y.L.; Li, L. Microwave-Assisted Extraction and In Vitro Antioxidant Evaluation of Polysaccharides from Enteromorpha prolifera. Appl. Mech. Mater. 2011, 79, 204–209. [Google Scholar] [CrossRef]
- Yuan, Y.; Xu, X.; Jing, C.; Zou, P.; Zhang, C.; Li, Y. Microwave assisted hydrothermal extraction of polysaccharides from Ulva prolifera: Functional properties and bioactivities. Carbohydr. Polym. 2018, 181, 902–910. [Google Scholar] [CrossRef] [PubMed]
- Abdullah Al-Dhabi, N.; Ponmurugan, K. Microwave assisted extraction and characterization of polysaccharide from waste jamun fruit seeds. Int. J. Biol. Macromol. 2020, 152, 1157–1163. [Google Scholar] [CrossRef]
- Rostami, H.; Gharibzahedi, S.M.T. Microwave-assisted extraction of jujube polysaccharide: Optimization, purification and functional characterization. Carbohydr. Polym. 2016, 143, 100–107. [Google Scholar] [CrossRef]
- Guo, Y.; Shang, H.; Zhao, J.; Zhang, H.; Chen, S. Enzyme-assisted extraction of a cup plant (Silphium perfoliatum L.) Polysaccharide and its antioxidant and hypoglycemic activities. Process Biochem. 2020, 92, 17–28. [Google Scholar] [CrossRef]
- Nadar, S.S.; Rao, P.; Rathod, V.K. Enzyme assisted extraction of biomolecules as an approach to novel extraction technology: A review. Food Res. Int. 2018, 108, 309–330. [Google Scholar] [CrossRef]
- Song, X.; Xu, Q.; Zhou, Y.; Lin, C.; Yang, H. Growth, feed utilization and energy budgets of the sea cucumber Apostichopus japonicus with different diets containing the green tide macroalgae Chaetomorpha linum and the seagrass Zostera marina. Aquaculture 2017, 470, 157–163. [Google Scholar] [CrossRef]
- Xiong, Q.; Song, Z.; Hu, W.; Liang, J.; Jing, Y.; He, L.; Li, S. Methods of extraction, separation, purification, structural characterization for polysaccharides from aquatic animals and their major pharmacological activities. Crit. Rev. Food Sci. Nutr. 2020, 60, 48–63. [Google Scholar] [CrossRef]
- Lü, H.; Gao, Y.; Shan, H.; Lin, Y. Preparation and antibacterial activity studies of degraded polysaccharide selenide from Enteromorpha prolifera. Carbohydr. Polym. 2014, 107, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Ren, R.; Yang, Z.; Zhao, A.; Huang, Y.; Lin, S.; Gong, J.; Lin, W. Sulfated polysaccharide from Enteromorpha prolifera increases hydrogen sulfide production and attenuates non-alcoholic fatty liver disease in high-fat diet rats. Food Funct. 2018, 9, 4376–4383. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Wu, H.; Pan, M.; Zhang, Y.; Wei, X.; Cheng, J. Separation, purification and component analysis of Enteromorpha polysaccharides from Jiangsu. Chin. J. New Drugs 2019, 28, 2274–2278. [Google Scholar]
- Lin, W.; Wang, W.; Liao, D.; Chen, D.; Zhu, P.; Cai, G.; Kiyoshi, A. Polysaccharides from Enteromorpha prolifera improve glucose metabolism in diabetic rats. J. Diabetes Res. 2015, 2015, 675201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, W.; Zhang, H.; Duan, R.; Xie, Q.; Hong, Z.; Xiao, Z. A rapid and scalable integrated membrane separation process for purification of polysaccharides from Enteromorpha prolifera. Nat. Prod. Res. 2019, 33, 3109–3119. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Gao, B.; Yue, Q.; Song, W.; Jia, R.; Liu, P. RETRACTED: Evaluation of floc properties and membrane fouling in coagulation–ultrafiltration system: The role of Enteromorpha polysaccharides. Desalination 2015, 367, 126–133. [Google Scholar] [CrossRef]
- Xu, J.; Xu, L.-L.; Zhou, Q.-W.; Hao, S.-X.; Zhou, T.; Xie, H.-J. Enhanced in Vitro Antioxidant Activity of Polysaccharides From Enteromorpha Prolifera by Enzymatic Degradation. J. Food Biochem. 2016, 40, 275–283. [Google Scholar] [CrossRef]
- Minton, N.P. Clostridia in cancer therapy. Nat. Rev. Microbiol. 2003, 1, 237–242. [Google Scholar] [CrossRef]
- Sawyers, C. Targeted cancer therapy. Nature 2004, 432, 294–297. [Google Scholar] [CrossRef]
- Kim, J.-K.; Cho, M.L.; Karnjanapratum, S.; Shin, I.-S.; You, S.G. In vitro and in vivo immunomodulatory activity of sulfated polysaccharides from Enteromorpha prolifera. Int. J. Biol. Macromol. 2011, 49, 1051–1058. [Google Scholar] [CrossRef]
- Delves, P.J.; Roitt, I.M. The Immune System. N. Engl. J. Med. 2000, 343, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Parkin, J.; Cohen, B. An overview of the immune system. Lancet 2001, 357, 1777–1789. [Google Scholar] [CrossRef]
- Levy, M.; Kolodziejczyk, A.A.; Thaiss, C.A.; Elinav, E. Dysbiosis and the immune system. Nat. Rev. Immunol. 2017, 17, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Liu, L.; Gamallat, Y.; Zhang, B.; Xin, Y. Enteromorpha and polysaccharides from Enteromorpha ameliorate loperamide-induced constipation in mice. Biomed. Pharmacother. 2017, 96, 1075–1081. [Google Scholar] [CrossRef] [PubMed]
- Zou, M.; Chen, Y.; Sun-Waterhouse, D.; Zhang, Y.; Li, F. Immunomodulatory acidic polysaccharides from Zizyphus jujuba cv. Huizao: Insights into their chemical characteristics and modes of action. Food Chem. 2018, 258, 35–42. [Google Scholar] [CrossRef]
- Mao, W.; Zang, X.; Li, Y.; Zhang, H. Sulfated polysaccharides from marine green algae Ulva conglobata and their anticoagulant activity. J. Appl. Phycol. 2006, 18, 9–14. [Google Scholar] [CrossRef]
- Yasantha, A.; KiWan, L.; SeKwon, K.; YouJin, J. Anticoagulant activity of marine green and brown algae collected from Jeju Island in Korea. Bioresour. Technol. 2007, 98, 1711–1716. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Z.; Yao, Z.; Zhao, M.; Qi, H. Sulfation, anticoagulant and antioxidant activities of polysaccharide from green algae Enteromorpha linza. Int. J. Biol. Macromol. 2013, 58, 225–230. [Google Scholar] [CrossRef]
- Jain, K.S.; Kathiravan, M.K.; Somani, R.S.; Shishoo, C.J. The biology and chemistry of hyperlipidemia. Bioorganic Med. Chem. 2007, 15, 4674–4699. [Google Scholar] [CrossRef]
- Ross, R.; Harker, L. Hyperlipidemia and Atherosclerosis: Chronic hyperlipidemia initiates and maintains lesions by endothelial cell desquamation and lipid accumulation. Science 1976, 193, 1094–1100. [Google Scholar] [CrossRef]
- Teng, Z.; Qian, L.; Zhou, Y. Hypolipidemic activity of the polysaccharides from Enteromorpha prolifera. Int. J. Biol. Macromol. 2013, 62, 254–256. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Xiong, Q.; Gan, D.; Jiao, Y.; Liu, J.; Ma, L.; Zeng, X. Antioxidant activity and potential hepatoprotective effect of polysaccharides from Cyclina sinensis. Carbohydr. Polym. 2013, 91, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Liu, S.; Xing, R.; Li, K.; Li, R.; Qin, Y.; Li, P. Degradation of sulfated polysaccharides from Enteromorpha prolifera and their antioxidant activities. Carbohydr. Polym. 2013, 92, 1991–1996. [Google Scholar] [CrossRef] [PubMed]
- Duan, K.; Shan, H.; Lin, Y.; Lv, H. Degradation of polysaccharide from Enteromorpha prolifera with hydrochloric acid and hydrogen peroxide assisted by microwave and its antioxidant activity. Food Sci. Technol. 2015, 40, 141–147. [Google Scholar]
- Li, Y.; Wang, J.; Yu, Y.; Li, X.; Jiang, X.; Hwang, H.; Wang, P. Production of enzymes by Alteromonas sp. A321 to degrade polysaccharides from Enteromorpha prolifera. Carbohydr. Polym. 2013, 98, 988–994. [Google Scholar] [CrossRef]
- Zhang, Z.; Han, X.; Xu, Y.; Li, J.; Li, Y.; Hu, Z. Biodegradation of Enteromorpha polysaccharides by intestinal micro-community from Siganus oramin. J. Ocean Univ. China 2016, 15, 1034–1038. [Google Scholar] [CrossRef]
- Li, Y.; Li, W.; Zhang, G.; Lü, X.; Hwang, H.; Aker, W.G.; Wang, P. Purification and characterization of polysaccharides degradases produced by Alteromonas sp. A321. Int. J. Biol. Macromol. 2016, 86, 96–104. [Google Scholar] [CrossRef]
- Patra, J.K.; Baek, K.-H. Antibacterial Activity and Action Mechanism of the Essential Oil from Enteromorpha linza L. against Foodborne Pathogenic Bacteria. Molecules 2016, 21, 388. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Keesing, J.K.; Dong, Z.; Zhen, Y.; Di, B.; Shi, Y.; Shi, P. Recurrence of the world’s largest green-tide in 2009 in Yellow Sea, China: Porphyra yezoensis aquaculture rafts confirmed as nursery for macroalgal blooms. Mar. Pollut. Bull. 2010, 60, 1423–1432. [Google Scholar] [CrossRef]
- Cui, J.; Li, Y.; Wang, S.; Chi, Y.; Hwang, H.; Wang, P. Directional preparation of anticoagulant-active sulfated polysaccharides from Enteromorpha prolifera using artificial neural networks. Sci. Rep. 2018, 8, 3062. [Google Scholar] [CrossRef]
- Jin, W.; He, X.; Long, L.; Fang, Q.; Wei, B.; Sun, J.; Linhardt, R.J. Structural characterization and anti-lung cancer activity of a sulfated glucurono-xylo-rhamnan from Enteromorpha prolifera. Carbohydr. Polym. 2020, 237, 116143. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; He, P.; Li, H.; Li, G.; Liu, J.; Jiao, F.; Jiao, N. Ulva prolifera green-tide outbreaks and their environmental impact in the Yellow Sea, China. Natl. Sci. Rev. 2019, 6, 825–838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Paula Silva, P.H.; McBride, S.; de Nys, R.; Paul, N.A. Integrating filamentous ‘green tide’ algae into tropical pond-based aquaculture. Aquaculture 2008, 284, 74–80. [Google Scholar] [CrossRef]
- Song, Y.; Han, A.; Park, S.; Cho, C.; Rhee, Y.; Hong, H. Effect of enzyme-assisted extraction on the physicochemical properties and bioactive potential of lotus leaf polysaccharides. Int. J. Biol. Macromol. 2020, 153, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, X.; Jin, W.; Guo, Y. Immunomodulatory Effects of a Low-Molecular Weight Polysaccharide from Enteromorpha prolifera on RAW 264.7 Macrophages and Cyclophosphamide-Induced Immunosuppression Mouse Models. Mar. Drugs 2020, 18, 340. [Google Scholar] [CrossRef] [PubMed]





| Ulva linza | Ulva prolifera | Ulva clathrata | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fraction | MCS | Main components | [→4)-α-L-Rhap-(1→] [→2,4)-α-L-Rhap-(1→] [→4)-β-D-Xylp-(1→] [→4)-β-D-GlcUAp-(1→] | Fraction | QC1S | Main components | [→4)-α-L-Rhap-(1→] [→4)-β-D-Xylp-(1→] | Fraction | XCS | Main components | [→2)-β-D-Galp-(1→] [→3)-β-D-Galp-(11→] [→4)-β-D-Galp-(1→] [→6)-β-D-Galp-(1→] |
| Other components | [→3)-α-L-Rhap-(1→] [→2)-α-L-Rhap-(1→] | Other components | [→2)-α-L-Rhap4S-(1→] [→3)-α-L-Rha4S-(1→] [→4)-α-L-Rhap2S-(1→] [→3,4)-α-L-Rhap-(1→] | Other components | [→4)-β-L-Arap-(1→] [→2)-α-L-Rhap-(1→] [→3)-α-L-Rhap-(1→] [→2)-α-L-Rhap-(1→] | ||||||
| Sulfated position | The C3 of [→4)-α-L-Rhap-(1→] | Sulfated position | The C6 or C2 [→4)-β-D-Galp-(1→], the C4 or C2 of [→6)-β-D-Galp-(1→] | ||||||||
| MHS | Main components | [→4)-α-L-Rhap-(1→] [→2,4)-α-L-Rhap-(1→] [→4)-β-D-Xylp-(1→] [→4)-β-D-GlcUAp-(1→] | QHS | Main components | [→4)-α-L-Rhap-(1→] [→4)-β-D-Xylp-(1→] [→4)-β-D-Glc UAp-(1→] | XH1S | Main components | [→4)-β-L-Arap-(1→] | |||
| Other components | [→3)-α-L-Rhap-(1→] [→2)-α-L-Rhap-(1→] | Other components | [→3)-α-L-Rhap-(1→] [→2,4)-α-L-Rhap-(1→] | Other components | [→3)-β-D-Galp-(1→] [→4)-β-D-Galp-(1→] [→6)-β-D-Galp-(1→] | ||||||
| Sulfated position | The C3 of [→4)-α-L-Rhap-(1→] | Sulfated position | The C3 of [→4)-α-L-Rhap-(1→] | Sulfated position | The C3 of [→4)-β-L-Arap-(1→] | ||||||
| SCS | Main components | [→3)-α-L-Rhap-(1→] [→2)-α-L-Rhap-(1→] [→4)-β-D-Xylp-(1→] [→4)-α-L-Rhap-(1→] [→2,4)-α-L-Rhap-(1→] | QCQ2 | - | XH2S | Main components | [→4)-β-L-Arap3S-(1→] | ||||
| Sulfated position | The C2 or C4 of [→3)-α-L-Rhap-(1→], the C3 or C4 [→2)-α-L-Rhap-(1→], the C2 of [→4)-α-L-Rhap-(1→] | Other components | [→4)-β-L-Arap-(1→] [→3)-α-L-Rhap4S-(1→] | ||||||||
| SH1S | Main components | [→4)-α-L-Rhap-(1→] [→3)-α-L-Rhap-(1→] [→2,4)-α-L-Rhap-(1→] [→4)-β-D-Xylp-(1→] [→4)-β-D-Glc UAp-(1→] | QCQ3 | - | |||||||
| Sulfated position | The C3 of [→4)-α-L-Rhap-(1→] | ||||||||||
| SH2S | Main components | [→4)-α-L-Rhap-(1→] [→4)-β-D-Xylp-(1→] | |||||||||
| Other components | [→2)-α-L-Rhap-(1→] [→3)-α-L-Rhap-(1→] [→3,4)-α-L-Rhap-(1→] [→2,3)-α-L-Rhap-(1→] [→2,4)-α-L-Rhap-(1→] | ||||||||||
| Sulfated position | The C3 of [→4)-α-L-Rhap-(1→] | ||||||||||
| [→4)-β-D-Xylp-(1→] | |||||||||||
| Other components | [→2)-α-L-Rhap-(1→] [→3)-α-L-Rhap-(1→] [→3,4)-α-L-Rhap-(1→] [→2,3)-α-L-Rhap-(1→] [→2,4)-α-L-Rhap-(1→] | ||||||||||
| Extraction Method | Procedure Time | Yield | Recovery | Reference |
|---|---|---|---|---|
| Hot water extraction with Hot water (90 °C) | 4 h | 21.96% | - | [25] |
| Hot water extraction with Hot water (80 °C) | 1.5 h | 18% | - | [12] |
| Hot solution extraction with 95% of alcohol (80 °C) | 2 h | - | 70% | [37] |
| Hot alkaline solution extraction with 0.5 M NaOH (90 °C) | 2 h | 33.3% | - | [38] |
| Acidic solution extraction with 0.05 M HCl | 2 h | 86.1% | [39] | |
| Ultrasonication treatment Ultrasonication treatment | 28 min | 25.84% | - | [41] |
| Ultrasonication treatment Ultrasonication (531.17 W) | 4.8 min | 17.42% | - | [15] |
| Ultrasonication treatment Ultrasonication (610 W) | - | - | 7.58% | [44] |
| Ultrasonication treatment Ultrasonication (610 W) | - | - | 4.04% | [45] |
| Enzymatic extraction with Protease | - | 27.75% | - | [52] |
| Enzymatic extraction with Cellulase | - | 20.22% | - | [25] |
| Purification Method | Column | Mobile Phase | Speed | Reference |
|---|---|---|---|---|
| IEC | Q Sepharose Fast Flow | 0~2 M NaCl | 0.5~2 mL/min | [31] |
| IEC | DEAE Sepharose Fast Flow | 0~2 M NaCl | 0.92 mL/min | [33] |
| IEC | DEAE Sepharose CL-6B | 0.9%NaCl | 0.18 mL/min | [35] |
| IEC | DEAE Cellulose 52 | 0.2~0.8 M NaCl | 0.5 mL/min | [25] |
| IEC | DEAE Sephadex A-25 | 0~4 M NaCl | 0.5 mL/min | [58] |
| GPC | Sephadex G-75 | H2O | 1.0 mL/min | [25] |
| GPC | Sephadex G-100 | H2O | 0.4 mL/min | [52] |
| GPC | SephacryTm S-300 HR | 0.9% NaCl | 0.5 mL/min | [58] |
| GPC | Sephacryl S-300 HR | 0.2 M NH4HCO3 | 0.5 mL/min | [30] |
| GPC | Sephacryl S-400/HR | 0.2 M NH4HCO3 | 0.3 mL/min | [28] |
| IEC+GPC | DEAE Cellulose 52, Bio-Gel P-2 | 0.7 M NaCl | 0.85 mL/min | [40] |
| IEC+GPC | DEAE-Sepharose CL-6B, Sephadex G-200 | 0.2~1.5 M NaCl | 0.8 mL/min | [11] |
| Preparation Method | Structure | Molecular Weight | Bioactivities | Reference |
|---|---|---|---|---|
| Microwave-assisted acid hydrolysis | - | 3.1 kDa | Antioxidant activity | [76] |
| Microwave-assisted acid hydrolysis | - | 53.59 kDa | Antioxidant activity | [75] |
| H2O2 degradation | - | - | Antioxidant activity | [26] |
| Enzymatic degradation | - | 243, 341, 401, 503, 665 Da | - | [77] |
| Enzymatic degradation | - | 103, 45.4, 9.8 kDa | Antioxidant activity | [25] |
| Enzymatic degradation | Rha1(SO3H)1, Rha1(SO3H)1Glc1, Rha2(SO3H)2Glc1, Rha3(SO3H)3Glc1Xyl1 | 244, 402, 628, 760 Da | - | [78] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ning, L.; Yao, Z.; Zhu, B. Ulva (Enteromorpha) Polysaccharides and Oligosaccharides: A Potential Functional Food Source from Green-Tide-Forming Macroalgae. Mar. Drugs 2022, 20, 202. https://doi.org/10.3390/md20030202
Ning L, Yao Z, Zhu B. Ulva (Enteromorpha) Polysaccharides and Oligosaccharides: A Potential Functional Food Source from Green-Tide-Forming Macroalgae. Marine Drugs. 2022; 20(3):202. https://doi.org/10.3390/md20030202
Chicago/Turabian StyleNing, Limin, Zhong Yao, and Benwei Zhu. 2022. "Ulva (Enteromorpha) Polysaccharides and Oligosaccharides: A Potential Functional Food Source from Green-Tide-Forming Macroalgae" Marine Drugs 20, no. 3: 202. https://doi.org/10.3390/md20030202
APA StyleNing, L., Yao, Z., & Zhu, B. (2022). Ulva (Enteromorpha) Polysaccharides and Oligosaccharides: A Potential Functional Food Source from Green-Tide-Forming Macroalgae. Marine Drugs, 20(3), 202. https://doi.org/10.3390/md20030202