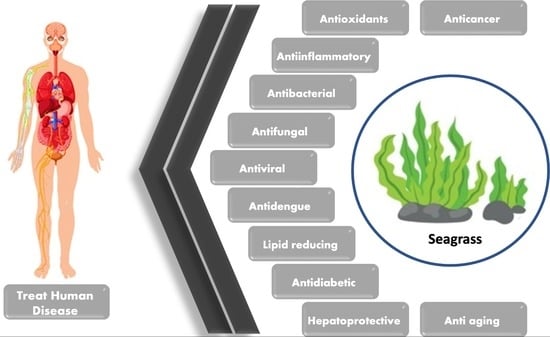
A Comprehensive Update on the Bioactive Compounds from Seagrasses
Abstract
:1. Introduction
2. Methods
| Family | Species |
|---|---|
| Cymodoceaceae | Cymodocea nodosa Cymodocea rotundata Cymodocea serrulata Halodule pinifolia Halodule uninervis Syringodium isoetifolium Syringodium filiforme Thalassodendron ciliatum |
| Hydrocharitaceae | Enhalus acoroides Halophila beccarii Halophila ovalis Halophila ovata Halophila stipulaceae Thalassia hemprichii Thalassia testudinum |
| Posidoniaceae | Posidonia oceanica |
| Zosteraceae | Zostera marina Zostera noltei |
3. Bioactivities of Extracts and Compounds Isolated from Seagrasses
3.1. Anticancer


| Species | Extract/Active Compound | Cell Line | Inhibition | References |
|---|---|---|---|---|
| C. serrulata | AgNPs (silver nanoparticles) | HeLa | IC50: 34.5 µg/mL | [26] |
| Aqueous extract | HeLa | IC50: 107.7 µg/mL | [26] | |
| H. stipulacea | Ethyl acetate extract (leaves) | MG63 | IC50: 29.4 μg/mL | [21] |
| SHSY5Y | IC50: 10.6 μg/mL | [21] | ||
| Ethyl acetate extract (stems) | MG63 | IC50: 19.1 μg/mL | [21] | |
| SHSY5Y | IC50: 18.7 μg/mL | [21] | ||
| Hexane extract (leaves) | HCT116 | IC50: 19.5 μg/mL | [21] | |
| Hexane extract (stems) | HCT116 | IC50: 7.6 μg/mL | [21] | |
| P. oceanica | EtOH/H2O (7:3) | SH-SY5Y | Inhibits 57% cell migration at 3 μg/mL after 7 h treatment | [33] |
| Hydrophilic extract | HT1080 | Inhibits 72.3% cell migration after 12 h treatment | [27] | |
| MeOH/H2O 7:3 (balls extract) | HepG2 | IC50: 24.3 µg/mL | [25] | |
| MCF7 | IC50: 22.6 µg/mL | [25] | ||
| HCT116 | IC50: 22.5 µg/mL | [25] | ||
| MeOH/H2O 7:3 (leaves extract) | HepG2 | IC50: 17 µg/mL | [25] | |
| HepG2 | IC50: 28.3 µg/mL | [25] | ||
| HCT116 | IC50: 27.8 µg/mL | [25] | ||
| S. filiforme | Chloroform fraction of hydroethanolic extract | A549 | Decreases the viability of A549 cells below 60% at 100 µg/mL | [34] |
| T. ciliatum | Methanolic extract | HCT-116 | IC50: 4.2 μg/mL | [17] |
| HeLa | IC50: 9.8 μg/mL | [17] | ||
| HepG2 | IC50: 8.12 μg/mL | [17] | ||
| MCF7 | IC50: 4.12 μg/mL | [17] | ||
| T. testudinum | Chloroform fraction of the hydroethanolic extract | A549 | IC50: 20.4 µg/mL | [28] |
| EA.hy926 | IC50: 248.4 µg/mL | [28] | ||
| Hydroethanolic extract | RKO | IC50: 174.9 µg/mL | [29] | |
| SW480 | IC50: 58.9 µg/mL | [29] | ||
| CT26 | IC50: 115.3 µg/mL | [29] | ||
| HepG2 | IC50: 102 μg/mL | [30] | ||
| PC12 | IC50: 135 μg/mL | [30] | ||
| Caco2 | IC50: 165 μg/mL | [30] | ||
| 4T1 | IC50: 129 μg/mL | [30] | ||
| Polyphenol fraction of hydroethanolic extract | HCT15 | IC50: 22.47 µg/mL | [23] | |
| HT29 | IC50: 93.11 µg/mL | [23] | ||
| HT29 | IC50: 121.71 µg/mL | [23] |
3.2. Antioxidant

| Species | Extract/Active Compound | % Inhibition | Assay | References |
|---|---|---|---|---|
| C. nodosa | Sulfated polysaccharide | OD: 0.3 mm at 1 mg/mL | FRAP | [51] |
| 82.44% inhibition at 0.5 mg/mL | DPPH | [51] | ||
| 82.6% inhibition at 2 mg/mL | ABTS | [51] | ||
| C. rotundata | Aqueous methanol (1:4) | 70.30% | DPPH | [52] |
| 53.74% | Hydroxyl radical scavenging activity | [52] | ||
| Ethyl acetate extract | 50% inhibition at 362.56 ppm | DPPH | [53] | |
| Methanolic extract | 50% inhibition at 214.68 ppm | DPPH | [53] | |
| C. serrulata | Aqueous extract | 65.68% inhibition at 100 μg/mL | DPPH | [26] |
| Aqueous fraction | 53.8% inhibition at 600 µg/mL | DPPH | [54] | |
| Aqueous methanol (1:4) | 41.28% | DPPH | [52] | |
| Butanol fraction | 82.6% inhibition at 600 µg/mL | DPPH | [54] | |
| Ethanolic extract | 28.423 mg Gallic acid/g | FRAP | [55] | |
| 61.85% | DPPH | [55] | ||
| Ethyl acetate fraction | 89.45% inhibition at 600 µg/mL | DPPH | [54] | |
| Petroleum ether fraction | 26.75% inhibition at 600 µg/mL | DPPH | [54] | |
| Silver nanoparticles | 87.99% inhibition at 100 μg/mL | DPPH | [26] | |
| E. acoroides | Aqueous extract | 30.68% inhibition at 200 µg/mL | DPPH | [56] |
| 83.67% inhibition at 200 µg/mL | ABTS | [56] | ||
| 44.91% inhibition at 200 µg/mL | SO assay | [56] | ||
| 56.64% inhibition at 200 µg/mL | NO assay | [56] | ||
| 0.42% inhibition at 200 µg/mL | FRAP | [56] | ||
| Aqueous fraction | 15.8% inhibition at 600 µg/mL | DPPH | [54] | |
| Aqueous methanol (1:4) | 35.80% | DPPH | [52] | |
| Butanol fraction | 19.4% inhibition at 600 µg/mL | DPPH | [54] | |
| Chloroform extract | 32.92% inhibition at 200 µg/mL | DPPH | [56] | |
| 60.52% inhibition at 200 µg/mL | ABTS | [56] | ||
| 52.18% inhibition at 200 µg/mL | SO assay | [56] | ||
| 22.6% inhibition at 200 µg/mL | NO assay | [56] | ||
| 0.21% inhibition at 200 µg/mL | FRAP | [56] | ||
| Ethanolic extract | 30% inhibition at 200 µg/mL | DPPH | [56] | |
| 42.93% inhibition at 200 µg/mL | ABTS | [56] | ||
| 36.94% inhibition at 200 µg/mL | SO assay | [56] | ||
| 39.7% inhibition at 200 µg/mL | NO assay | [56] | ||
| 0.17% inhibition at 200 µg/mL | FRAP | [56] | ||
| 3.373 mg gallic acid/g | FRAP | [55] | ||
| 24.13% | DPPH | [55] | ||
| Ethyl acetate extract | 50% inhibition at 153.4 ppm | DPPH | [53] | |
| 30.72% inhibition at 200 µg/mL | DPPH | [56] | ||
| 78.31% inhibition at 200 µg/mL | ABTS | [56] | ||
| 44.56% inhibition at 200 µg/mL | SO assay | [56] | ||
| 29.33% inhibition at 200 µg/mL | NO assay | [56] | ||
| 0.21% inhibition at 200 µg/mL | FRAP | [56] | ||
| Ethyl acetate fraction | 80.57% inhibition at 600 µg/mL | DPPH | [54] | |
| Hexane extract | 26.88% inhibition at 200 µg/mL | DPPH | [56] | |
| 61.43% inhibition at 200 µg/mL | ABTS | [56] | ||
| 42.7% inhibition at 200 µg/mL | SO assay | [56] | ||
| 25.98% inhibition at 200 µg/mL | NO assay | [56] | ||
| 0.21% inhibition at 200 µg/mL | FRAP | [56] | ||
| Methanolic extract | 50% inhibition at 115.79 ppm | DPPH | [53] | |
| 70.2 mg Trolox equivalents (TE)/g DM | ORAC | [57] | ||
| Petroleum ether fraction | 33.75% inhibition at 600 µg/mL | DPPH | [54] | |
| H. beccarii | Aqueous fraction | 24.4% inhibition at 600 µg/mL | DPPH | [54] |
| Aqueous fraction of aqueous methanol 1:1 extract | IC50: 31.8 µg/mL | DPPH | [45] | |
| Butanol fraction | 13.9% inhibition at 600 µg/mL | DPPH | [54] | |
| Ethyl acetate fraction | 84.56% inhibition at 600 µg/mL | DPPH | [54] | |
| Petroleum ether fraction | 14.33% inhibition at 600 µg/mL | DPPH | [54] | |
| H. ovalis | Acetone extract | 73.55% | DPPH | [58] |
| 23.58% | Hydrogen peroxide scavenging activity | [58] | ||
| Aqueous fraction | 5.2% inhibition at 600 µg/mL | DPPH | [54] | |
| Butanol fraction | 12.2% inhibition at 600 µg/mL | DPPH | [54] | |
| Ethanolic extract | 12.042 mg gallic acid/g | FRAP | [55] | |
| Ethyl acetate fraction | 6.68% inhibition at 600 µg/mL | DPPH | [54] | |
| Hexane extract | 8.20% | DPPH | [58] | |
| 21.21% | DPPH | [55] | ||
| Methanolic extract | IC50: 0.13 mg/mL | DPPH | [39] | |
| IC50: 0.65 mg/mL | Superoxide radicals scavenged | [39] | ||
| 72.5 mg Trolox equivalents (TE)/g DM | ORAC | [57] | ||
| Petroleum ether fraction | 4.77% inhibition at 600 µg/mL | DPPH | [54] | |
| H. ovata | Ethanolic extract | 5.856 mg gallic acid/g | FRAP | [55] |
| 16.93% | DPPH | [55] | ||
| H. pinifolia | Acetone extract | 66.98% | DPPH | [58] |
| 10.63% | NO scavenging activity | [58] | ||
| Aqueous fraction | 22.2% inhibition at 600 µg/mL | DPPH | [54] | |
| Aqueous methanol (1:4) | 58.60% | DPPH | [52] | |
| 51.05% | hydroxyl radical scavenging activity | [52] | ||
| Butanol fraction | 28.4% inhibition at 600 µg/mL | DPPH | [54] | |
| Ethanolic extract | 42.611 mg gallic acid/g | FRAP | [55] | |
| 68.07% | DPPH | [55] | ||
| Ethyl acetate fraction | 80.25% inhibition at 600 µg/mL | DPPH | [54] | |
| Hexane extract | 68.64% | Hydrogen peroxide scavenging activity | [58] | |
| 23.45% | DPPH | [58] | ||
| Methanolic extract | 87.81% | DPPH | [58] | |
| 71.49% | Hydrogen peroxide scavenging activity | [58] | ||
| 97.7 mg Trolox equivalents (TE)/g DM | ORAC | [57] | ||
| Petroleum ether fraction | 21.02% inhibition at 600 µg/mL | DPPH | [54] | |
| H. stipulacea | Ethanolic extract | 46.289 mg gallic acid/g | FRAP | [55] |
| 67.41% | DPPH | [55] | ||
| H. stipulacea (old leaf extract) | EtOH/H2O (3:1) | 85% inhibition at 100 µg/mL | DPPH | [41] |
| H. stipulacea (young leaf extract) | 45% inhibition at 100 µg/mL | DPPH | [41] | |
| S. filiforme | MeOH/H2O (1:1) | IC50: 0.8 mg/mL | DPPH | [59] |
| S. isoetifolium | Acetone extract | 45.69% | DPPH | [58] |
| 49.24% | NO scavenging activity | [58] | ||
| Aqueous fraction | 16.2% inhibition at 600 µg/mL | DPPH | [54] | |
| Aqueous methanol (1:4) | 51.56% | DPPH | [52] | |
| 48.42% | Hydroxyl radical scavenging activity | [52] | ||
| Butanol fraction | 6.2% inhibition at 600 µg/mL | DPPH | [54] | |
| Ethanolic extract | 26.557 mg gallic acid/g | FRAP | [55] | |
| 23.68% | DPPH | [55] | ||
| Ethyl acetate | 50% inhibition at 96.34 ppm | DPPH | [53] | |
| Ethyl acetate fraction | 6.36% inhibition at 600 µg/mL | DPPH | [54] | |
| Hexane extract | 15.19% | DPPH | [58] | |
| 51.49% | NO Scavenging Activity | [58] | ||
| Methanolic extract | 83.03% | DPPH | [58] | |
| 50% inhibition at 520.91 ppm | DPPH | [53] | ||
| 5.39 mgTE/g | DPPH | [60] | ||
| 9.56 mgTE/g | ABTS | [60] | ||
| 18.66 mgTE/g | CUPRAC | [60] | ||
| 9.53 mgTE/g | FRAP | [60] | ||
| 0.33 mmolTE/g | PHPD | [60] | ||
| 9.17 mgEDTAE/g | Chelating ability | [60] | ||
| Petroleum ether fraction | 10.2% inhibition at 600 µg/mL | DPPH | [54] | |
| T. ciliatum | Catechins | 50% inhibition at 3.82 mM | DPPH | [17] |
| Methanolic extract | 71% inhibition at 1 mg/mL | DPPH | [17] | |
| T. hemprichii | Aqueous fraction | 26.6% inhibition at 600 µg/mL | DPPH | [54] |
| Aqueous methanol (1:4) | 38.62% | DPPH | [52] | |
| 35.25% | Hydroxyl radical scavenging activity | [52] | ||
| Butanol fraction | 84.9% inhibition at 600 µg/mL | DPPH | [54] | |
| Ethanolic extract | 27.979 mg gallic acid/g | FRAP | [55] | |
| 61.64% | DPPH | [55] | ||
| Ethyl acetate extract | IC50: 25.98 µg/mL | DPPH | [61] | |
| 50% inhibition at 250.72 ppm | DPPH | [53] | ||
| Ethyl acetate fraction | 94.34% inhibition at 600 µg/mL | DPPH | [54] | |
| Hexane extract | IC50: 139.5 µg/mL | DPPH | [61] | |
| Methanolic extract | 50% inhibition at 123.72 ppm | DPPH | [53] | |
| Petroleum ether fraction | 42.67% inhibition at 600 µg/mL | DPPH | [54] | |
| T. testudinum | MeOH/H2O (1:1) | IC50: 0.8 mg/mL | DPPH | [59] |
3.3. Anti-Inflammatory Effects

3.4. Antibacterial Activity

| Species | Extract Type/Active Compound | Bacteria | Inhibition | References |
|---|---|---|---|---|
| C. nodosa | Sulfated polysaccharide | E. coli | MIC: 25 mg/mL 20 mm zone inhibition | [51] |
| L. monocytogene | MIC: 25 mg/mL 18 mm zone inhibition | [51] | ||
| S. enterica | MIC: 6.25 mg/mL 21.5 mm zone inhibition | [51] | ||
| B. subtilis | MIC: 6.25 mg/mL 18.6 mm zone inhibition | [51] | ||
| B. amyloliquefaciens | MIC: 50 mg/mL 17 mm zone inhibition | [51] | ||
| S. aureus | MIC: 25 mg/mL 23 mm zone inhibition | [51] | ||
| M. luteus | MIC: 6.25 mg/mL 24 mm zone inhibition | [51] | ||
| C. rotundata | Aqueous methanol (1:4) | S. dysenteriae | MIC: 68 μg/mL | [90] |
| S. boydii | MIC: 68 μg/mL | [90] | ||
| S. paratyphi | MIC: 34 μg/mL | [90] | ||
| Urinary tract infection (UTI) bacteria | MIC: 10 μg/mL MBC: 50 μg/mL | [91] | ||
| E. coli | 10 mm zone inhibition | [91] | ||
| P. mirabilis | 12 mm zone inhibition | [91] | ||
| S. saprophyticus | 11.6 mm zone inhibition | [91] | ||
| K. pneumonia | 11.3 mm zone inhibition | [91] | ||
| P. aeruginosa | 12.3 mm zone inhibition | [91] | ||
| E. aerogenes | 9.7 mm zone inhibition | [91] | ||
| Serratia sp. | 10 mm zone inhibition | [91] | ||
| Aqueous methanolic extracts | S. dysenteriae | MBC: 68 μg/mL | [90] | |
| S. boydii | MBC: 34 μg/mL | [90] | ||
| Butanolic extract | S. aureus | 6 mm zone inhibition | [78] | |
| Ethanolic extract | Shigella | 7 mm zone inhibition | [78] | |
| P. fluorescens | 7 mm zone inhibition | [78] | ||
| Methanolic extract | S. aureus | 17 mm zone inhibition | [92] | |
| S. faecalis | 13 mm zone inhibition | [92] | ||
| S. enteric | 8 mm zone inhibition | [92] | ||
| B. subtilis | 14 mm zone inhibition | [92] | ||
| E. coli | 15 mm zone inhibition | [92] | ||
| S. boydii | 8 mm zone inhibition | [92] | ||
| V. cholera | 8 mm zone inhibition | [92] | ||
| C. serrulata | Acetone extract | P. aeruginosa | MIC: 25 μg/mL | [88] |
| H. aquamarina | MIC: 50 μg/mL | [88] | ||
| P. agglomerans | MIC: 25 μg/mL | [88] | ||
| S. marcescens | MIC: 50 μg/mL | [88] | ||
| S. liquefaciens | MIC: 10 μg/mL | [88] | ||
| V. fischeri | MIC: 25 μg/mL | [88] | ||
| V. parahaemolyticus | MIC: 50 μg/mL | [88] | ||
| S. flexneri | MIC: 50 μg/mL | [88] | ||
| Aqueous methanol (1:4) | S. dysenteriae | MIC: 130 μg/mL | [90] | |
| S. paratyphi | MIC: 131 μg/mL | [90] | ||
| Urinary tract infection (UTI) bacteria | MIC: 100 μg/mL | [12] | ||
| S. saprophyticus | 6 mm zone inhibition | [91] | ||
| P. aeruginosa | 6.3 mm zone inhibition | [91] | ||
| Aqueous methanolic extracts | S. dysenteriae | MBC: 130 μg/mL | [90] | |
| S. paratyphi | MBC: 130 μg/mL | [90] | ||
| Chloroform extract | Corynebacterium | MIC: 850 μg/mL 7 mm zone inhibition | [93] | |
| E. coli | MIC: 90 μg/mL 8.66 mm zone inhibition | [93] | ||
| Dichloromethane extract | P. aeruginosa | MIC: 10 μg/mL | [88] | |
| H. aquamarina | MIC: 25 μg/mL | [88] | ||
| P. agglomerans | MIC: 25 μg/mL | [88] | ||
| S. marcescens | MIC: 10 μg/mL | [88] | ||
| S. liquefaciens | MIC: 50 μg/mL | [88] | ||
| V. fischeri | MIC: 10 μg/mL | [88] | ||
| V. parahaemolyticus | MIC: 25 μg/mL | [88] | ||
| S. flexneri | MIC: 25 μg/mL | [88] | ||
| A. hydrophila | MIC: 10 μg/mL | [88] | ||
| Ethanolic extract | E. coli | MIC: 90 μg/mL 7.33 mm zone inhibition | [93] | |
| Ethyl acetate extract | Corynebacterium | MIC: 875 μg/mL 8 mm zone inhibition | [93] | |
| E. coli | MIC: 75 μg/mL 9 mm zone inhibition | [93] | ||
| Methanolic extract | P. aeruginosa | MIC: 10 μg/mL | [88] | |
| H. aquamarina | MIC: 1 μg/mL | [88] | ||
| V. alginolyticus | MIC: 10 μg/mL | [88] | ||
| P. agglomerans | MIC: 1 μg/mL | [88] | ||
| S. marcescens | MIC: 1 μg/mL | [88] | ||
| S. liquefaciens | MIC: 10 μg/mL | [88] | ||
| V. fischeri | MIC: 1 μg/mL | [88] | ||
| V. parahaemolyticus | MIC: 10 μg/mL | [88] | ||
| S. flexneri | MIC: 25 μg/mL | [88] | ||
| A. hydrophila | MIC: 1 μg/mL | [88] | ||
| E. acoroides | Aqueous methanol (1:4) | Urinary tract infection (UTI) bacteria | MIC: 25 μg/mL MBC: 100 μg/mL | [91] |
| P. mirabilis | 9.3 mm zone inhibition | [91] | ||
| K. pneumonia | 8.3 mm zone inhibition | [91] | ||
| P. aeruginosa | 9.3 mm zone inhibition | [91] | ||
| E. aerogenes | 8.7 mm zone inhibition | [91] | ||
| Serratia sp. | 6.3 mm zone inhibition | [91] | ||
| Ethanolic extract | E. coli | MIC: 250 μg/mL | [94] | |
| S. aureus | MIC: 62.5 μg/mL | [94] | ||
| B. subtilis | MIC: 250 μg/mL | [94] | ||
| Ethanolic extract (leaves) | S. aureus | 8.37 mm at 400 ppm | [95] | |
| Ethanolic extract (roots) | S. aureus | 8.63 mm at 400 ppm | [95] | |
| Ethyl acetate | E. coli | MIC: 31.25 μg/mL | [94] | |
| S. aureus | MIC: 31.25 μg/mL | [94] | ||
| B. subtilis | MIC: 62.5 μg/mL | [94] | ||
| Hexane extract | S. aureus | MIC: 15.625 μg/mL 5.6 mm zone inhibition at 1000 ppm 5.2 mm zone inhibition at 500 ppm | [89,94] | |
| 19 mm zone inhibition | [96] | |||
| E. coli | MIC: 31.25 μg/mL | [94] | ||
| B. subtilis | MIC: 250 μg/mL | [94] | ||
| Methanolic extract | S. aureus | 5.9 mm zone inhibition at 1000 ppm | [89] | |
| 5.2 mm zone inhibition at 500 ppm | [89] | |||
| E. coli | 60.86% inhibition at 10 mg/mL | [57] | ||
| H. ovalis | Chloroform extract | Corynebacterium | MIC: 65 μg/mL 11.66 mm zone inhibition | [93] |
| E. coli | MIC: 225 μg/mL 7.6 mm zone inhibition | [93] | ||
| Ethanolic extract | E. coli | MIC: 90 μg/mL 7 mm zone inhibition | [93] | |
| Ethyl acetate extract | Corynebacterium | MIC: 65 μg/mL 13 mm zone inhibition | [93] | |
| E. femelis | MIC: 85 μg/mL 7.66 mm zone inhibition | [93] | ||
| E. coli | MIC: 90 μg/mL 7.33 mm zone inhibition | [93] | ||
| Hexane extract | Corynebacterium | MIC: 50 μg/mL 14.66 mm zone inhibition | [93] | |
| E. coli | MIC: 435 μg/mL 7 mm zone inhibition | [93] | ||
| Methanolic extract | B. cereus | MIC: 50 μg/mL 17.16 mm zone inhibition at 200 μg/mL | [39] | |
| A. baumannii | MIC: 75 μg/mL 13.83 mm zone inhibition at 200 μg/mL | [39] | ||
| V. vulnificus | MIC: 100 μg/mL 10.36 mm zone inhibition at 200 μg/mL | [39] | ||
| V. parahaemolyticus | MIC: 75 μg/mL 10.16 mm zone inhibition at 200 μg/mL | [39] | ||
| V. anguillarum | MIC: 75 μg/mL 10.16 mm zone inhibition at 200 μg/mL | [39] | ||
| V. fischeri | MIC: 75 μg/mL 10 mm zone inhibition at 200 μg/mL | [39] | ||
| E. coli | MIC: 75 μg/mL 8.53 mm zone inhibition at 200 μg/mL | [39] | ||
| M. luteus | MIC: 50 μg/mL | [39] | ||
| H. pinifolia | Aqueous methanol (1:4) | Urinary tract infection (UTI) bacteria | MIC: 1 μg/mL MBC: 25 μg/mL | [91] |
| E. coli | 12.3 mm zone inhibition | [91] | ||
| P. mirabilis | 13.7 mm zone inhibition | [91] | ||
| S. saprophyticus | 10.7 mm zone inhibition | [91] | ||
| K. pneumonia | 11.7 mm zone inhibition | [91] | ||
| P. aeruginosa | 10.3 mm zone inhibition | [91] | ||
| E. aerogenes | 14.3 mm zone inhibition | [91] | ||
| Serratia sp. | 11.3 mm zone inhibition | [91] | ||
| S. dysenteriae | MIC: 34 μg/mL | [90] | ||
| S. paratyphi | MIC: 509 μg/mL | [90] | ||
| S. boydii | MIC: 510 μg/mL | [90] | ||
| Aqueous methanolic extracts | S. dysenteriae | MBC: 34 μg/mL | [90] | |
| S. paratyphi | MBC: 510 μg/mL | [90] | ||
| S. boydii | MBC: 510 μg/mL | [90] | ||
| Chloroform extract | Corynebacterium | MIC: 55 μg/mL 13.66 mm zone inhibition | [93] | |
| E. coli | MIC: 90 μg/mL 8.33 mm zone inhibition | [93] | ||
| Ethanolic extract | E. coli | MIC: 80 μg/mL 8 mm zone inhibition | [93] | |
| Ethyl acetate extract | Corynebacterium | MIC: 35 μg/mL 11 mm zone inhibition | [93] | |
| E. coli | MIC: 70 μg/mL 9 mm zone inhibition | [93] | ||
| Hexane extract | Corynebacterium | MIC: 50 μg/mL 14.33 mm zone inhibition | [93] | |
| H. stipulaceae | Aqueous extract | B. subtilis | 15 mm zone inhibition | [97] |
| H. uninervis | Chloroform extract | B. subtilis | 17 mm zone inhibition | [75] |
| MRSA | 18.33 mm zone inhibition | [75] | ||
| M. luteus | 15/67 mm zone inhibition | [75] | ||
| S. aureus | 13.67 mm zone inhibition | [75] | ||
| E. coli | 16.33 mm zone inhibition | [75] | ||
| K. pneumoniae | 17.67 mm zone inhibition | [75] | ||
| P. aeruginosa | 18.33 mm zone inhibition | [75] | ||
| Distilled water | P. aeruginosa | 14.67 mm zone inhibition | [75] | |
| Ethanolic extract | B. subtilis | 24.67 mm zone inhibition | [75] | |
| MRSA | 20 mm zone inhibition | [75] | ||
| M. luteus | 17.33 mm zone inhibition | [75] | ||
| S. aureus | 15.67 mm zone inhibition | [75] | ||
| E. coli | 17.33 mm zone inhibition | [75] | ||
| K. pneumoniae | 18.67 mm zone inhibition | [75] | ||
| P. aeruginosa | 33.33 mm zone inhibition | [75] | ||
| Ethyl acetate extract | B. subtilis | 15.67 mm zone inhibition | [75] | |
| MRSA | 16.67 mm zone inhibition | [75] | ||
| M. luteus | 15 mm zone inhibition | [75] | ||
| S. aureus | 12.67 mm zone inhibition | [75] | ||
| E. coli | 15.33 mm zone inhibition | [75] | ||
| K. pneumoniae | 16 mm zone inhibition | [75] | ||
| P. aeruginosa | 16.67 mm zone inhibition | [75] | ||
| Petroleum ether extract | B. subtilis | 15.67 mm zone inhibition | [75] | |
| MRSA | 16 mm zone inhibition | [75] | ||
| M. luteus | 14.33 mm zone inhibition | [75] | ||
| S. aureus | 11.67 mm zone inhibition | [75] | ||
| E. coli | 14.33 mm zone inhibition | [75] | ||
| K. pneumoniae | 14.47 mm zone inhibition | [75] | ||
| P. aeruginosa | 15.67 mm zone inhibition | [75] | ||
| S. filiforme | Chloroform fraction | S. aureus | MIC: 0.7 mg/mL | [34] |
| E. coli | MIC: 0.7 mg/mL | [34] | ||
| C. albicans | MIC: 1.5 mg/mL | [34] | ||
| P. aeruginosa | MIC: 1.5 mg/mL | [34] | ||
| S. typhii | MIC: 0.7 mg/mL | [34] | ||
| Ethanolic extract | S. aureus | MIC: 47.7 mg/mL | [34] | |
| E. coli | MIC: 38.1 mg/mL | [34] | ||
| C. albicans | MIC: 190.5 mg/mL | [34] | ||
| S. isoetifolium | Acetone extract | P. aeruginosa | MIC: 25 μg/mL | [88] |
| H. aquamarina | MIC: 25 μg/mL | [88] | ||
| V. alginolyticus | MIC: 50 μg/mL | [88] | ||
| S. marcescens | MIC: 25 μg/mL | [88] | ||
| S. liquefaciens | MIC: 50 μg/mL | [88] | ||
| V. parahaemolyticus | MIC: 50 μg/mL | [88] | ||
| S. flexneri | MIC: 25 μg/mL | [88] | ||
| A. hydrophila | MIC: 50 μg/mL | [88] | ||
| Aqueous methanol (1:4) | Urinary tract infection (UTI) bacteria | MIC: 50 μg/mL MBC: 100 μg/mL | [91] | |
| P. mirabilis | 8.7 mm zone inhibition | [91] | ||
| S. saprophyticus | 8.3 mm zone inhibition | [91] | ||
| E. aerogenes | 7 mm zone inhibition | [91] | ||
| Ethyl acetate extract | K. pneumoniae | 14 mm zone inhibition at 100 μg/mL | [87] | |
| E. coli | 13 mm zone inhibition at 100 μg/mL | [87] | ||
| S. typhii | 11 mm zone inhibition at 100 μg/mL | [87] | ||
| Dichlorometahane extract | A. hydrophila | MIC: 10 μg/mL | [88] | |
| P. aeruginosa | MIC: 10 μg/mL | [88] | ||
| H. aquamarina | MIC: 10 μg/mL | [88] | ||
| V. alginolyticus | MIC: 25 μg/mL | [88] | ||
| P. agglomerans | MIC: 25 μg/mL | [88] | ||
| S. marcescens | MIC: 10 μg/mL | [88] | ||
| S. liquefaciens | MIC: 50 μg/mL | [88] | ||
| V. fischeri | MIC: 10 μg/mL | [88] | ||
| S. flexneri | MIC: 25 μg/mL | [88] | ||
| Methanolic extract | P. aeruginosa | MIC: 1 μg/mL | [88] | |
| H. aquamarina | MIC: 10 μg/mL | [88] | ||
| V. alginolyticus | MIC: 25 μg/mL | [88] | ||
| P. agglomerans | MIC: 1 μg/mL | [88] | ||
| S. marcescens | MIC: 10 μg/mL | [88] | ||
| S. liquefaciens | MIC: 10 μg/mL | [88] | ||
| V. fischeri | MIC: 10 μg/mL | [88] | ||
| V. parahaemolyticus | MIC: 25 μg/mL | [88] | ||
| S. flexneri | MIC: 1 μg/mL | [88] | ||
| A. hydrophila | MIC: 10 μg/mL | [88] | ||
| S. aureus | 15 mm zone inhibition | [92] | ||
| S. faecalis | 10 mm zone inhibition | [92] | ||
| S. enteric | 6 mm zone inhibition | [92] | ||
| B. subtilis | 10 mm zone inhibition | [92] | ||
| E. coli | 8 mm zone inhibition | [92] | ||
| V. cholera | 6 mm zone inhibition | [92] | ||
| T. hemprichii | Aqueous methanol (1:4) | Urinary tract infection (UTI) bacteria | MIC: 25 μg/mL MBC: 50 μg/mL | [91] |
| E. coli | 9.3 mm zone inhibition | [91] | ||
| P. mirabilis | 10.3 mm zone inhibition | [91] | ||
| S. saprophyticus | 9.3 mm zone inhibition | [91] | ||
| K. pneumonia | 11.3 mm zone inhibition | [91] | ||
| P. aeruginosa | 10.6 mm zone inhibition | [91] | ||
| E. aerogenes | 9.3 mm zone inhibition | [91] | ||
| Serratia sp. | 8.7 mm zone inhibition | [91] | ||
| Ethanolic extract | E. coli | MIC: 500 μg/mL | [94] | |
| S. aureus | MIC: 125 μg/mL | [94] | ||
| B. subtilis | MIC: 500 μg/mL | [94] | ||
| Ethyl acetate extract | E. coli | MIC: 125 μg/mL | [94] | |
| S. aureus | MIC: 250 μg/mL | [94] | ||
| B. subtilis | MIC: 125 μg/mL | [94] | ||
| Hexane extract | E. coli | MIC: 62.5 μg/mL | [94] | |
| S. aureus | MIC: 62.5 μg/mL | [94] | ||
| B. subtilis | MIC: 125 μg/mL | [94] |
3.5. Antifungal Treatments
| Species | Extract/Active Compounds | Fungus | Activity | References |
|---|---|---|---|---|
| C. nodosa | Sulfated polysaccharide | A. niger | Zone of inhibition: 15 mm MIC: 6.25 mg/mL | [51] |
| F. oxysporum | Zone of inhibition: 14.3 mm MIC: 12.5 mg/mL | [51] | ||
| C. albicans | Zone of inhibition: 18 mm MIC: 12.5 mg/mL | [51] | ||
| C. neoformans (flucytosine sensitive) | MIC: 16 μg/mL MBC > 200 μg/mL | [105] | ||
| C. neoformans (flucytosine resistant) | MIC: 8 μg/mL MBC: 128 μg/mL | [105] | ||
| M. gypseum | MIC: 2 μg/mL MBC: 16 μg/mL | [105] | ||
| C. rotundata | Methanolic extract | A. niger | Zone of inhibition: 15 mm antifungal activity index: 83% | [92] |
| A. fumigates | Zone of inhibition: 8 mm antifungal activity index: 67% | [92] | ||
| Fusarium | Zone of inhibition: 10 mm antifungal activity index: 10% | [92] | ||
| E. acoroides | Methanolic extract | C. albicans | Reduces fungal coverage up to 73.89 ± 1.01% at 0.01 mg/L | [57] |
| H. ovalis | Methanolic extract | C. albicans | Reduces fungal coverage up to 68.37 ± 2.49% at 1 mg/L | [57] |
| H. stipulaceae | Aqueous extract | A. niger | Zone of inhibition: 20 mm | [97] |
| C. albicans | Zone of inhibition: 15 mm | [97] | ||
| S. isoetifolium | Methanolic extract | A. niger | Zone of inhibition: 12 mm antifungal activity index: 67% | [92] |
| A. fumigates | Zone of inhibition: 6 mm antifungal activity index: 50% | [92] | ||
| Fusarium | Zone of inhibition: 8 mm antifungal activity index: 8% | [92] | ||
| T. hemprichii | Hexane/ethanol (3:1) | F. acuminatum | Zone of inhibition: 2.5 mm | [106] |
| A. niger | Zone of inhibition: 1.7 mm | [106] | ||
| P. expansum | Zone of inhibition: 2.1 mm | [106] | ||
| A. terrus | Zone of inhibition: 3.2 mm | [106] | ||
| A. fumigatus | Zone of inhibition: 1.5 mm | [106] |
3.6. Antiviral Activity

| Species | Extract/Active Compound | Virus | Inhibition | References |
|---|---|---|---|---|
| P. oceanica | MeOH/H2O 7:3 (balls extract) | H5N1 | 45% inhibition at 100 μg/mL | [25] |
| T. ciliatum | Methanolic extract | Hepatitis A (HAV) and herpes simplex (HSV-1) | 100% inhibition at 20 μg/mL | [17] |
| T. hemprichii | Methanolic extract | HCV | 50% inhibition at 23 μg/mL | [109] |
| Zosteraceae family | Polyphenol complex | Tick-borne encephalitis (TBE) | Suppressed accumulation of the pathogen in the cell culture at 100 μg/mL concentration. | [113] |
3.7. Anti-Dengue Activity
| Species | Extract/Active Compound | Mosquito | LC50 (μg/mL) | LC90 (μg/mL) | References |
|---|---|---|---|---|---|
| C. serrulata (leaves) | EtOH/water (3:1) | A. aegypti | 0.0780 | 0.1675 | [115] |
| 70% ethanol | A. aegypti | 42.9 | - | [116] | |
| E. acorodies | Distilled water | A. aegypti | 0.0852 | 0.1369 | [117] |
| H. ovalis | Distilled water | A. aegypti | 0.067 | 0.128 | [117] |
| H. pinifolia (roots) | 70% ethanol | A. aegypti | 22.0 | 54.2 | [116] |
| S. isoetifolium (leaves) | EtOH/water (3:1) | A. aegypti | 0.0620 | 0.8970 | [115] |
| S. isoetifolium (root) | EtOH/water (3:1) | A. aegypti | 0.0604 | 0.9090 | [115] |
| T. hemprichii | Ethanolic extract | A. aegypti | 201.7 | - | [116] |
| T. testudinum (leaves) | 70% ethanol | A. aegypti | 44.8 | 81.2 | [116] |
3.8. Lipid-Reducing Activity
3.9. Antidiabetic Activity
3.10. Hepatoprotective
3.11. Anti-Aging Effects
4. Bioactive Compounds from Seagrass under the Clinical Trial
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Blunt, J.W.; Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine Natural Products. Nat. Prod. Rep. 2018, 35, 8–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghareeb, M.A.; Tammam, M.A.; El-Demerdash, A.; Atanasov, A.G. Insights about Clinically Approved and Preclinically Investigated Marine Natural Products. Curr. Res. Biotechnol. 2020, 2, 88–102. [Google Scholar] [CrossRef]
- Marine Pharmacologhy. Available online: https://www.marinepharmacology.org/ (accessed on 2 May 2022).
- Short, F.; Short, C.; Novak, A. Seagrasses. In The Wetland Book: II: Distribution, Description and Conservation; Springer Science: New York, NY, USA, 2016; ISBN 978-94-007-6173-5. [Google Scholar]
- Danaraj, J.; Saravanakumar, A.; Mariasingarayan, Y. Seagrass Metabolomics: A New Insight towards Marine Based Drug Discovery. In Metabolomics-Methodology and Applications in Medical Sciences and Life Sciences; IntechOpen: London, UK, 2021; ISBN 978-1-83969-083-9. [Google Scholar]
- Mehari Ghebretinsae, M.; Awet Adhanom, B.; Henok Ghebremedhin, G.; Biniam Tsegay, B. A Review on Bioactive Secondary Metabolites of Seagrass of the Southern Red Sea, Eritrea. Int. J. Adv. Res. 2019, 7, 306–314. [Google Scholar] [CrossRef] [Green Version]
- Unsworth, R.; van Keulen, M.; Coles, R. Seagrass Meadows in a Globally Changing Environment. Mar. Pollut. Bull. 2014, 83, 383–386. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Mahomoodally, M.F.; Sadeer, N.B.; Seok, P.G.; Zengin, G.; Palaniveloo, K.; Khalil, A.A.; Rauf, A.; Rengasamy, K.R. Nutritional and Bioactive Potential of Seagrasses: A Review. S. Afr. J. Bot. 2021, 137, 216–227. [Google Scholar] [CrossRef]
- Grignon-Dubois, M.; Rezzonico, B. Phenolic Fingerprint of the Seagrass Posidonia oceanica from Four Locations in the Mediterranean Sea: First Evidence for the Large Predominance of Chicoric Acid. Bot. Mar. 2015, 58, 379–391. [Google Scholar] [CrossRef]
- Jafriati, J.; Hatta, M.; Yuniar, N.; Junita, A.; Dwiyanti, R.; Sabir, M.; Primaguna, M. Thalassia hemprichii Seagrass Extract as Antimicrobial and Antioxidant Potential on Human: A Mini Review of the Benefits of Seagrass. J. Biol. Sci. 2019, 19, 363–371. [Google Scholar] [CrossRef] [Green Version]
- Mansoori, B.; Mohammadi, A.; Davudian, S.; Shirjang, S.; Baradaran, B. The Different Mechanisms of Cancer Drug Resistance: A Brief Review. Adv. Pharm. Bull. 2017, 7, 339–348. [Google Scholar] [CrossRef]
- Feitelson, M.A.; Arzumanyan, A.; Kulathinal, R.J.; Blain, S.W.; Holcombe, R.F.; Mahajna, J.; Marino, M.; Martinez-Chantar, M.L.; Nawroth, R.; Sanchez-Garcia, I.; et al. Sustained Proliferation in Cancer: Mechanisms and Novel Therapeutic Targets. Semin. Cancer Biol. 2015, 35, S25–S54. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: Globocan Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Greenwell, M.; Rahman, P.K.S.M. Medicinal Plants: Their Use in Anticancer Treatment. Int. J. Pharm. Sci. Res. 2015, 6, 4103–4112. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, S.A.M.; Elias, N.; Farag, M.A.; Chen, L.; Saeed, A.; Hegazy, M.-E.F.; Moustafa, M.S.; Abd El-Wahed, A.; Al-Mousawi, S.M.; Musharraf, S.G.; et al. Marine Natural Products: A Source of Novel Anticancer Drugs. Mar. Drugs 2019, 17, 491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, S.; Cheng, X.; Wink, M. Cytotoxicity of Arctigenin and Matairesinol against the T-Cell Lymphoma Cell Line CCRF-CEM. J. Pharm. Pharmacol. 2015, 67, 1316–1323. [Google Scholar] [CrossRef] [PubMed]
- Hamdy, A.-H.A.; Mettwally, W.S.A.; El Fotouh, M.A.; Rodriguez, B.; El-Dewany, A.I.; El-Toumy, S.A.A.; Hussein, A.A. Bioactive Phenolic Compounds from the Egyptian Red Sea Seagrass Thalassodendron Ciliatum. Z. Nat. C J. Biosci. 2012, 67, 291–296. [Google Scholar] [CrossRef]
- Li, Y.; Grauso, L.; Scarpato, S.; Cacciola, N.A.; Borrelli, F.; Zidorn, C.; Mangoni, A. Stable Catechol Keto Tautomers in Cytotoxic Heterodimeric Cyclic Diarylheptanoids from the Seagrass Zostera Marina. Org. Lett. 2021, 23, 7134–7138. [Google Scholar] [CrossRef]
- Abdelhameed, R.; Ibrahim, A.; Yamada, K.; Ahmed, S. Cytotoxic and Anti-Inflammatory Compounds from Red Sea Grass Thalassodendron Ciliatum. Med. Chem. Res. 2018, 27, 1238–1244. [Google Scholar] [CrossRef]
- Wang, Y.; Kong, D.; Wang, X.; Dong, X.; Tao, Y.; Gong, H. Molecular Mechanisms of Luteolin Induced Growth Inhibition and Apoptosis of Human Osteosarcoma Cells. Iran. J. Pharm. Res. IJPR 2015, 14, 531–538. [Google Scholar]
- Bel Mabrouk, S.; Reis, M.; Sousa, M.L.; Ribeiro, T.; Almeida, J.R.; Pereira, S.; Antunes, J.; Rosa, F.; Vasconcelos, V.; Achour, L.; et al. The Marine Seagrass Halophila Stipulacea as a Source of Bioactive Metabolites against Obesity and Biofouling. Mar. Drugs 2020, 18, 88. [Google Scholar] [CrossRef] [Green Version]
- Choi, H.; Mevers, E.; Byrum, T.; Valeriote, F.A.; Gerwick, W.H. Lyngbyabellins K-N from Two Palmyra Atoll Collections of the Marine Cyanobacterium Moorea Bouillonii. Eur. J. Org. Chem. 2012, 2012, 5141–5150. [Google Scholar] [CrossRef]
- Delgado-Roche, L.; González, K.; Mesta, F.; Couder, B.; Tavarez, Z.; Zavala, R.; Hernandez, I.; Garrido, G.; Rodeiro, I.; Vanden Berghe, W. Polyphenolic Fraction Obtained From Thalassia Testudinum Marine Plant and Thalassiolin B Exert Cytotoxic Effects in Colorectal Cancer Cells and Arrest Tumor Progression in a Xenograft Mouse Model. Front. Pharmacol. 2020, 11, 1939. [Google Scholar] [CrossRef]
- Shailaja, V.L.; Christina, V.S.; Mohanapriya, C.D.; Sneha, P.; Lakshmi Sundaram, R.; Magesh, R.; George Priya Doss, C.; Gnanambal, K.M.E. A Natural Anticancer Pigment, Pheophytin α, from a Seagrass Acts as a High Affinity Human Mitochondrial Translocator Protein (TSPO) Ligand, in Silico, to Reduce Mitochondrial Membrane Potential (∆ψmit) in Adenocarcinomic A549 Cells. Phytomedicine 2019, 61, 152858. [Google Scholar] [CrossRef] [PubMed]
- Farid, M.; Marzouk, M.; Hussein, S.; Elkhateeb, A.; Saleh, E.-S. Comparative Study of Posidonia oceanica L.: LC/ESI/MS Analysis, Cytotoxic Activity and Chemosystematic Significance. J. Mater. Environ. Sci. 2018, 9, 1676–1682. [Google Scholar]
- Chanthini, A.B.; Balasubramani, G.; Ramkumar, R.; Sowmiya, R.; Balakumaran, M.D.; Kalaichelvan, P.T.; Perumal, P. Structural Characterization, Antioxidant and in Vitro Cytotoxic Properties of Seagrass, Cymodocea serrulata (R.Br.) Asch. & Magnus Mediated Silver Nanoparticles. J. Photochem. Photobiol. B 2015, 153, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Barletta, E.; Ramazzotti, M.; Fratianni, F.; Pessani, D.; Degl’Innocenti, D. Hydrophilic Extract from Posidonia oceanica Inhibits Activity and Expression of Gelatinases and Prevents HT1080 Human Fibrosarcoma Cell Line Invasion. Cell Adhes. Migr. 2015, 9, 422–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riera-Romo, M.; Marrero-Delange, D.; Hernandez-Balmaseda, I.; González, K.; Pérez-Martínez, D.; Manso-Vargas, A.; Labrada, M.; Rodeiro, I.; Berghe, W. Chemical Characterization and Cytotoxic Potential of a Chloroform Fraction Obtained from Marine Plant Thalassia Testudinum. J. Chromatogr. Sep. Tech. 2018, 9, 1–6. [Google Scholar] [CrossRef]
- Hernández-Balmaseda, I.; Guerra, I.R.; Declerck, K.; Herrera Isidrón, J.A.; Pérez-Novo, C.; Van Camp, G.; De Wever, O.; González, K.; Labrada, M.; Carr, A.; et al. Marine Seagrass Extract of Thalassia Testudinum Suppresses Colorectal Tumor Growth, Motility and Angiogenesis by Autophagic Stress and Immunogenic Cell Death Pathways. Mar. Drugs 2021, 19, 52. [Google Scholar] [CrossRef]
- Rodeiro, I.; Hernández, I.; Herrera, J.A.; Riera, M.; Donato, M.T.; Tolosa, L.; González, K.; Ansoar, Y.; Gómez-Lechón, M.J.; Vanden Berghe, W.; et al. Assessment of the Cytotoxic Potential of an Aqueous-Ethanolic Extract from Thalassia Testudinum Angiosperm Marine Grown in the Caribbean Sea. J. Pharm. Pharmacol. 2018, 70, 1553–1560. [Google Scholar] [CrossRef]
- Kim, W.; Lee, S.; Seo, D.; Kim, D.; Kim, K.; Kim, E.; Kang, J.; Seong, K.M.; Youn, H.; Youn, B. Cellular Stress Responses in Radiotherapy. Cells 2019, 8, 1105. [Google Scholar] [CrossRef] [Green Version]
- Mei, D.; Zhu, Y.; Zhang, L.; Wei, W. The Role of CTHRC1 in Regulation of Multiple Signaling and Tumor Progression and Metastasis. Mediat. Inflamm. 2020, 2020, e9578701. [Google Scholar] [CrossRef]
- Piazzini, V.; Vasarri, M.; Degl’Innocenti, D.; Guastini, A.; Barletta, E.; Salvatici, M.C.; Bergonzi, M.C. Comparison of Chitosan Nanoparticles and Soluplus Micelles to Optimize the Bioactivity of Posidonia oceanica Extract on Human Neuroblastoma Cell Migration. Pharmaceutics 2019, 11, 655. [Google Scholar] [CrossRef] [Green Version]
- Garcia, K.G.; Delange, D.M.; Rivera, Y.H.; Suárez, Y.A.; Cuesta, R.G.; Riera-Romo, M.; Echemendia, O.; Dutra, L.M.; Almeida, J.R.G.D.S.; Pérez-martínez, D.; et al. Chemical Composition and Biological Potential of a Chloroform Fraction from the Leaves of Marine Plant Syringodium Filiforme Kützing. Pharmacogn. Mag. 2020, 16, 750. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative Stress, Aging, and Diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conti, V.; Izzo, V.; Corbi, G.; Russomanno, G.; Manzo, V.; De Lise, F.; Di Donato, A.; Filippelli, A. Antioxidant Supplementation in the Treatment of Aging-Associated Diseases. Front. Pharmacol. 2016, 7, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ighodaro, O.M.; Akinloye, O.A. First Line Defence Antioxidants-Superoxide Dismutase (SOD), Catalase (CAT) and Glutathione Peroxidase (GPX): Their Fundamental Role in the Entire Antioxidant Defence Grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef] [Green Version]
- Zhong, Q.; Wei, B.; Wang, S.; Ke, S.; Chen, J.; Zhang, H.; Wang, H. The Antioxidant Activity of Polysaccharides Derived from Marine Organisms: An Overview. Mar. Drugs 2019, 17, 674. [Google Scholar] [CrossRef] [Green Version]
- Yuvaraj, N.; Kanmani, P.; Satishkumar, R.; Paari, A.; Pattukumar, V.; Arul, V. Seagrass as a Potential Source of Natural Antioxidant and Anti-Inflammatory Agents. Pharm. Biol. 2012, 50, 458–467. [Google Scholar] [CrossRef] [Green Version]
- Menajang, F.S.I.; Mahmudi, M.; Yanuhar, U.; Herawati, E.Y. Evaluation of Phytochemical and Superoxide Dismutase Activities of Enhalus Acoroides (L.f.) Royle from Coastal Waters of North Sulawesi, Indonesia. Vet. World 2020, 13, 676–680. [Google Scholar] [CrossRef] [Green Version]
- Sansone, C.; Galasso, C.; Lo Martire, M.; Fernández, T.V.; Musco, L.; Dell’Anno, A.; Bruno, A.; Noonan, D.M.; Albini, A.; Brunet, C. In Vitro Evaluation of Antioxidant Potential of the Invasive Seagrass Halophila Stipulacea. Mar. Drugs 2021, 19, 37. [Google Scholar] [CrossRef]
- Custódio, L.; Laukaityte, S.; Engelen, A.H.; João Rodrigues, M.; Pereira, H.; Vizetto-Duarte, C.; Barreira, L.; Rodríguez, H.; Alberício, F. A Comparative Evaluation of Biological Activities and Bioactive Compounds of the Seagrasses Zostera Marina and Zostera Noltei from Southern Portugal. Nat. Prod. Res. 2016, 30, 724–728. [Google Scholar] [CrossRef] [Green Version]
- Simos, Y.V.; Verginadis, I.I.; Toliopoulos, I.K.; Velalopoulou, A.P.; Karagounis, I.V.; Karkabounas, S.C.; Evangelou, A.M. Effects of Catechin and Epicatechin on Superoxide Dismutase and Glutathione Peroxidase Activity, in Vivo. Redox Rep. 2012, 17, 181–186. [Google Scholar] [CrossRef] [Green Version]
- Zduńska, K.; Dana, A.; Kolodziejczak, A.; Rotsztejn, H. Antioxidant Properties of Ferulic Acid and Its Possible Application. Skin Pharmacol. Physiol. 2018, 31, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Enerstvedt, K.H. Analysis of Polyphenolic Content in Marine and Aquatic Angiosperms from Norwegian Coastal Waters. Ph.D. Thesis, University of Bergen, Bergen, Norway, 2018. [Google Scholar]
- Velika, B.; Kron, I. Antioxidant Properties of Benzoic Acid Derivatives against Superoxide Radical. Free Radic. Antioxid. 2012, 2, 62–67. [Google Scholar] [CrossRef] [Green Version]
- Farhoosh, R.; Johnny, S.; Asnaashari, M.; Molaahmadibahraseman, N.; Sharif, A. Structure–Antioxidant Activity Relationships of o-Hydroxyl, o-Methoxy, and Alkyl Ester Derivatives of p-Hydroxybenzoic Acid. Food Chem. 2016, 194, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Hu, J.; Hu, D.; Yang, X. A Role of Gallic Acid in Oxidative Damage Diseases: A Comprehensive Review. Nat. Prod. Commun. 2019, 14, 1934578X19874174. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, D. Biological Activities of Characterized Isolates of N-Hexane Extract of Azadirachta Indica A. Juss (Neem) Leaves. Nat. Sci. 2013, 11, 141–147. [Google Scholar]
- Jones, D.A. Rosacea, Reactive Oxygen Species, and Azelaic Acid. J. Clin. Aesthetic Dermatol. 2009, 2, 26–30. [Google Scholar]
- Kolsi, R.B.A.; Gargouri, B.; Sassi, S.; Frikha, D.; Lassoued, S.; Belghith, K. In Vitro Biological Properties and Health Benefits of a Novel Sulfated Polysaccharide Isolated from Cymodocea Nodosa. Lipids Health Dis. 2017, 16, 252. [Google Scholar] [CrossRef] [Green Version]
- Ragupathi Raja Kannan, R.; Arumugam, R.; Thangaradjou, T.; Anantharaman, P. Phytochemical Constituents, Antioxidant Properties and p-Coumaric Acid Analysis in Some Seagrasses. Food Res. Int. 2013, 54, 1229–1236. [Google Scholar] [CrossRef]
- Santoso, J.; Anwariyah, S.; Rumiantin, R.O.; Putri, A.P.; Ukhty, N.; Stark, Y.Y. Phenol Content, Antioxidant Activity and Fibers Profile of Four Tropical Seagrasses from Indonesia. J. Coast. Dev. 2012, 15, 189–196. [Google Scholar]
- Jeyapragash, D.; Subhashini, P.; Raja, S.; Abirami, K.; Thangaradjou, T. Evaluation of in-vitro antioxidant activity of seagrasses: Signals for potential alternate source. Free Radic. Antioxid. 2016, 6, 77–89. [Google Scholar] [CrossRef]
- Kannan Rengasamy, R.R.; Rajasekaran, A.; Micheline, G.-D.; Perumal, A. Antioxidant Activity of Seagrasses of the Mandapam Coast, India. Pharm. Biol. 2012, 50, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathy, A.; Varadharaj, V.; Bharathi, N.; Jayalakshmi, M.; Sugumar, M. Phytochemical Analysis and Invitro Antioxidant Screening of Sea Grass-Enhalus Acoroides. Int. J. Res. Pharm. Sci. 2017, 8, 251–258. [Google Scholar]
- De Vincenti, L.; Glasenapp, Y.; Cattò, C.; Villa, F.; Cappitelli, F.; Papenbrock, J. Hindering the Formation and Promoting the Dispersion of Medical Biofilms: Non-Lethal Effects of Seagrass Extracts. BMC Complement. Altern. Med. 2018, 18, 168. [Google Scholar] [CrossRef] [Green Version]
- Karthik, G.; Chermapandi, P.; Hemalatha, A.; Saranya, C.; Anantharaman, P. Evaluation of Antioxidant Activities and Preliminary Phytochemical Analysis of Seagrasses Halodule pinifolia, Halophila ovalis and Syringodium isoetifolium. J. Phytochem. 2013, 114, 181–187. [Google Scholar]
- González, K.L.; Richard, G.; Hernández, Y.; Valdés-Iglesias, O.; Rodriguez, M. Determination of the Antioxidant Capacity of Two Seagrass Species According to the Extraction Method. J. Pharm. Pharmacogn. Res. 2016, 4, 199–205. [Google Scholar]
- Rengasamy, K.R.R.; Sadeer, N.B.; Zengin, G.; Mahomoodally, M.F.; Cziáky, Z.; Jekő, J.; Diuzheva, A.; Abdallah, H.H.; Kim, D.H. Biopharmaceutical Potential, Chemical Profile and in Silico Study of the Seagrass—Syringodium isoetifolium (Asch.) Dandy. S. Afr. J. Bot. 2019, 127, 167–175. [Google Scholar] [CrossRef]
- Tristanto, R.; Putri, M.A.; Situmorang, A.P.; Suryanti, S. Optimalizatiom Use of Seagrass Leafs Thalassia hemprichii As Natural Antioksidan Source. Saintek Perikan. Indones. J. Fish. Sci. Technol. 2014, 10, 26–29. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory Responses and Inflammation-Associated Diseases in Organs. Oncotarget 2017, 9, 7204–7218. [Google Scholar] [CrossRef] [Green Version]
- Micheli, L.; Vasarri, M.; Barletta, E.; Lucarini, E.; Ghelardini, C.; Degl’Innocenti, D.; Di Cesare Mannelli, L. Efficacy of Posidonia oceanica Extract against Inflammatory Pain: In Vivo Studies in Mice. Mar. Drugs 2021, 19, 48. [Google Scholar] [CrossRef]
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef]
- Gordon, S.; Martinez-Pomares, L. Physiological Roles of Macrophages. Pflugers Arch. 2017, 469, 365–374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farhana, A.; Khan, Y.S. Biochemistry, Lipopolysaccharide. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Vasarri, M.; Leri, M.; Barletta, E.; Ramazzotti, M.; Marzocchini, R.; Degl’Innocenti, D. Anti-Inflammatory Properties of the Marine Plant Posidonia oceanica (L.) Delile. J. Ethnopharmacol. 2020, 247, 112252. [Google Scholar] [CrossRef] [PubMed]
- Weimann, E.; Silva, M.B.B.; Murata, G.M.; Bortolon, J.R.; Dermargos, A.; Curi, R.; Hatanaka, E. Topical Anti-Inflammatory Activity of Palmitoleic Acid Improves Wound Healing. PLoS ONE 2018, 13, e0205338. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Zhang, Y.; Tang, C.; Hou, Y.; Ai, X.; Chen, X.; Zhang, Y.; Wang, X.; Meng, X. Gallic Acid: Pharmacological Activities and Molecular Mechanisms Involved in Inflammation-Related Diseases. Biomed. Pharmacother. 2021, 133, 110985. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.T.; Ali, E.S.; Uddin, S.J.; Shaw, S.; Islam, M.A.; Ahmed, M.I.; Chandra Shill, M.; Karmakar, U.K.; Yarla, N.S.; Khan, I.N.; et al. Phytol: A Review of Biomedical Activities. Food Chem. Toxicol. 2018, 121, 82–94. [Google Scholar] [CrossRef]
- Sieber, M.A.; Hegel, J.K.E. Azelaic Acid: Properties and Mode of Action. Skin Pharmacol. Physiol. 2014, 27, 9–17. [Google Scholar] [CrossRef]
- Alam, M.B.; Ju, M.-K.; Kwon, Y.-G.; Lee, S.H. Protopine Attenuates Inflammation Stimulated by Carrageenan and LPS via the MAPK/NF-ΚB Pathway. Food Chem. Toxicol. 2019, 131, 110583. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, C.; Zhou, C.; Hong, P.; Zhang, Y.; Sun, S.; Qian, Z.-J. 2′-Hydroxy-5′-Methoxyacetophenone Attenuates the Inflammatory Response in LPS-Induced BV-2 and RAW264.7 Cells via NF-ΚB Signaling Pathway. J. Neuroimmunol. 2019, 330, 143–151. [Google Scholar] [CrossRef]
- Chassagne, F.; Samarakoon, T.; Porras, G.; Lyles, J.T.; Dettweiler, M.; Marquez, L.; Salam, A.M.; Shabih, S.; Farrokhi, D.R.; Quave, C.L. A Systematic Review of Plants With Antibacterial Activities: A Taxonomic and Phylogenetic Perspective. Front. Pharmacol. 2021, 11, 6548. [Google Scholar] [CrossRef]
- Gumgumjee, N.M.; Bukhari, D.A.; Alshehri, W.A.; Hajar, A.S. Antibacterial Activity of Halodule Uninervis Leaves Extracts Against Some Bacterial Pathogens Strains—Pharmacophore. Available online: https://pharmacophorejournal.com/article/antibacterial-activity-of-halodule-uninervis-leaves-extracts-against-some-bacterial-pathogens-strains (accessed on 15 January 2022).
- Manandhar, S.; Luitel, S.; Dahal, R.K. In Vitro Antimicrobial Activity of Some Medicinal Plants against Human Pathogenic Bacteria. J. Trop. Med. 2019, 2019, 1895340. [Google Scholar] [CrossRef] [Green Version]
- Kapoor, G.; Saigal, S.; Elongavan, A. Action and Resistance Mechanisms of Antibiotics: A Guide for Clinicians. J. Anaesthesiol. Clin. Pharmacol. 2017, 33, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Mani, A.E.; Bharathi, V.; Patterson, J.; Devadason, S. Antibacterial Activity and Preliminary Phytochemical Analysis of Sea Grass Cymodocea Rotundata. Int. J. Microbiol. Res. 2012, 3, 99–103. [Google Scholar] [CrossRef]
- Shin, B.; Park, C.; Imlay, J.A.; Park, W. 4-Hydroxybenzaldehyde Sensitizes Acinetobacter Baumannii to Amphenicols. Appl. Microbiol. Biotechnol. 2018, 102, 2323–2335. [Google Scholar] [CrossRef] [PubMed]
- Hawas, U. A New 8-Hydroxy Flavone O-Xyloside Sulfate and Antibacterial Activity from the Egyptian Seagrass Thalassia hemprichii. Chem. Nat. Compd. 2014, 50, 629–632. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, Z.; Li, J.; Yang, X.; Fei, B.; Leung, P.H.M.; Tao, X. A New Antimicrobial Agent: Poly (3-Hydroxybutyric Acid) Oligomer. Macromol. Biosci. 2019, 19, 1800432. [Google Scholar] [CrossRef] [PubMed]
- Galdiero, E.; Ricciardelli, A.; D’Angelo, C.; de Alteriis, E.; Maione, A.; Albarano, L.; Casillo, A.; Corsaro, M.M.; Tutino, M.L.; Parrilli, E. Pentadecanoic Acid against Candida Albicans-Klebsiella Pneumoniae Biofilm: Towards the Development of an Anti-Biofilm Coating to Prevent Polymicrobial Infections. Res. Microbiol. 2021, 172, 103880. [Google Scholar] [CrossRef]
- Anzaku, A.A.; Akyala, J.I.; Juliet, A.; Obianuju, E.C. Antibacterial Activity of Lauric Acid on Some Selected Clinical Isolates. Ann. Clin. Lab. Res. 2017, 5. [Google Scholar] [CrossRef] [Green Version]
- Tao, C.; Wu, J.; Liu, Y.; Liu, M.; Yang, R.; Lv, Z. Antimicrobial Activities of Bamboo (Phyllostachys Heterocycla Cv. Pubescens) Leaf Essential Oil and Its Major Components. Eur. Food Res. Technol. 2018, 244, 881–891. [Google Scholar] [CrossRef]
- Zhang, H.; Dolan, H.L.; Ding, Q.; Wang, S.; Tikekar, R.V. Antimicrobial Action of Octanoic Acid against Escherichia Coli O157:H7 during Washing of Baby Spinach and Grape Tomatoes. Food Res. Int. 2019, 125, 108523. [Google Scholar] [CrossRef]
- Yoon, B.K.; Jackman, J.A.; Valle-González, E.R.; Cho, N.-J. Antibacterial Free Fatty Acids and Monoglycerides: Biological Activities, Experimental Testing, and Therapeutic Applications. Int. J. Mol. Sci. 2018, 19, 1114. [Google Scholar] [CrossRef] [Green Version]
- Sathyanathan, C.V.; Jyothirmayi, B.; Sundaram, L.R.; Abhinand, P.A.; Eswaramoorthy, R.; Gnanambal, K.M.E. Pheophytin a Isolated from the Seagrass Syringodium isoetifolium Plausibly Blocks UmuC Proteins of Select Bacterial Pathogens, in Silico. J. Appl. Microbiol. 2016, 121, 1592–1602. [Google Scholar] [CrossRef] [PubMed]
- Iyapparaj, P.; Revathi, P.; Ramasubburayan, R.; Prakash, S.; Palavesam, A.; Immanuel, G.; Anantharaman, P.; Sautreau, A.; Hellio, C. Antifouling and Toxic Properties of the Bioactive Metabolites from the Seagrasses Syringodium isoetifolium and Cymodocea serrulata. Ecotoxicol. Environ. Saf. 2014, 103, 54–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nur, R.M.; Koroy, K.; Alwi, D.; Wahab, I.; Sulistiawati, S.; Dewi, R.; Rorano, M. The Antibacterial Activity of Seagrass Enhalus Acoroides against Staphylococcus Aureus. IOP Conf. Ser. Earth Environ. Sci. 2021, 890, 012013. [Google Scholar] [CrossRef]
- Ragupathi Raja Kannan, R.; Arumugam, R.; Iyapparaj, P.; Thangaradjou, T.; Anantharaman, P. In Vitro Antibacterial, Cytotoxicity and Haemolytic Activities and Phytochemical Analysis of Seagrasses from the Gulf of Mannar, South India. Food Chem. 2013, 136, 1484–1489. [Google Scholar] [CrossRef] [PubMed]
- Ragupathi Raja Kannan, R.; Arumugam, R.; Anantharaman, P. Chemical Composition and Antibacterial Activity of Indian Seagrasses against Urinary Tract Pathogens. Food Chem. 2012, 135, 2470–2473. [Google Scholar] [CrossRef]
- Selva, J.; Albert, M.; Gayathri, K.; Sornaraj, R. Assessment and Screening of Phyto-Chemical Components of the Sea Grasses Cymodocea rotundata and Syringodium isoetifolium and Its Antibiotic Potential. Biology 2015. [Google Scholar]
- Sangeetha, J.; Asokan, S. Phytochemical analysis and antibacterial activity of the three different seagrass extracts. Int. J. Adv. Res. 2016, 4, 1451–1457. [Google Scholar] [CrossRef] [Green Version]
- Purnama, A.; Brahmana, E. Bioaktivitas Antibakteri Lamun Thalassia hemprichii Dan Enhalus Acoroides. J. Biol. UNAND 2018, 6, 45. [Google Scholar] [CrossRef] [Green Version]
- Setyoningrum, D.; Yamindago, A.; Maftuch, M.S. Phytochemical Analysis and in Vitro Antibacterial Activities of Seagrass Enhalus Acoroides against Staphylococcus Aureus. Res. J. Life Sci. 2021, 7, 85–91. [Google Scholar] [CrossRef]
- Notarte, K.I.; Yaguchi, T.; Suganuma, K.; dela Cruz, T.E. Antibacterial, Cytotoxic and Trypanocidal Activities of Marine-Derived Fungi Isolated from Philippine Macroalgae and Seagrasses. Acta Bot. Croat. 2018, 77, 141–151. [Google Scholar] [CrossRef] [Green Version]
- El-Hady, H.; Hamed, E.; Shehata, A.N. Molecular Identification, Antimicrobial and Antioxidant Activities of the Tropical Seagrass Halophila Stipulacea Grown in El-Bardawil Lake, Egypt. Aust. J. Basic Appl. Sci. 2012, 6, 474–481. [Google Scholar]
- Nazzaro, F.; Fratianni, F.; Coppola, R.; De Feo, V. Essential Oils and Antifungal Activity. Pharmaceuticals 2017, 10, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badiee, P.; Hashemizadeh, Z. Opportunistic Invasive Fungal Infections: Diagnosis & Clinical Management. Indian J. Med. Res. 2014, 139, 195–204. [Google Scholar] [PubMed]
- Puebla, L.E.J. Fungal Infections in Immunosuppressed Patients|IntechOpen. Available online: https://www.intechopen.com/chapters/39805 (accessed on 24 January 2022).
- Bongomin, F.; Gago, S.; Oladele, R.O.; Denning, D.W. Global and Multi-National Prevalence of Fungal Diseases—Estimate Precision. J. Fungi 2017, 3, 57. [Google Scholar] [CrossRef]
- Hasim, S.; Coleman, J.J. Targeting the Fungal Cell Wall: Current Therapies and Implications for Development of Alternative Antifungal Agents. Future Med. Chem. 2019, 11, 869–883. [Google Scholar] [CrossRef]
- Arastehfar, A.; Gabaldón, T.; Garcia-Rubio, R.; Jenks, J.D.; Hoenigl, M.; Salzer, H.J.F.; Ilkit, M.; Lass-Flörl, C.; Perlin, D.S. Drug-Resistant Fungi: An Emerging Challenge Threatening Our Limited Antifungal Armamentarium. Antibiotics 2020, 9, 877. [Google Scholar] [CrossRef]
- Ross, C.; Puglisi-Weening, M.; Paul, V. Antifungal Defenses of Seagrasses from the Indian River Lagoon, Florida. Aquat. Bot. 2008, 88, 134–141. [Google Scholar] [CrossRef]
- Supaphon, P.; Phongpaichit, S.; Rukachaisirikul, V.; Sakayaroj, J. Antimicrobial Potential of Endophytic Fungi Derived from Three Seagrass Species: Cymodocea serrulata, Halophila ovalis and Thalassia hemprichii. PLoS ONE 2013, 8, e72520. [Google Scholar] [CrossRef]
- Jebasingh, S.E.J.; Lakshmikandan, M.; Sivaraman, K.; Uthiralingam, M. Assessment of Antibacterial, Antifungal Property and Purification of Bioactive Compounds from Seagrass, Thalassia hemprichii. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2015, 86, 905–910. [Google Scholar] [CrossRef]
- Bachar, S.C.; Mazumder, K.; Bachar, R.; Aktar, A.; Al Mahtab, M. A Review of Medicinal Plants with Antiviral Activity Available in Bangladesh and Mechanistic Insight Into Their Bioactive Metabolites on SARS-CoV-2, HIV and HBV. Front. Pharmacol. 2021, 12, 732891. [Google Scholar] [CrossRef]
- Mohammed, M.M.D.; Hamdy, A.-H.A.; El-Fiky, N.M.; Mettwally, W.S.A.; El-Beih, A.A.; Kobayashi, N. Anti-Influenza A Virus Activity of a New Dihydrochalcone Diglycoside Isolated from the Egyptian Seagrass Thalassodendron Ciliatum (Forsk.) Den Hartog. Nat. Prod. Res. 2014, 28, 377–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hawas, U.W.; Abou El-Kassem, L.T. Thalassiolin D: A New Flavone O-Glucoside Sulphate from the Seagrass Thalassia hemprichii. Nat. Prod. Res. 2017, 31, 2369–2374. [Google Scholar] [CrossRef] [PubMed]
- Tumilaar, S.G.; Fatimawali, F.; Niode, N.J.; Effendi, Y.; Idroes, R.; Adam, A.A.; Rakib, A.; Emran, T.B.; Tallei, T.E. The Potential of Leaf Extract of Pangium Edule Reinw as HIV-1 Protease Inhibitor: A Computational Biology Approach. J. Appl. Pharm. Sci. 2021, 11, 101–110. [Google Scholar] [CrossRef]
- Liang, X.; Huang, Y.; Pan, X.; Hao, Y.; Chen, X.; Jiang, H.; Li, J.; Zhou, B.; Yang, Z. Erucic Acid from Isatis Indigotica Fort. Suppresses Influenza A Virus Replication and Inflammation in Vitro and in Vivo through Modulation of NF-ΚB and P38 MAPK Pathway. J. Pharm. Anal. 2020, 10, 130–146. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.L.; Siddiqui, F.A. Beta-Sitosterol: As Immunostimulant, Antioxidant and Inhibitor of SARS-CoV-2 Spike Glycoprotein. Arch. Pharmacol. Ther. 2020, 2, 12–16. [Google Scholar] [CrossRef]
- Krylova, N.V.; Leonova, G.N.; Maystrovskaya, O.S.; Popov, A.M.; Artyukov, A.A. Mechanisms of Antiviral Activity of the Polyphenol Complex from Seagrass of the Zosteraceae Family against Tick-Borne Encephalitis Virus. Bull. Exp. Biol. Med. 2018, 165, 61–63. [Google Scholar] [CrossRef]
- Kularatne, S.A.M. Dengue Fever. BMJ 2015, 351, h4661. [Google Scholar] [CrossRef]
- Ali, M.S.; Ravikumar, S.; Beula, J.M. Bioactivity of Seagrass against the Dengue Fever Mosquito Aedes Aegypti Larvae. Asian Pac. J. Trop. Biomed. 2012, 2, 570–573. [Google Scholar] [CrossRef] [Green Version]
- Mahyoub, J.; Hawas, U.; Alghamdi, K.; Aljameeli, M.M.E.; Shaher, F.M.; Bamakhrama, M.A.; Alkenani, N. The Biological Effects of Some Marine Extracts against Aedes Aegypti (L.) Mosquito Vector of the Dengue Fever in Jeddah Governorate, Saudi Arabia. J. Pure Appl. Microbiol. 2016, 10, 1949–1956. [Google Scholar]
- Ali, M.S.; Ravikumar, S.; Beula, J.M. Larvicidal Potential of Seagrass Extracts Against Dengue Vector Aedes Aegypti (Insecta: Diptera: Culicidae). Int. J. Pharma Bio Sci. 2013, 4, 62–67. [Google Scholar]
- Urbatzka, R.; Freitas, S.; Palmeira, A.; Almeida, T.; Moreira, J.; Azevedo, C.; Afonso, C.; Correia-da-Silva, M.; Sousa, E.; Pinto, M.; et al. Lipid Reducing Activity and Toxicity Profiles of a Library of Polyphenol Derivatives. Eur. J. Med. Chem. 2018, 151, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Packard, C.J.; Boren, J.; Taskinen, M.-R. Causes and Consequences of Hypertriglyceridemia. Front. Endocrinol. 2020, 11, 252. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakash, B. Antidiabetic Activity of Thalassia hemprichii (Seagrass) in Alloxan Induced Diabetic Mice. IOSR J. Pharm. Biol. Sci. 2018, 12, 24–33. [Google Scholar] [CrossRef]
- Vani, M.; Vasavi, T.; Uma Maheswari Devi, P. Evaluation of in Vitro Antidiabetic Activity of Methanolic Extract of Seagrass Halophila Beccarii. Evaluation 2018, 11, 150–153. [Google Scholar] [CrossRef]
- Wu, Y.; Ding, Y.; Tanaka, Y.; Zhang, W. Risk Factors Contributing to Type 2 Diabetes and Recent Advances in the Treatment and Prevention. Int. J. Med. Sci. 2014, 11, 1185–1200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karthikeyan, R.; Sundarapandian, M. Antidiabetic Activity of Methanolic Extract of Halodule Uninervis in Streptozotocin-Induced Diabetic Mice—ProQuest. Available online: https://www.proquest.com/openview/bc426276888c3a1cdeba4b86e5e60822/1?pq-origsite=gscholar&cbl=54977 (accessed on 8 January 2022).
- Wang, J.; Huang, M.; Yang, J.; Ma, X.; Zheng, S.; Deng, S.; Huang, Y.; Yang, X.; Zhao, P. Anti-Diabetic Activity of Stigmasterol from Soybean Oil by Targeting the GLUT4 Glucose Transporter. Food Nutr. Res. 2017, 61, 1364117. [Google Scholar] [CrossRef] [Green Version]
- Ponnulakshmi, R.; Shyamaladevi, B.; Vijayalakshmi, P.; Selvaraj, J. In Silico and in Vivo Analysis to Identify the Antidiabetic Activity of Beta Sitosterol in Adipose Tissue of High Fat Diet and Sucrose Induced Type-2 Diabetic Experimental Rats. Toxicol. Mech. Methods 2019, 29, 276–290. [Google Scholar] [CrossRef]
- Hegazi, N.M.; Saad, H.H.; Marzouk, M.M.; Abdel Rahman, M.F.; El Bishbishy, M.H.; Zayed, A.; Ulber, R.; Ezzat, S.M. Molecular Networking Leveraging the Secondary Metabolomes Space of Halophila stipulaceae (Forsk.) Aschers. and Thalassia hemprichii (Ehrenb. Ex Solms) Asch. in Tandem with Their Chemosystematics and Antidiabetic Potentials. Mar. Drugs 2021, 19, 279. [Google Scholar] [CrossRef]
- Vasarri, M.; Barletta, E.; Ramazzotti, M.; Degl’Innocenti, D. In Vitro Anti-Glycation Activity of the Marine Plant Posidonia oceanica (L.) Delile. J. Ethnopharmacol. 2020, 259, 112960. [Google Scholar] [CrossRef]
- Mujeeb, M.; Alam Khan, S.; Aeri, V.; Ali, B. Hepatoprotective Activity of the Ethanolic Extract of Ficus CaricaLinn. LeavesinCarbon Tetrachloride-Induced Hepatotoxicityin Rats. Iran. J. Pharm. Res. IJPR 2011, 10, 301–306. [Google Scholar]
- Mettwally, W.S.A.; Ragab, T.I.M.; Hamdy, A.-H.A.; Helmy, W.A.; Hassan, S.A. Preliminary Study on the Possible Impact of Thalassodendron Ciliatum (Forss.) Den Hartog Acidic Polysaccharide Fractions against TAA Induced Liver Failure. Biomed. Pharmacother. 2021, 138, 111502. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Cho, W.C.; Upadhyay, G. Drug-Induced Liver Toxicity and Prevention by Herbal Antioxidants: An Overview. Front. Physiol. 2016, 6, 363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dewi, R.; Malika, R.; Meilani, A.; Saputri, F. Hepatoprotective Effect of 50% Ethanol Extract of Seagrass Rhizome (Cymodocea Rotundata) against Liver Tissues in Paracetamol-Induced Rats. Int. J. Appl. Pharm. 2018, 10, 240. [Google Scholar] [CrossRef] [Green Version]
- Limtrakul, P.; Yodkeeree, S.; Thippraphan, P.; Punfa, W.; Srisomboon, J. Anti-Aging and Tyrosinase Inhibition Effects of Cassia Fistula Flower Butanolic Extract. BMC Complement. Altern. Med. 2016, 16, 497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cornara, L.; Pastorino, G.; Borghesi, B.; Salis, A.; Clericuzio, M.; Marchetti, C.; Damonte, G.; Burlando, B. Posidonia oceanica (L.) Delile Ethanolic Extract Modulates Cell Activities with Skin Health Applications. Mar. Drugs 2018, 16, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, L.; Chen, C.; Cheng, C.; Dai, H.; Ai, Y.; Lin, C.; Chung, Y. Evaluation of Tyrosinase Inhibitory, Antioxidant, Antimicrobial, and Antiaging Activities of Magnolia Officinalis Extracts after Aspergillus Niger Fermentation. BioMed Res. Int. 2018, 2018, 5201786. [Google Scholar] [CrossRef] [Green Version]
- Ragheb, S.R.; El Wakeel, L.M.; Nasr, M.S.; Sabri, N.A. Impact of Rutin and Vitamin C Combination on Oxidative Stress and Glycemic Control in Patients with Type 2 Diabetes. Clin. Nutr. ESPEN 2020, 35, 128–135. [Google Scholar] [CrossRef]
- Bumrungpert, A.; Lilitchan, S.; Tuntipopipat, S.; Tirawanchai, N.; Komindr, S. Ferulic Acid Supplementation Improves Lipid Profiles, Oxidative Stress, and Inflammatory Status in Hyperlipidemic Subjects: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients 2018, 10, 713. [Google Scholar] [CrossRef] [Green Version]
- Pierro, F.D.; Derosa, G.; Maffioli, P.; Bertuccioli, A.; Togni, S.; Riva, A.; Allegrini, P.; Khan, A.; Khan, S.; Khan, B.A.; et al. Possible Therapeutic Effects of Adjuvant Quercetin Supplementation Against Early-Stage COVID-19 Infection: A Prospective, Randomized, Controlled, and Open-Label Study. Int. J. Gen. Med. 2021, 14, 2359–2366. [Google Scholar] [CrossRef]
- Kozan, A.; Guner, R.Y.; Akyol, M. A Retrospective Assessment and Comparison of the Effectiveness of Benzoyl Peroxide; the Combination of Topical Niacinamide, Gallic Acid, and Lauric Acid; and the Combination of Benzoyl Peroxide and Erythromycin in Acne Vulgaris. Dermatol. Ther. 2020, 33, e13534. [Google Scholar] [CrossRef]
- Dall’Oglio, F.; Tedeschi, A.; Lacarrubba, F.; Fabbrocini, G.; Skroza, N.; Chiodini, P.; Micali, G. A Novel Azelaic Acid Formulation for the Topical Treatment of Inflammatory Rosacea: A Multicentre, Prospective Clinical Trial. J. Cosmet. Dermatol. 2021, 20, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Noguchi-Shinohara, M.; Ono, K.; Hamaguchi, T.; Nagai, T.; Kobayashi, S.; Komatsu, J.; Samuraki-Yokohama, M.; Iwasa, K.; Yokoyama, K.; Nakamura, H.; et al. Safety and Efficacy of Melissa Officinalis Extract Containing Rosmarinic Acid in the Prevention of Alzheimer’s Disease Progression. Sci. Rep. 2020, 10, 18627. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gono, C.M.P.; Ahmadi, P.; Hertiani, T.; Septiana, E.; Putra, M.Y.; Chianese, G. A Comprehensive Update on the Bioactive Compounds from Seagrasses. Mar. Drugs 2022, 20, 406. https://doi.org/10.3390/md20070406
Gono CMP, Ahmadi P, Hertiani T, Septiana E, Putra MY, Chianese G. A Comprehensive Update on the Bioactive Compounds from Seagrasses. Marine Drugs. 2022; 20(7):406. https://doi.org/10.3390/md20070406
Chicago/Turabian StyleGono, Christina Mutiara Putri, Peni Ahmadi, Triana Hertiani, Eris Septiana, Masteria Yunovilsa Putra, and Giuseppina Chianese. 2022. "A Comprehensive Update on the Bioactive Compounds from Seagrasses" Marine Drugs 20, no. 7: 406. https://doi.org/10.3390/md20070406
APA StyleGono, C. M. P., Ahmadi, P., Hertiani, T., Septiana, E., Putra, M. Y., & Chianese, G. (2022). A Comprehensive Update on the Bioactive Compounds from Seagrasses. Marine Drugs, 20(7), 406. https://doi.org/10.3390/md20070406