Antioxidant Activity of Gracilaria lemaneiformis Polysaccharide Degradation Based on Nrf-2/Keap-1 Signaling Pathway in HepG2 Cells with Oxidative Stress Induced by H2O2
Abstract
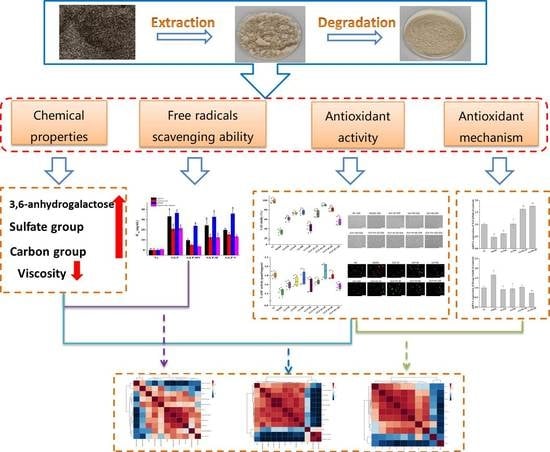
:1. Introduction
2. Results
2.1. The Content of 3,6-Anhydrogalactose and Sulfate and Carbon Groups of GLP and GLP-HV
2.2. The Viscosity of Polysaccharides of G. lemaneiformis
2.3. Scavenging Ability of Polysaccharides of G. lemaneiformis on Free Radicals
2.4. Cell Viability of GLP, GLP-HV, and H2O2 on HepG2 Cells and Establishment of HepG2 Cells with Oxidative Damage Model Induced by H2O2
2.5. Effects of GLP and GLP-HV on T-AOC, CAT, GSH-PX, SOD, and MDA Activity in HepG2 Cells
2.6. Effects of GLP and GLP-HV on ROS in HepG2 Cells
2.7. The Calcium Ion Intensity of GLP and GLP-HV in HepG2 Cells
2.8. AO/EB Fluorescence Staining of GLP and GLP-HV in HepG2 Cells
2.9. Antioxidant Mechanism of GLP and GLP-HV Based on the Nrf-2/Keap-1 Signaling Pathway in HepG2 Cells
2.10. The Correlation between Chemical Properties and Antioxidant Mechanism of GLP-HV
3. Discussion
4. Materials and Methods
4.1. Materials and Chemicals
4.2. Extraction and Degradation of G. lemaneiformis Polysaccharide
4.3. The Content of 3,6-Anhydrogalactose of GLP and GLP-HV
4.4. The Content of Sulfate and Carbon Groups of GLP and GLP-HV
4.5. Viscosity Determination of GLP and GLP-HV
4.6. Scavenging Ability of GLP and GLP-HV on Free Radicals
4.6.1. Scavenging Ability of GLP and GLP-HV on ABTS+
4.6.2. Chelating Capacity of Ferrous Ion of GLP and GLP-HV
4.6.3. Scavenging Ability of GLP and GLP-HV on DPPH
4.6.4. Scavenging Ability of GLP and GLP-HV on Superoxide Anion Radical
4.6.5. Scavenging Ability of GLP and GLP-HV on Hydroxyl Radical
4.7. Cell Culture
4.8. Cell Viability Assay
4.9. Analysis of Antioxidant Indexes of GLP and GLP-HV in HepG2 Cells
4.10. Effects of GLP and GLP-HV on ROS in HepG2 Cells
4.11. The Calcium Ion + Intensity of GLP and GLP-HV in HepG2 Cells
4.12. AO/EB Fluorescence Staining of GLP and GLP-HV in HepG2 Cells
4.13. Real-Time Quantitative PCR Detection
4.14. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, X.; Wang, X.; Shen, M.; Chen, Y.; Yu, Q.; Yang, J.; Xie, J. Combined RNA-Seq and molecular biology technology revealed the protective effect of Cyclocarya paliurus polysaccharide on H2O2-induced oxidative damage in L02 cells thought regulating mitochondrial function, oxidative stress and PI3K/Akt and MAPK signalin. Food Res. Int. 2022, 155, 111080. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Zhao, J.; Song, X. Protective effects of fraxin on cerebral ischemia-reperfusion injury by mediating neuroinflammation and oxidative stress through PPAR-γ/NF-ΚB pathway. Brain Res. Bull. 2022, 187, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Vel, L.; Quiñones, J.; Inostroza, K.; Rommy, D.; Scheuermann, E.; Lorenzo, J.M.; Vel, C. Maqui (Aristotelia Chilensis (Mol.) Stuntz): A natural antioxidant to improve quality of meat patties. Antioxidants 2022, 11, 1405. [Google Scholar]
- Washim, K.; Omkar, R.; Prasad, P.B. Enrichment of dimerumic acid in Monascus-fermented rice and its in vivo antioxidant activity. Food Front. 2021, 2, 547–556. [Google Scholar] [CrossRef]
- Characterization, P.; Activity, A.; Figueroa, F.A.; Abdala-d, R.T.; Claudia, P.; Casas-arrojo, V.; Nesic, A.; Tapia, C.; Dur, C.; Valdes, O.; et al. Sulfated polysaccharide extracted from the green algae. Mar. Drugs 2022, 20, 458. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; González-Rodríguez, R.M.; Sánchez, M.; Amado, I.R.; Franco, D. Effects of natural (grape seed and chestnut extract) and synthetic antioxidants (buthylatedhydroxytoluene, BHT) on the physical, chemical, microbiological and sensory characteristics of dry cured sausage “Chorizo”. Food Res. Int. 2013, 54, 611–620. [Google Scholar] [CrossRef]
- Gunathilake, T.; Akanbi, T.O.; Suleria, H.A.R.; Nalder, T.D.; Francis, D.S.; Barrow, C.J. Seaweed phenolics as natural antioxidants, aquafeed additives, veterinary treatments and cross-linkers for microencapsulation. Mar. Drugs 2022, 20, 445. [Google Scholar] [CrossRef]
- Liao, S.; Long, X.; Zou, Y.; Liu, F.; Li, Q. Mulberry leaf phenolics and fiber exert anti-obesity through the gut microbiota-host metabolism pathway. J. Food Sci. 2021, 86, 1432–1447. [Google Scholar] [CrossRef]
- Di, T.; Chen, G.; Sun, Y.; Ou, S.; Zeng, X.; Ye, H. Antioxidant and immunostimulating activities in vitro of sulfated polysaccharides isolated from Gracilaria rubra. J. Funct. Foods 2017, 28, 64–75. [Google Scholar] [CrossRef]
- Khan, H.; Ullah, H.; Tundis, R.; Belwal, T.; Devkota, H.P.; Daglia, M.; Cetin, Z.; Saygili, E.I.; Campos, M.D.; Capanoglu, E.; et al. Dietary flavonoids in the management of huntington’s disease: Mechanism and clinical perspective. eFood 2020, 1, 38–52. [Google Scholar] [CrossRef]
- Long, X.; Hu, X.; Liu, S.; Pan, C.; Chen, S.; Li, L.; Qi, B.; Yang, X. Insights on preparation, structure and activities of Gracilaria Lemaneiformis polysaccharide. Food Chem. X 2021, 12, 100153. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Zhang, Y.; Sun-Waterhouse, D.; You, L.; Fu, X. Advantages of the polysaccharides from Gracilaria lemaneiformis over metformin in antidiabetic effects on streptozotocin-induced diabetic mice. RSC Adv. 2017, 7, 9141–9151. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, Y.; Shu, Z.; Liu, M.; Zeng, R.; Wang, Y.; Liu, H.; Cao, M.; Su, W.; Liu, G. Sulfated oligosaccharide of Gracilaria lemaneiformis protect against food allergic response in mice by up-regulating immunosuppression. Carbohydr. Polym. 2020, 230, 115567. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Ma, Y.; Cheung, P.C.K.; You, L.; Liao, L.; Pedisić, S.; Kulikouskaya, V. Structural characteristics and anti-inflammatory activity of UV/H2O2-treated algal sulfated polysaccharide from Gracilaria lemaneiformis. Food Chem. Toxicol. 2021, 152, 112157. [Google Scholar] [CrossRef]
- Tang, L.; Luo, X.; Wang, M.; Wang, Z.; Guo, J.; Kong, F.; Bi, Y. Synthesis, characterization, in vitro antioxidant and hypoglycemic activities of selenium nanoparticles decorated with polysaccharides of Gracilaria lemaneiformis. Int. J. Biol. Macromol. 2021, 193, 923–932. [Google Scholar] [CrossRef]
- Chen, X.; You, L.; Ma, Y.; Zhao, Z.; Kulikouskaya, V. Influence of UV/H2O2 treatment on polysaccharides from Sargassum fusiforme: Physicochemical properties and RAW 264.7 cells responses. Food Chem. Toxicol. 2021, 153, 112246. [Google Scholar] [CrossRef]
- Qiu, Y.; Gao, X.; Chen, R.; Lu, S.; Wan, X.; Farag, M.A. Food chemistry: X metabolomics and biochemical insights on the regulation of aging-related diabetes by a low-molecular-weight polysaccharide from green microalga Chlorella pyrenoidosa. Food Chem. X 2022, 14, 100316. [Google Scholar] [CrossRef]
- Long, X.; Hu, X.; Xiang, H.; Chen, S.; Li, L.; Qi, B.; Li, C.; Liu, S.; Yang, X. Structural characterization and hypolipidemic activity of Gracilaria lemaneiformis polysaccharide and its degradation products. Food Chem. X 2022, 14, 100314. [Google Scholar] [CrossRef]
- Long, X.; Hu, X.; Zhou, S.; Xiang, H.; Chen, S.; Li, L.; Liu, S.; Yang, X. Optimized degradation and inhibition of α-glucosidase activity by Gracilaria lemaneiformis polysaccharide and its production in vitro. Mar. Drugs 2022, 20, 13. [Google Scholar] [CrossRef]
- Rahman, M.S.; Hee Choi, Y.; Seok Choi, Y.; Alam, M.B.; Han Lee, S.; Cheol Yoo, J. A novel antioxidant peptide, purified from Bacillus amyloliquefaciens, showed strong antioxidant potential via Nrf-2 mediated heme oxygenase-1 expression. Food Chem. 2018, 239, 502–510. [Google Scholar] [CrossRef]
- Derangula, K.; Javalgekar, M.; Kumar Arruri, V.; Gundu, C.; Kumar Kalvala, A. Probucol attenuates NF-ΚB/NLRP3 signalling and augments Nrf-2 mediated antioxidant defence in nerve injury induced neuropathic pain. Int. Immunopharmacol. 2022, 102, 108397. [Google Scholar] [CrossRef] [PubMed]
- Uribe-carretero, E.; Martinez-chac, G.; Yakhine-diop, S.M.S.; Duque-gonz, G.; Rodr, M.; Alegre-cort, E.; Paredes-barquero, M.; Canales-cort, S.; Pizarro-estrella, E.; Cuadrado, A.; et al. Loss of KEAP1 causes an accumulation of nondegradative organelles. Antioxidants 2022, 11, 1398. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Wang, R.; Yin, Z.; Sun, J.; Wang, B.; Zhao, D.; Zeng, X.A.; Li, H.; Huang, M.; Sun, B. Optimization of Jiuzao protein hydrolysis conditions and antioxidant activity in vivo of Jiuzao tetrapeptide Asp-Arg-Glu-Leu by elevating the Nrf2/Keap1-P38/PI3K-MafK signaling pathway. Food Funct. 2021, 12, 4808–4824. [Google Scholar] [CrossRef]
- Yuan, Y.; Xu, X.; Jing, C.; Zou, P.; Zhang, C.; Li, Y. Microwave assisted hydrothermal extraction of polysaccharides from Ulva prolifera: Functional properties and bioactivities. Carbohydr. Polym. 2018, 181, 902–910. [Google Scholar] [CrossRef]
- Hu, S.M.; Zhou, J.M.; Zhou, Q.Q.; Li, P.; Xie, Y.Y.; Zhou, T.; Gu, Q. Purification, characterization and biological activities of exopolysaccharides from Lactobacillus Rhamnosus ZFM231 isolated from milk. LWT 2021, 147, 111561. [Google Scholar] [CrossRef]
- Chen, X.; Shen, M.; Yang, J.; Yu, Q.; Chen, Y.; Wang, X.; Lu, H.; Tao, X.; Li, H.; Xie, J. RNA-Seq based elucidation of mechanism underlying Mesona chinensis Benth polysaccharide protected H2O2-induced oxidative damage in L02 cells. Food Res. Int. 2022, 157, 111383. [Google Scholar] [CrossRef]
- Hu, Y.M.; Lu, S.Z.; Li, Y.S.; Wang, H.; Shi, Y.; Zhang, L.; Tu, Z.C. Protective effect of antioxidant peptides from grass carp scale gelatin on the H2O2-mediated oxidative injured HepG2 cells. Food Chem. 2022, 373, 131539. [Google Scholar] [CrossRef]
- Ren, Y.; Zheng, G.; You, L.; Wen, L.; Li, C.; Fu, X.; Zhou, L. Structural characterization and macrophage immunomodulatory activity of a polysaccharide isolated from Gracilaria lemaneiformis. J. Funct. Foods 2017, 33, 286–296. [Google Scholar] [CrossRef]
- Wang, M.M.; Wang, F.; Li, G.; Tang, M.T.; Wang, C.; Zhou, Q.Q.; Zhou, T.; Gu, Q. Antioxidant and hypolipidemic activities of pectin isolated from citrus canning processing water. LWT 2022, 159, 113203. [Google Scholar] [CrossRef]
- Sakanashi, Y.; Oyama, K.; Matsui, H.; Oyama, T.B.; Oyama, T.M.; Nishimura, Y.; Sakai, H.; Oyama, Y. Possible use of quercetin, an antioxidant, for protection of cells suffering from overload of intracellular Ca2+: A model experiment. Life Sci. 2008, 83, 164–169. [Google Scholar] [CrossRef]
- Sivaraj, D.; Vijayalakshmi, K.; Ganeshkumar, A.; Rajaram, R. Tailoring Cu substituted hydroxyapatite/functionalized multiwalled carbon nanotube composite coating on 316L SS implant for enhanced corrosion resistance, antibacterial and bioactive properties. Int. J. Pharm. 2020, 590, 119946. [Google Scholar] [CrossRef] [PubMed]
- Bano, F.; Mohanty, B. Corrigendum to “thyroxine modulation of immune toxicity induced by mixture pesticides mancozeb and fipronil in mice” [Life Sci. 240 (2020) 117078]. Life Sci. 2020, 260, 118539. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Zou, L.; Liu, Q.; Geng, Y.; Xu, G.; Chen, L.; Pan, P.; Chen, J. Construction of chitosan-based asymmetric antioxidant and anti-inflammatory repair film for acceleration of wound healing. Int. J. Biol. Macromol. 2022, 215, 377–386. [Google Scholar] [CrossRef]
- Divakar, D.D.; Jastaniyah, N.T.; Altamimi, H.G.; Alnakhli, Y.O.; Muzaheed; Alkheraif, A.A.; Haleem, S. Enhanced antimicrobial activity of naturally derived bioactive molecule chitosan conjugated silver nanoparticle against dental implant pathogens. Int. J. Biol. Macromol. 2018, 108, 790–797. [Google Scholar] [CrossRef]
- Wang, M.; Wu, J.; Jiao, H.; Oluwabiyi, C.; Li, H.; Zhao, J.; Zhou, Y.; Wang, X.; Lin, H. Enterocyte synthesizes and secrets uric acid as antioxidant to protect against oxidative stress via the involvement of Nrf pathway. Free Radic. Biol. Med. 2022, 179, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Chen, J.; Wang, H.; Tu, Z.; Yin, J.; Nie, S. Mechanism of viscosity reduction of okra pectic polysaccharide by ascorbic acid. Carbohydr. Polym. 2022, 284, 119196. [Google Scholar] [CrossRef] [PubMed]
- Olawuyi, I.F.; Lee, W.Y. Structural characterization, functional properties and antioxidant activities of polysaccharide extract obtained from okra leaves (Abelmoschus esculentus). Food Chem. 2021, 354, 129437. [Google Scholar] [CrossRef]
- Chen, J.; Tian, J.; Ge, H.; Liu, R.; Xiao, J. Effects of tetramethylpyrazine from Chinese black vinegar on antioxidant and hypolipidemia activities in HepG2 cells. Food Chem. Toxicol. 2017, 109, 930–940. [Google Scholar] [CrossRef]
- Lee, M.S.; Lee, B.; Park, K.E.; Utsuki, T.; Shin, T.; Oh, C.W.; Kim, H.R. Dieckol enhances the expression of antioxidant and detoxifying enzymes by the activation of Nrf2-MAPK signalling pathway in HepG2 cells. Food Chem. 2015, 174, 538–546. [Google Scholar] [CrossRef]
- Bondre, N.; Zhang, Y.; Geddes, C.D. Metal-enhanced fluorescence based calcium detection: Greater than 100-fold Increase in signal/noise using Fluo-3 or Fluo-4 and silver nanostructures. Sens. Actuators B Chem. 2011, 152, 82–87. [Google Scholar] [CrossRef]
- Hagen, B.M.; Boyman, L.; Kao, J.P.Y.; Lederer, W.J. A Comparative assessment of Fluo Ca2+ indicators in rat ventricular myocytes. Cell Calcium 2012, 52, 170–181. [Google Scholar] [CrossRef] [Green Version]
- Wan, F.; Cai, X.; Wang, M.; Chen, L.; Zhong, R.; Liu, L.; Yi, B.; Hou, F.; Zhang, H. Chlorogenic acid supplementation alleviates dextran sulfate sodium (DSS)-induced colitis via inhibiting inflammatory responses and oxidative stress, improving gut barrier integrity and Nrf-2/HO-1 pathway. J. Funct. Foods 2021, 87, 104808. [Google Scholar] [CrossRef]
- Ferreira, L.G.; da Silva, A.C.R.; Noseda, M.D.; Fuly, A.L.; de Carvalho, M.M.; Fujii, M.T.; Sanchez, E.F.; Carneiro, J.; Duarte, M.E.R. Chemical structure and snake antivenom properties of sulfated agarans obtained from Laurencia dendroidea (Ceramiales, Rhodophyta). Carbohydr. Polym. 2019, 218, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; Ma, Y.; Xiao, J.; You, L.; Pedisić, S.; Liao, L. The possible mechanism of the protective effect of a sulfated polysaccharide from Gracilaria lemaneiformis against colitis induced by dextran sulfate sodium in mice. Food Chem. Toxicol. 2021, 149, 112001. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, K.; Shibuta, S.; Judai, K. Viscosity study of tert-butyl alcohol aqueous solution by brownian motion and gravimetric capillaries. J. Mol. Liq. 2020, 319, 114170. [Google Scholar] [CrossRef]
- Balkrishna, A.; Solleti, S.K.; Singh, H.; Verma, S.; Sharma, N.; Nain, P.; Varshney, A. Herbal decoction Divya-Swasari-Kwath Attenuates airway inflammation and remodeling through Nrf-2 mediated antioxidant lung defence in mouse model of allergic asthma. Phytomedicine 2020, 78, 153295. [Google Scholar] [CrossRef]












| Content | GLP | GLP-HV | GLP-H | GLP-V |
|---|---|---|---|---|
| 3,6-Anhydrogalactose (%) | 35.69 ± 0.34 c | 37.18 ± 0.48 b | 34.04 ± 0.77 d | 43.98 ± 1.07 a |
| Standard curve equation of 3,6-anhydrogalactose | Y = 1.5813 X + 0.1831 R2 = 0.9921 | |||
| Sulfate group (%) | 4.22 ± 0.08 c | 7.53 ± 0.0.05 a | 5.38 ± 0.25 b | 5.48 ± 0.13 b |
| Standard curve of equation sulfate group | Y = 0.60024 X + 0.2273 R2 = 0.9981 | |||
| Carbon group (μmol/g) | 3.31 ± 0.19 b | 4.22 ± 0.32 a | 4.32 ± 0.14 a | 4.41 ± 0.02 a |
| Gene | NCBI Reference Sequence | Primer Sequences | |
|---|---|---|---|
| Forward | Reverse | ||
| GAPDH | NM_001289726.1 | GGAGAAACCTGCCAAGTATGATGAC | GAGACAACCTGGTCCTCAGTGTA |
| Nrf-2 | NM_010902.5 | CAGTGCTCCTATGCGTGAATCCC | TGCCCTAAGCTCATCTCGTGTGA |
| Keap-1 | NM_016679.4 | CGAAGAGGCGGCAGAAGAAG | GACGCTCCAGGGCTATGACA |
| HO-1 | NM_010442.2 | ACCGCCTTCCTGCTCAACATTG | CTCTGACGAAGTGACGCCATCTG |
| NQO-1 | NM_008706.5 | GTCTGGAAACCGTCTGGGAGGA | GCCCACAGAGAGGCCAAACTTG |
| ZO-1 | NM_009386.2 | GGTGCCCTGAAAGAAGCGAT | CTGACAGGTAGGACAGACGA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Long, X.; Hu, X.; Pan, C.; Xiang, H.; Chen, S.; Qi, B.; Liu, S.; Yang, X. Antioxidant Activity of Gracilaria lemaneiformis Polysaccharide Degradation Based on Nrf-2/Keap-1 Signaling Pathway in HepG2 Cells with Oxidative Stress Induced by H2O2. Mar. Drugs 2022, 20, 545. https://doi.org/10.3390/md20090545
Long X, Hu X, Pan C, Xiang H, Chen S, Qi B, Liu S, Yang X. Antioxidant Activity of Gracilaria lemaneiformis Polysaccharide Degradation Based on Nrf-2/Keap-1 Signaling Pathway in HepG2 Cells with Oxidative Stress Induced by H2O2. Marine Drugs. 2022; 20(9):545. https://doi.org/10.3390/md20090545
Chicago/Turabian StyleLong, Xiaoshan, Xiao Hu, Chuang Pan, Huan Xiang, Shengjun Chen, Bo Qi, Shucheng Liu, and Xianqing Yang. 2022. "Antioxidant Activity of Gracilaria lemaneiformis Polysaccharide Degradation Based on Nrf-2/Keap-1 Signaling Pathway in HepG2 Cells with Oxidative Stress Induced by H2O2" Marine Drugs 20, no. 9: 545. https://doi.org/10.3390/md20090545
APA StyleLong, X., Hu, X., Pan, C., Xiang, H., Chen, S., Qi, B., Liu, S., & Yang, X. (2022). Antioxidant Activity of Gracilaria lemaneiformis Polysaccharide Degradation Based on Nrf-2/Keap-1 Signaling Pathway in HepG2 Cells with Oxidative Stress Induced by H2O2. Marine Drugs, 20(9), 545. https://doi.org/10.3390/md20090545