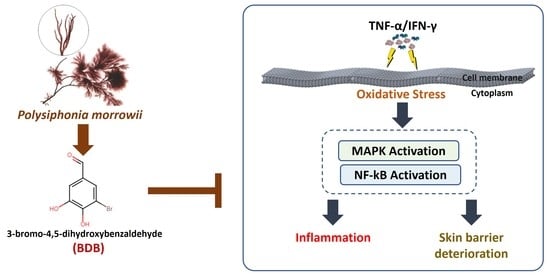
3-Bromo-4,5-dihydroxybenzaldehyde Isolated from Polysiphonia morrowii Suppresses TNF-α/IFN-γ-Stimulated Inflammation and Deterioration of Skin Barrier in HaCaT Keratinocytes
Abstract
:1. Introduction
2. Results
2.1. BDB Effectively Increases Cell Viability and Suppresses the Intracellular ROS Production in TNF-α/IFN-γ-Stimulated HaCaT Keratinocytes
2.2. BDB Downregulates the Expression Levels of Inflammatory Cytokines and Chemokines in TNF-α/IFN-γ-Stimulated HaCaT Keratinocytes
2.3. BDB Effectively Suppresses the Activation of NF-κB and MAPK Signaling Pathways in TNF-α/IFN-γ-Stimulated HaCaT Keratinocytes
2.4. BDB Activates the Nrf2/HO-1 Signaling in TNF-α/IFN-γ-Stimulated HaCaT Keratinocytes
2.5. BDB Ameliorates the Deterioration of Skin Barrier Proteins in TNF-α/IFN-γ-Stimulated HaCaT Keratinocytes
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture and Viability Assay
4.3. Evaluation of Intracellular ROS Production
4.4. Western Blot Analysis
4.5. ELISA Analysis
4.6. RT-PCR Analysis
4.7. Immunofluorescence Analysis
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, L.; Jayawardena, T.U.; Yang, H.-W.; Lee, H.G.; Kang, M.-C.; Sanjeewa, K.; Oh, J.Y.; Jeon, Y.-J. Isolation, characterization, and antioxidant activity evaluation of a fucoidan from an enzymatic digest of the edible seaweed, Hizikia fusiforme. Antioxidants 2020, 9, 363. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Wang, S.-H.; Huang, C.-Y.; Dong, C.-D.; Huang, C.-Y.; Chang, C.-C.; Chang, J.-S. Effect of molecular mass and sulfate content of fucoidan from Sargassum siliquosum on antioxidant, anti-lipogenesis, and anti-inflammatory activity. J. Biosci. Bioeng. 2021, 132, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.K.H.M.; Madusanka, D.M.D.; Han, E.J.; Kim, H.-S.; Jeon, Y.-J.; Jee, Y.; Kim, K.-N.; Lee, K.; Fernando, I.P.S.; Ahn, G. Sargassum horneri (Turner) C. Agardh ethanol extract attenuates fine dust-induced inflammatory responses and impaired skin barrier functions in HaCaT keratinocytes. J. Ethnopharmacol. 2021, 273, 114003. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Oh, J.-Y.; Lee, W.; Jeon, Y.-J. Fucoidan isolated from Hizikia fusiforme suppresses ultraviolet B-induced photodamage by down-regulating the expressions of matrix metalloproteinases and pro-inflammatory cytokines via inhibiting NF-κB, AP-1, and MAPK signaling pathways. Int. J. Biol. Macromol. 2021, 166, 751–759. [Google Scholar] [CrossRef]
- Han, E.J.; Fernando, I.P.S.; Kim, E.-A.; Kim, J.; Jung, K.; Kim, S.-Y.; Cha, S.-H.; Kim, K.-N.; Heo, S.-J.; Ahn, G. 5-Bromo-3, 4-dihydroxybenzaldehyde from Polysiphonia morrowii attenuate IgE/BSA-stimulated mast cell activation and passive cutaneous anaphylaxis in mice. Biochem. Pharmacol. 2020, 178, 114087. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Kim, S.R.; Oh, M.-J.; Jung, S.-J.; Kang, S.Y. In vitro antiviral activity of red alga, Polysiphonia morrowii extract and its bromophenols against fish pathogenic infectious hematopoietic necrosis virus and infectious pancreatic necrosis virus. J. Microbiol. 2011, 49, 102–106. [Google Scholar] [CrossRef]
- Liu, M.; Hansen, P.E.; Lin, X. Bromophenols in marine algae and their bioactivities. Mar. Drugs 2011, 9, 1273–1292. [Google Scholar] [CrossRef]
- Duan, X.-J.; Li, X.-M.; Wang, B.-G. Highly brominated mono-and bis-phenols from the marine red alga Symphyocladia latiuscula with radical-scavenging activity. J. Nat. Prod. 2007, 70, 1210–1213. [Google Scholar] [CrossRef]
- Xu, N.; Fan, X.; Yan, X.; Li, X.; Niu, R.; Tseng, C. Antibacterial bromophenols from the marine red alga Rhodomela confervoides. Phytochemistry 2003, 62, 1221–1224. [Google Scholar] [CrossRef]
- Lee, J.-H.; Lee, T.-K.; Kang, R.-S.; Shin, H.-J.; Lee, H.-S. The in vitro antioxidant activities of the bromophenols from the red alga Tichocarpus crinitus and phenolic derivatives. J. Korean Magn. Reson. Soc. 2007, 11, 56–63. [Google Scholar]
- Kang, N.-J.; Han, S.-C.; Kang, H.-J.; Ko, G.; Yoon, W.-J.; Kang, H.-K.; Yoo, E.-S. Anti-inflammatory effect of 3-bromo-4, 5-dihydroxybenzaldehyde, a component of Polysiphonia morrowii, in vivo and in vitro. Toxicol. Res. 2017, 33, 325–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, S.-H.; Ko, E.-Y.; Heo, S.-J.; Kim, S.-Y.; Ahn, J.; Kim, K.-N. Anti-senescence and anti-wrinkle activities of 3-Bromo-4,5-dihydroxybenzaldehyde from Polysiphonia morrowii Harvey in human dermal fibroblasts. Asian Pac. J. Trop. Biomed. 2021, 11, 74–80. [Google Scholar]
- Hyun, Y.J.; Piao, M.J.; Zhang, R.; Choi, Y.H.; Chae, S.; Hyun, J.W. Photo-protection by 3-bromo-4, 5-dihydroxybenzaldehyde against ultraviolet B-induced oxidative stress in human keratinocytes. Ecotoxicol. Environ. Saf. 2012, 83, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Jayasinghe, A.M.K.; Kirindage, K.G.I.S.; Fernando, I.P.S.; Han, E.J.; Oh, G.-W.; Jung, W.-K.; Ahn, G. Fucoidan Isolated from Sargassum confusum Suppresses Inflammatory Responses and Oxidative Stress in TNF-α/IFN-γ-Stimulated HaCaT Keratinocytes by Activating Nrf2/HO-1 Signaling Pathway. Mar. Drugs 2022, 20, 117. [Google Scholar] [CrossRef]
- Han, E.J.; Kim, H.-S.; Jung, K.; Sanjeewa, K.K.A.; Herath, K.H.I.N.M.; Lee, W.; Jee, Y.; Jeon, Y.-J.; Lee, J.; Kim, T. Sargassum horneri ethanol extract ameliorates TNF-α/IFN-γ-induced inflammation in human keratinocytes and TPA-induced ear edema in mice. Food Biosci. 2021, 39, 100831. [Google Scholar] [CrossRef]
- Kirindage, K.G.I.S.; Fernando, I.P.S.; Jayasinghe, A.M.K.; Han, E.-J.; Dias, M.K.H.M.; Kang, K.-P.; Moon, S.-I.; Shin, T.-S.; Ma, A.; Ahn, G. Moringa oleifera Hot Water Extract Protects Vero Cells from Hydrogen Peroxide-Induced Oxidative Stress by Regulating Mitochondria-Mediated Apoptotic Pathway and Nrf2/HO-1 Signaling. Foods 2022, 11, 420. [Google Scholar] [CrossRef]
- Saha, S.; Buttari, B.; Panieri, E.; Profumo, E.; Saso, L. An overview of Nrf2 signaling pathway and its role in inflammation. Molecules 2020, 25, 5474. [Google Scholar] [CrossRef]
- Han, E.-J.; Fernando, I.P.S.; Kim, H.-S.; Lee, D.-S.; Kim, A.; Je, J.-G.; Seo, M.-J.; Jee, Y.-H.; Jeon, Y.-J.; Kim, S.-Y. (–)-Loliolide Isolated from Sargassum horneri Suppressed Oxidative Stress and Inflammation by Activating Nrf2/HO-1 Signaling in IFN-γ/TNF-α-Stimulated HaCaT Keratinocytes. Antioxidants 2021, 10, 856. [Google Scholar] [CrossRef]
- Cho, S.-H.; Kim, H.-S.; Lee, W.; Han, E.J.; Kim, S.-Y.; Fernando, I.S.; Ahn, G.; Kim, K.-N. Eckol from Ecklonia cava ameliorates TNF-α/IFN-γ-induced inflammatory responses via regulating MAPKs and NF-κB signaling pathway in HaCaT cells. Int. Immunopharmacol. 2020, 82, 106146. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Dias, M.K.H.M.; Madusanka, D.M.D.; Han, E.J.; Kim, M.J.; Jeon, Y.-J.; Ahn, G. Fucoidan refined by Sargassum confusum indicate protective effects suppressing photo-oxidative stress and skin barrier perturbation in UVB-induced human keratinocytes. Int. J. Biol. Macromol. 2020, 164, 149–161. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Dias, M.K.H.M.; Madusanka, D.M.D.; Han, E.J.; Kim, M.J.; Heo, S.-J.; Lee, K.; Cheong, S.H.; Ahn, G. Low molecular weight fucoidan fraction ameliorates inflammation and deterioration of skin barrier in fine-dust stimulated keratinocytes. Int. J. Biol. Macromol. 2021, 168, 620–630. [Google Scholar] [CrossRef] [PubMed]
- Sanjeewa, K.A.; Jayawardena, T.U.; Kim, H.-S.; Kim, S.-Y.; Fernando, I.S.; Wang, L.; Abetunga, D.; Kim, W.-S.; Lee, D.-S.; Jeon, Y.-J. Fucoidan isolated from Padina commersonii inhibit LPS-induced inflammation in macrophages blocking TLR/NF-κB signal pathway. Carbohydr. Polym. 2019, 224, 115195. [Google Scholar] [CrossRef] [PubMed]
- Piao, M.J.; Kang, K.A.; Ryu, Y.S.; Shilnikova, K.; Park, J.E.; Hyun, Y.J.; Zhen, A.X.; Kang, H.K.; Koh, Y.S.; Ahn, M.J. The red algae compound 3-bromo-4, 5-dihydroxybenzaldehyde protects human keratinocytes on oxidative stress-related molecules and pathways activated by UVB irradiation. Mar. Drugs 2017, 15, 268. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.C.; Hyun, Y.J.; Hewage, S.R.K.M.; Piao, M.J.; Kang, K.A.; Kang, H.K.; Koh, Y.S.; Ahn, M.J.; Hyun, J.W. 3-Bromo-4,5-dihydroxybenzaldehyde enhances the level of reduced glutathione via the Nrf2-mediated pathway in human keratinocytes. Mar. Drugs 2017, 15, 291. [Google Scholar] [CrossRef]
- Park, J.-H.; Kim, M.-S.; Jeong, G.-S.; Yoon, J. Xanthii fructus extract inhibits TNF-α/IFN-γ-induced Th2-chemokines production via blockade of NF-κB, STAT1 and p38-MAPK activation in human epidermal keratinocytes. J. Ethnopharmacol. 2015, 171, 85–93. [Google Scholar] [CrossRef]
- Sung, Y.-Y.; Kim, Y.S.; Kim, H.K. Illicium verum extract inhibits TNF-α-and IFN-γ-induced expression of chemokines and cytokines in human keratinocytes. J. Ethnopharmacol. 2012, 144, 182–189. [Google Scholar] [CrossRef]
- Yi, L.; Cheng, D.; Zhang, K.; Huo, X.; Mo, Y.; Shi, H.; Di, H.; Zou, Y.; Zhang, H.; Zhao, J. Intelectin contributes to allergen-induced IL-25, IL-33, and TSLP expression and type 2 response in asthma and atopic dermatitis. Mucosal Immunol. 2017, 10, 1491–1503. [Google Scholar] [CrossRef]
- Fort, M.M.; Cheung, J.; Yen, D.; Li, J.; Zurawski, S.M.; Lo, S.; Menon, S.; Clifford, T.; Hunte, B.; Lesley, R. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity 2001, 15, 985–995. [Google Scholar] [CrossRef]
- Al-Shami, A.; Spolski, R.; Kelly, J.; Keane-Myers, A.; Leonard, W.J. A role for TSLP in the development of inflammation in an asthma model. J. Exp. Med. 2005, 202, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Ryu, M.J.; Chung, H.S. Anti-inflammatory activity of fucoidan with blocking NF-κB and STAT1 in human keratinocytes cells. Nat. Prod. Sci. 2015, 21, 205–209. [Google Scholar]
- Hashizume, M.; Mihara, M. The roles of interleukin-6 in the pathogenesis of rheumatoid arthritis. Arthritis 2011, 2011, 765624. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.-S.; Yeji, K.; Seo, C.-S.; Yoo, S.-R.; Jin, S.-E.; Shin, H.-K.; Jeong, S.-J. Chungsimyeonja-eum inhibits inflammatory responses in RAW 264.7 macrophages and HaCaT keratinocytes. BMC Complement. Altern. Med. 2015, 15, 371. [Google Scholar] [CrossRef] [PubMed]
- Frattaruolo, L.; Carullo, G.; Brindisi, M.; Mazzotta, S.; Bellissimo, L.; Rago, V.; Curcio, R.; Dolce, V.; Aiello, F.; Cappello, A. Antioxidant and Anti-Inflammatory Activities of Flavanones from Glycyrrhiza glabra L.(licorice) Leaf Phytocomplexes: Identification of Licoflavanone as a Modulator of NF-kB/MAPK Pathway. Antioxidants 2019, 8, 186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arbabi, S.; Maier, R.V. Mitogen-activated protein kinases. Crit. Care Med. 2002, 30, S74–S79. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.-F.; Shen, X.-Y.; Lio, C.K.; Dai, Y.; Cheng, C.-S.; Liu, J.-X.; Yao, Y.-D.; Yu, Y.; Xie, Y.; Luo, P. Activation of Nrf2/HO-1 pathway by nardochinoid C inhibits inflammation and oxidative stress in lipopolysaccharide-stimulated macrophages. Front. Pharmacol. 2018, 9, 911. [Google Scholar] [CrossRef]
- Li, R.; Jia, Z.; Zhu, H. Regulation of Nrf2 signaling. React. Oxyg. Species 2019, 8, 312–322. [Google Scholar] [CrossRef]
- Hayes, J.D.; McMahon, M.; Chowdhry, S.; Dinkova-Kostova, A.T. Cancer chemoprevention mechanisms mediated through the Keap1–Nrf2 pathway. Antioxid. Redox Signal. 2010, 13, 1713–1748. [Google Scholar] [CrossRef]
- Hänel, K.H.; Cornelissen, C.; Lüscher, B.; Baron, J.M. Cytokines and the skin barrier. Int. J. Mol. Sci. 2013, 14, 6720–6745. [Google Scholar] [CrossRef]
- Furue, M. Regulation of filaggrin, loricrin, and involucrin by IL-4, IL-13, IL-17A, IL-22, AHR, and NRF2: Pathogenic implications in atopic dermatitis. Int. J. Mol. Sci. 2020, 21, 5382. [Google Scholar] [CrossRef]
- Morizane, S.; Sunagawa, K.; Nomura, H.; Ouchida, M. Aberrant Serine Protease Activities in Atopic Dermatitis. J. Dermatol. Sci. 2022, 107, 2–7. [Google Scholar] [CrossRef]
- Deraison, C.; Bonnart, C.; Lopez, F.; Besson, C.; Robinson, R.; Jayakumar, A.; Wagberg, F.; Brattsand, M.; Hachem, J.P.; Leonardsson, G. LEKTI fragments specifically inhibit KLK5, KLK7, and KLK14 and control desquamation through a pH-dependent interaction. Mol. Biol. Cell 2007, 18, 3607–3619. [Google Scholar] [CrossRef]
- Joo, Y.A.; Chung, H.; Yoon, S.; Park, J.I.; Lee, J.E.; Myung, C.H.; Hwang, J.S. Skin barrier recovery by protease-activated receptor-2 antagonist lobaric acid. Biomol. Ther. 2016, 24, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.J.; McLean, W.I. One remarkable molecule: Filaggrin. J. Investig. Dermatol. 2012, 132, 751–762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Razia, S.; Park, H.; Shin, E.; Shim, K.-S.; Cho, E.; Kim, S.-Y. Effects of Aloe vera flower extract and its active constituent isoorientin on skin moisturization via regulating involucrin expression: In vitro and molecular docking studies. Molecules 2021, 26, 2626. [Google Scholar] [CrossRef]
- Günzel, D.; Yu, A.S. Claudins and the modulation of tight junction permeability. Physiol. Rev. 2013, 93, 525–569. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Mariscal, L.; Betanzos, A.; Nava, P.; Jaramillo, B. Tight junction proteins. Prog. Biophys. Mol. Biol. 2003, 81, 1–44. [Google Scholar] [CrossRef]
- Fanning, A.S.; Anderson, J.M. Zonula occludens-1 and-2 are cytosolic scaffolds that regulate the assembly of cellular junctions. Ann. N. Y. Acad. Sci. 2009, 1165, 113–120. [Google Scholar] [CrossRef]
- Draelos, Z.D. New treatments for restoring impaired epidermal barrier permeability: Skin barrier repair creams. Clin. Dermatol. 2012, 30, 345–348. [Google Scholar] [CrossRef]
- Litwiniuk, M.; Krejner, A.; Speyrer, M.S.; Gauto, A.R.; Grzela, T. Hyaluronic acid in inflammation and tissue regeneration. Wounds 2016, 28, 78–88. [Google Scholar]
- Kirindage, K.G.I.S.; Jayasinghe, A.M.K.; Han, E.-J.; Jee, Y.; Kim, H.-J.; Do, S.G.; Fernando, I.P.S.; Ahn, G. Fucosterol Isolated from Dietary Brown Alga Sargassum horneri Protects TNF-α/IFN-γ-Stimulated Human Dermal Fibroblasts Via Regulating Nrf2/HO-1 and NF-κB/MAPK Pathways. Antioxidants 2022, 11, 1429. [Google Scholar] [CrossRef]





| Target Gene | Primer Sequence (5′ to 3′ Direction) | |
|---|---|---|
| IL-25 | Forward | CTC AAC AGC AGG GCC ACT C |
| Reverse | GTC TGT AGG CTG ACG CAG TGT G | |
| IL-33 | Forward | GAT GAG ATG TCT CGG CTG CTT G |
| Reverse | AGC CGT TAC GGA TAT GGT GGT C | |
| TSLP | Forward | TAT GAG TGG GAC CAA AAG TAC CG |
| Reverse | GGG ATT GAA GGT TAG GCT CTG G | |
| IL-6 | Forward | GAT GGC TGA AAA AGA TGG ATG C |
| Reverse | TGG TTG GGT CAG GGG TGG TT | |
| IL-8 | Forward | ACA CTG CGC CAA CAC AGA AAT TA |
| Reverse | CAG GCA GTT GGG CAT TGG TG | |
| IL-13 | Forward | ACA CTG CGC CAA CAC AGA AAT TA |
| Reverse | CAG GCA GTT GGG CAT TGG TG | |
| TNF-α | Forward | GGC AGT CAG ATC ATC TTC TCG AA |
| Reverse | GAA GGC CTA AGG TCC ACT TGT GT | |
| IFN-γ | Forward | TCT TGG CTT TTC AGC TCT GCA TCG |
| Reverse | GCT GGC GAC AGT TCA GCC ATC A | |
| Eotaxin | Forward | AAC ATG GCG GGC TCT GCT AC |
| Reverse | CCT GCC TTG GGA CAG ATG CT | |
| MDC | Forward | AGG ACA GAG CAT GGC TCG CCT ACA GA |
| Reverse | TAA TGG CAG GGA GGT AGG GCT CCT GA | |
| RANTES | Forward | CCC CGT GCC GAG ATC AAG GAG TAT TT |
| Reverse | CGT CCA GCC TGG GGA AGG TTT TTG TA | |
| TARC | Forward | ACT GCT CCA GGG ATG CCA TCG TTT TT |
| Reverse | ACA AGG GGA TGG GAT CTC CCT CAC TG | |
| GAPDH | Forward | CGT CTA GAA AAA CCT GCC AA |
| Reverse | TGA AGT CAA AGG AGA CCA CC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jayasinghe, A.M.K.; Han, E.-J.; Kirindage, K.G.I.S.; Fernando, I.P.S.; Kim, E.-A.; Kim, J.; Jung, K.; Kim, K.-N.; Heo, S.-J.; Ahn, G. 3-Bromo-4,5-dihydroxybenzaldehyde Isolated from Polysiphonia morrowii Suppresses TNF-α/IFN-γ-Stimulated Inflammation and Deterioration of Skin Barrier in HaCaT Keratinocytes. Mar. Drugs 2022, 20, 563. https://doi.org/10.3390/md20090563
Jayasinghe AMK, Han E-J, Kirindage KGIS, Fernando IPS, Kim E-A, Kim J, Jung K, Kim K-N, Heo S-J, Ahn G. 3-Bromo-4,5-dihydroxybenzaldehyde Isolated from Polysiphonia morrowii Suppresses TNF-α/IFN-γ-Stimulated Inflammation and Deterioration of Skin Barrier in HaCaT Keratinocytes. Marine Drugs. 2022; 20(9):563. https://doi.org/10.3390/md20090563
Chicago/Turabian StyleJayasinghe, Arachchige Maheshika Kumari, Eui-Jeong Han, Kirinde Gedara Isuru Sandanuwan Kirindage, Ilekuttige Priyan Shanura Fernando, Eun-A Kim, Junseong Kim, Kyungsook Jung, Kil-Nam Kim, Soo-Jin Heo, and Ginnae Ahn. 2022. "3-Bromo-4,5-dihydroxybenzaldehyde Isolated from Polysiphonia morrowii Suppresses TNF-α/IFN-γ-Stimulated Inflammation and Deterioration of Skin Barrier in HaCaT Keratinocytes" Marine Drugs 20, no. 9: 563. https://doi.org/10.3390/md20090563
APA StyleJayasinghe, A. M. K., Han, E. -J., Kirindage, K. G. I. S., Fernando, I. P. S., Kim, E. -A., Kim, J., Jung, K., Kim, K. -N., Heo, S. -J., & Ahn, G. (2022). 3-Bromo-4,5-dihydroxybenzaldehyde Isolated from Polysiphonia morrowii Suppresses TNF-α/IFN-γ-Stimulated Inflammation and Deterioration of Skin Barrier in HaCaT Keratinocytes. Marine Drugs, 20(9), 563. https://doi.org/10.3390/md20090563