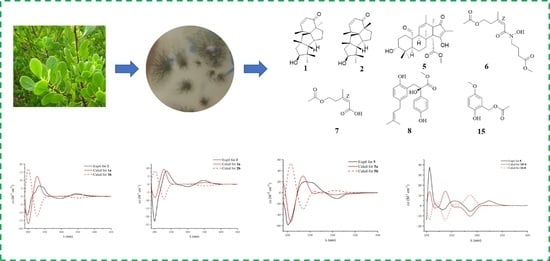
Biological Secondary Metabolites from the Lumnitzera littorea-Derived Fungus Penicillium oxalicum HLLG-13
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Fungal Materials
3.3. Fermentation, Extraction, and Isolation
3.4. Biological Assays
3.4.1. Antibacterial Activity
3.4.2. Growth Inhibition Activities against Newly Hatched H. armigera Hubner Larvae
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Darwish, A.G.G.; Samy, M.N.; Sugimoto, S.; Otsuka, H.; Abdel-Salam, H.; Matsunami, K. Effects of Hepatoprotective Compounds from the Leaves of Lumnitzera racemosa on Acetaminophen-Induced Liver Damage in Vitro. Chem. Pharm. Bull. 2016, 64, 360–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, G.G.D.; Mamdouh, N.S.; Sachiko, S.; Hideaki, O.; Hosni, A.S.; Katsuyoshi, M. A New macrolactone, racemolide along with seven known compounds with biological activities from mangrove plant, Lumnitzera racemose. Nat. Prod. Commun. 2019, 14. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, P.T.; Bui, T.T.L.; Chau, N.D.; Bui, H.T.; Eun, J.K.; Hee, K.K.; Sang, H.L.; Hae, D.J.; Nguyen, T.C.; Nguyen, V.T.; et al. In vitro evaluation of the antioxidant and cytotoxic activities of constituents of the mangrove Lumnitzera racemosa Willd. Arch. Pharm. Res. 2015, 38, 446–455. [Google Scholar] [PubMed]
- Yu, S.Y.; Wang, S.W.; Hwang, T.L.; Wei, B.L.; Su, C.J.; Chang, F.R.; Cheng, Y.B. Components from the Leaves and Twigs of Mangrove Lumnitzera racemosa with Anti-Angiogenic and Anti-Inflammatory Effects. Mar. Drugs 2018, 16, 404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, L.H.; Li, X.M.; Liu, Y.; Wang, B.G. Penicibilaenes A and B, sesquiterpenes with a tricyclo[6.3.1.01,5]dodecane skeleton from the marine isolate of Penicillium bilaiae MA-267. Org. Lett. 2014, 16, 6052–6055. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.H.; Li, X.M.; Lv, C.T.; Li, C.S.; Xu, G.M.; Huang, C.G.; Wang, B.G. Sulfur-containing cytotoxic curvularin macrolides from Penicillium sumatrense MA-92, a fungus obtained from the rhizosphere of the mangrove Lumnitzera racemose. J. Nat. Prod. 2013, 76, 2145–2149. [Google Scholar] [CrossRef]
- Qin, Y.Y.; Zou, L.H.; Lei, X.G.; Su, J.W.; Yang, R.X.; Xie, W.J.; Li, W.S.; Chen, G.Y. OSMAC strategy integrated with molecular networking discovery peniciacetals A−I, nine new meroterpenoids from the mangrove-derived fungus Penicillium sp. HLLG-122. Bioorg. Chem. 2023, 130, 106271. [Google Scholar] [CrossRef]
- Yang, J.Y.; Tang, M.M.; Chen, L.; Lai, X.Y.; Zhuo, X.; Zhou, X.M.; Chen, G.Y. Study on the secondary metabolites of endophytic Penicillium sclerotiorum HLL113. Chin. J. Org. Chem. 2022, 42, 896–900. [Google Scholar] [CrossRef]
- Zheng, C.J.; Bai, M.; Zhou, X.M.; Huang, G.L.; Shao, T.M.; Luo, Y.P.; Niu, Z.G.; Niu, Y.Y.; Chen, G.Y.; Han, C.R. Penicilindoles A-C, Cytotoxic Indole Diterpenes from the Mangrove-Derived Fungus Eupenicillium sp. HJ002. J. Nat. Prod. 2018, 81, 1045–1049. [Google Scholar] [CrossRef]
- Cai, J.; Zhu, X.C.; Zeng, W.N.; Wang, B.; Luo, Y.P.; Liu, J.; Chen, M.J.; Li, G.Y.; Huang, G.L.; Chen, G.Y.; et al. Talaromarins A–F: Six New Isocoumarins from Mangrove-Derived Fungus Talaromyces flavus TGGP35. Mar. Drugs 2022, 20, 361. [Google Scholar] [CrossRef]
- Bai, M.; Zheng, C.J.; Tang, D.Q.; Zhang, F.; Wang, H.Y.; Chen, G.Y. Two new secondary metabolites from a mangrove-derived fungus Cladosporium sp. JS1-2. J. Antibiot. 2019, 72, 779–782. [Google Scholar] [CrossRef]
- Bai, M.; Huang, G.L.; Mei, R.Q.; Wang, B.; Luo, Y.P.; Nong, X.H.; Chen, G.Y.; Zheng, C.J. Bioactive Lactones from the Mangrove-Derived Fungus Penicillium sp. TGM112. Mar. Drugs 2019, 17, 433. [Google Scholar] [CrossRef] [Green Version]
- Du, L.; Li, D.; Zhu, T.; Cai, S.; Wang, F.; Xiao, X.; Gu, Q. New alkaloids and diterpenes from a deep ocean sediment derived fungus Penicillium sp. Tetrahedron 2009, 65, 1033–1039. [Google Scholar] [CrossRef]
- Yudai, M.; Takayoshi, A.; Ikuro, A. Reconstituted biosynthesis of fungal meroterpenoid andrastin A. Tetrahedron 2013, 69, 8199–8204. [Google Scholar]
- Saima, K.; Muhammad, I.T.; Naheed, R.; Mamona, N.; Mahreen, M.; Liaquat, A.; Rasool, B.T.; Muhammad, S.J. Rarely occurring natural products isolated from Vincetoxicum stocksii. Chem. Soc. Pak. 2019, 41, 695–700. [Google Scholar]
- Tian, T.; Li, L.Q.; Xue, J.J.; Zhang, J.; Li, Y.J. Enantioselective Syntheses of Spiroketals via a Tandem Reaction of Cu(I)-Catalyzed Cycloetherification and Hydrogen-Bond-Induced [4 + 2] Cyclization. Org. Chem. 2015, 80, 4189–4200. [Google Scholar] [CrossRef]
- Li, X.; Xue, J.J.; Huang, C.S.; Li, Y. Copper(I)-Catalyzed hydroalkoxylation/hydrogen-bonding-induced asymmetric hetero-diels–alder cycloaddition cascade: An approach to aromatic spiroketals. Chem. Asian J. 2012, 7, 903–906. [Google Scholar] [CrossRef]
- Selvanathan, A.; Vladimir, V.P. Dual Reactivity of Hydroxy- and Methoxy- Substituted o-Quinone Methides in Aqueous Solutions: Hydration versus Tautomerization. J. Org. Chem. 2010, 75, 7338–7346. [Google Scholar]
- Bray, C.D. An Approach to Benzannelated [5,6]-Spiroketals. Synlett 2008, 16, 2500–2502. [Google Scholar] [CrossRef]
- Wang, C.F.; Huang, X.F.; Xiao, H.X.; Hao, Y.J.; Xu, L.; Yan, Q.X.; Zou, Z.B.; Xie, C.L.; Xu, Y.Q.; Yang, X.W. Chemical Constituents of the Marine Fungus Penicillium sp. MCCC 3A00228. Chem. Biodivers. 2021, 18, 2100697. [Google Scholar] [CrossRef]
- Abdelhameed, R.F.A.; Habib, E.S.; Goda, M.S.; Fahim, J.R.; Hassanean, H.A.; Eltamany, E.E.; Ibrahim, A.K.; AboulMagd, A.M.; Fayez, S.; Abd, E.; et al. Thalassosterol, a New Cytotoxic Aromatase Inhibitor Ergosterol Derivative from the Red Sea Seagrass Thalassodendron ciliatum. Mar. Drugs 2020, 18, 354. [Google Scholar] [CrossRef] [PubMed]
- Tettamanzi, M.C.; Jares, E.A.; Iannone, L.M.; Pomilio, A.B. Constituents of Senecio crassiflorus. Fitoterapia 1994, 65, 468. [Google Scholar]
- Lorensi, G.H.; Oliveira, R.S.; Leal, A.P.; Zanatta, A.P.; de Almea, C.G.M.; Barreto, Y.C.; Rosa, M.E.; Vieira, P.B.; Ramos, C.J.B.; Victoria, F.C.; et al. Entomotoxic Activity of Prasiola crispa (Antarctic Algae) in Nauphoeta cinerea Cockroaches: Identification of Main Steroidal Compounds. Mar. Drugs 2019, 17, 573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.; Su, M.; Song, S.J.; Hong, J.; Chung, H.Y.; Jung, J.H. An anti-inflammatory PPAR-γ agonist from the jellyfish-derived fungus Penicillium chrysogenum J08NF-4. J. Nat. Prod. 2018, 81, 356–363. [Google Scholar] [CrossRef]
- Kusakabe, K.; Honmura, Y.; Uesugi, S.; Tonouchi, A.; Maeda, H.; Kimura, K.; Koshino, H.; Hashimoto, M. Neomacrophorin X, a [4.4.3]Propellane-Type Meroterpenoid from Trichoderma sp. 1212-03. J. Nat. Prod. 2017, 80, 1484–1492. [Google Scholar] [CrossRef]
- Zhang, S.T.; He, Y.; Li, F.L.; Lin, S.; Yang, B.Y.; Mo, S.Y.; Li, H.Q.; Wang, J.P.; Qi, C.X.; Hu, Z.X. Bioassay-directed isolation of antibacterial metabolites from an arthropod-derived Penicillium chrysogenum. J. Nat. Prod. 2020, 83, 3397–3403. [Google Scholar] [CrossRef]
- Li, F.L.; Sun, W.G.; Zhang, S.T.; Gao, W.X.; Lin, S.; Yang, B.Y.; Chai, C.W.; Li, H.Q.; Wang, J.P.; Hu, Z.X. New cyclopiane diterpenes with anti-inflammatory activity from the sea sediment-derived fungus Penicillium sp. TJ403-2. Chin. Chem. Lett. 2020, 31, 197–201. [Google Scholar] [CrossRef]
- Mazlan, N.W.; Clements, C.; Edrada, E.R.A. Targeted Isolation of Anti-Trypanosomal Naphthofuran-Quinone Compounds from the Mangrove Plant Avicennia lanata. Mar. Drugs 2020, 18, 661. [Google Scholar] [CrossRef]
- Cheng, Z.B.; Li, Y.L.; Xu, W.; Liu, W.; Liu, L.J.; Zhu, D.G.; Kang, Y.; Luo, Z.H.; Li, Q. Three new cyclopiane-type diterpenes from a deep-sea derived fungus Penicillium sp. YPGA11 and their effects against human esophageal carcinoma cells. Bioorg. Chem. 2019, 91, 103129. [Google Scholar] [CrossRef]
- Xie, C.L.; Zhang, D.; Xia, J.M.; Hu, C.C.; Lin, T.; Lin, Y.K.; Wang, G.H.; Tian, W.J.; Li, Z.P.; Zhang, X.K.; et al. Steroids from the Deep-Sea-Derived Fungus Penicillium granulatum MCCC 3A00475 Induced Apoptosis via Retinoid X Receptor (RXR)-α Pathway. Mar. Drugs 2019, 17, 178. [Google Scholar] [CrossRef] [Green Version]
- Pierce, C.G.; Uppuluri, P.; Tristan, A.R.; Wormley, F.L.; Mowat, E.; Ramage, G.; Lopez-Ribot, J.L. A simple and reproducible 96-well plate-based method for the formation of fungal biofilms and its application to antifungal susceptibility testing. Nat. Protoc. 2008, 3, 1494–1500. [Google Scholar] [CrossRef]
- Guo, Z.K.; Gai, C.J.; Cai, C.H.; Chen, L.L.; Liu, S.B.; Zeng, Y.B.; Yuan, J.Z.; Mei, W.L.; Dai, H.F. Metabolites with Insecticidal Activity from Aspergillus fumigatus JRJ111048 Isolated from Mangrove Plant Acrostichum specioum Endemic to Hainan Island. Mar. Drugs 2017, 15, 381. [Google Scholar] [CrossRef]




| Position | 1 | 2 | 5 | 6 | 7 | 8 | 15 |
|---|---|---|---|---|---|---|---|
| 1 | 2.07, t (13.3) | 1.98, s | |||||
| 1.36, t (13.3) | |||||||
| 2 | 5.94, dd (10.0, 1.2) | 5.96, dd (10.0, 0.8) | 1.36, m | 5.77, s | 6.75, d (3.2) | ||
| 1.02, m | |||||||
| 3 | 7.08, dd (10.0, 5.6) | 7.13, dd (10.0, 6.0) | 3.17, m | 4.11, t (7.2) | 3.42, d (8.5) | ||
| 4 | 2.79, m | 2.74, m | 2.79, m | 2.95, t (6.8) | |||
| 5 | 1.76, m | 4.21, t (6.8) | 6.80, d (8.8) | ||||
| 6 | 2.41, m | 2.30, dd (9.4, 5.2) | 2.00, m | 6.30, br s | 6.48, d (8.2) | 6.70, dd (3.2, 8.8) | |
| 1.53, d (13.2) | |||||||
| 7 | 1.63, m | 1.57, m | 2.93, t (13.2) | 2.01, s | 6.55, dd (2.0, 8.2) | 3.77, s | |
| 1.23, m | 1.21, m | 2.06, t (13.2) | |||||
| 8 | 2.06, m | 2.04, m | 3.53, t (6.8) | 1.95, br s | 5.06, s | ||
| 1.73, m | 1.72, m | ||||||
| 9 | 2.00, q (2.7) | 1.77, m | 6.41, d (2.0) | ||||
| 10 | 1.74, m | 2.06, m | 2.30, t (7.6) | 3.06, d (7.2) | 2.07, s | ||
| 1.68, d (14.4) | |||||||
| 11 | 5.27, s | 5.06, m | |||||
| 12 | 2.18, d (14.8) | 2.02, m | 3.58, s | ||||
| 1.49, d (14.8) | 1.66, m | ||||||
| 13 | 4.08, dd (10.4, 7.2) | 3.94, dd (9.7, 8.6) | 1.87, br s | 1.57, s | |||
| 14 | 1.66, s | ||||||
| 15 | 1.64, d (6.1) | 1.52, d (5.2) | |||||
| 16 | 1.26, d (7.3) | 1.24, d (7.2) | 7.61, d (8.7) | ||||
| 17 | 1.20, s | 1.16, s | 6.88, d (8.7) | ||||
| 18 | 1.12, s | 1.35, s | 0.89, s | ||||
| 19 | 1.12, s | 0.98, s | 0.67, s | 6.88, d (8.7) | |||
| 20 | 0.98, s | 1.00, s | 1.11, s | 7.61, d (8.7) | |||
| 21 | 10.07, s | 3.78, s | |||||
| 22 | 1.65, s | ||||||
| 23 | 1.02, s | ||||||
| 24 | |||||||
| 25 | 3.49, s | ||||||
| 26 | 1.50, s |
| Position | 1 | 2 | 5 | 6 | 7 | 8 | 15 |
|---|---|---|---|---|---|---|---|
| 1 | 208.0, C | 208.2, C | 27.0, CH2 | 20.7, CH3 | 169.4, C | 171.7, C | 152.2, C |
| 2 | 128.0, CH | 128.0, CH | 25.8, CH2 | 170.2, C | 119.5, CH | 86.9, C | 117.3, CH |
| 3 | 157.1, CH | 157.2, CH | 73.5, CH | 62.4, CH2 | 157.1, C | 39.6, CH2 | 126.5, C |
| 4 | 40.2, CH | 45.8, CH | 37.4, C | 31.9, CH2 | 33.3, CH2 | 125.0, C | 152.1, C |
| 5 | 61.9, C | 59.8, C | 46.4, CH | 149.5, C | 63.8, CH2 | 155.0, C | 113.1, CH |
| 6 | 55.8, CH | 49.9, CH | 16.5, CH2 | 117.6, CH | 172.7, C | 115.0, CH | 116.3, CH |
| 7 | 35.6, CH2 | 35.5, CH2 | 32.2, CH2 | 166.2, C | 20.8, CH3 | 129.8, CH | 56.6, CH3 |
| 8 | 40.0, CH2 | 40.0, CH2 | 41.2, C | 46.2, CH2 | 25.7, CH3 | 128.4, C | 62.7, CH2 |
| 9 | 58.7, C | 58.7, C | 53.1, CH | 21.8, CH2 | 132.4, CH | 172.8, C | |
| 10 | 50.1, CH2 | 47.8, CH2 | 51.7, C | 30.4, CH2 | 28.7, CH2 | 20.8, CH3 | |
| 11 | 36.6, C | 38.9, C | 122.0, CH | 173.0, C | 123.5, CH | ||
| 12 | 43.5, CH2 | 47.7, CH2 | 135.5, C | 51.2, CH3 | 133.0, C | ||
| 13 | 78.5, CH | 80.8, CH | 55.7, C | 25.2, CH3 | 17.8, CH3 | ||
| 14 | 57.4, C | 57.6, C | 66.9, C | 25.9, CH3 | |||
| 15 | 74.0, CH | 73.9, CH | 186.2, C | 128.4, C | |||
| 16 | 18.8, CH3 | 19.2, CH3 | 111.5, C | 130.3, CH | |||
| 17 | 21.5, CH3 | 21.5, CH3 | 195.5, C | 116.6, CH | |||
| 18 | 35.1, CH3 | 33.8, CH3 | 27.4, CH3 | 159.2, C | |||
| 19 | 23.1, CH3 | 24.1, CH3 | 21.3, CH3 | 116.6, CH | |||
| 20 | 29.5, CH3 | 24.8, CH3 | 19.1, CH3 | 130.3, CH | |||
| 21 | 206.0, CH | 53.8, CH3 | |||||
| 22 | 19.7, CH3 | ||||||
| 23 | 15.5, CH3 | ||||||
| 24 | 170.8, C | ||||||
| 25 | 51.6, CH3 | ||||||
| 26 | 6.9, CH3 |
| Compounds | MIC (μg/ mL) | |
|---|---|---|
| S. epidermidis | C. albicans | |
| 5 | 12.5 | 6.25 |
| 9 | 6.25 | 6.25 |
| 10 | 25 | 25 |
| 11 | 12.5 | 25 |
| 12 | >50 | 25 |
| 13 | 12.5 | 6.25 |
| 14 | 6.25 | 6.25 |
| Ciprofloxacin a | 0.313 | 0.313 |
| Compounds | IC50 (μg/ mL) |
|---|---|
| 1 | 200 |
| 2 | 200 |
| 3 | 100 |
| 4 | 100 |
| 5 | 50 |
| 6 | 200 |
| 9 | 200 |
| 10 | 100 |
| 11 | 200 |
| 12 | 100 |
| 13 | 200 |
| 14 | 200 |
| Azadirachtin b | 50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Chen, W.; Xu, Z.; Bai, Q.; Zhou, X.; Zheng, C.; Bai, M.; Chen, G. Biological Secondary Metabolites from the Lumnitzera littorea-Derived Fungus Penicillium oxalicum HLLG-13. Mar. Drugs 2023, 21, 22. https://doi.org/10.3390/md21010022
Wang Y, Chen W, Xu Z, Bai Q, Zhou X, Zheng C, Bai M, Chen G. Biological Secondary Metabolites from the Lumnitzera littorea-Derived Fungus Penicillium oxalicum HLLG-13. Marine Drugs. 2023; 21(1):22. https://doi.org/10.3390/md21010022
Chicago/Turabian StyleWang, Yue, Wenhao Chen, Zhefei Xu, Qiqi Bai, Xueming Zhou, Caijuan Zheng, Meng Bai, and Guangying Chen. 2023. "Biological Secondary Metabolites from the Lumnitzera littorea-Derived Fungus Penicillium oxalicum HLLG-13" Marine Drugs 21, no. 1: 22. https://doi.org/10.3390/md21010022
APA StyleWang, Y., Chen, W., Xu, Z., Bai, Q., Zhou, X., Zheng, C., Bai, M., & Chen, G. (2023). Biological Secondary Metabolites from the Lumnitzera littorea-Derived Fungus Penicillium oxalicum HLLG-13. Marine Drugs, 21(1), 22. https://doi.org/10.3390/md21010022