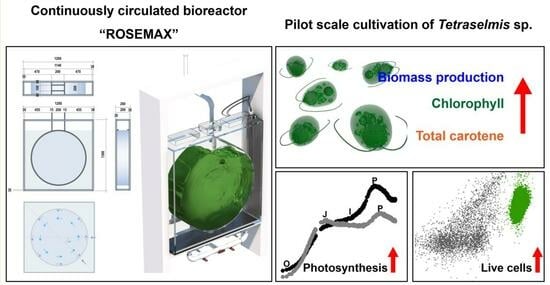
Enhanced Photosynthetic Pigment Production Using a Scaled-Up Continuously Circulated Bioreactor
Abstract
:1. Introduction
2. Results
2.1. Biomass Concentration and Phytochemical Composition
2.2. Flow Cytometric Analysis
2.3. Impact on the Photosynthetic Performance
3. Discussion
4. Materials and Methods
4.1. Strain and Culture Medium
4.2. Culture Conditions
4.3. Bioreactors
4.4. Biomass Concentration
4.5. Cell Harvesting and Phytochemical Analysis
4.6. Photosynthetic Performance
4.7. Flow Cytometric Measurement
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Aschemann-Witzel, J.; Gantriis, R.F.; Fraga, P.; Perez-Cueto, F.J.A. Plant-based food and protein trend from a business perspective: Markets, consumers, and the challenges and opportunities in the future. Crit. Rev. Food Sci. Nutr. 2021, 61, 3119–3128. [Google Scholar] [CrossRef] [PubMed]
- Leandro, A.; Pacheco, D.; Cotas, J.; Marques, J.C.; Pereira, L.; Gonçalves, A.M.M. Seaweed’s bioactive candidate compounds to food industry and global food security. Life 2020, 10, 140. [Google Scholar] [CrossRef] [PubMed]
- Oplatowska-Stachowiak, M.; Elliott, C.T. Food colors: Existing and emerging food safety concerns. Crit. Rev. Food Sci. Nutr. 2017, 57, 524–548. [Google Scholar] [CrossRef]
- Begum, H.; Yusoff, F.M.D.; Banerjee, S.; Khatoon, H.; Shariff, M. Availability and utilization of pigments from microalgae. Crit. Rev. Food Sci. Nutr. 2016, 56, 2209–2222. [Google Scholar] [CrossRef]
- Lu, W.; Shi, Y.; Wang, R.; Su, D.; Tang, M.; Liu, Y.; Li, Z. Antioxidant activity and healthy benefits of natural pigments in fruits: A review. Int. J. Mol. Sci. 2021, 22, 4945. [Google Scholar] [CrossRef] [PubMed]
- Jurić, S.; Jurić, M.; Król-Kilińska, Ż.; Vlahoviček-Kahlina, K.; Vinceković, M.; Dragović-Uzelac, V.; Donsì, F. Sources, stability, encapsulation and application of natural pigments in foods. Food Rev. Int. 2022, 38, 1735–1790. [Google Scholar] [CrossRef]
- Patel, A.K.; Albarico, F.P.J.B.; Perumal, P.K.; Vadrale, A.P.; Nian, C.T.; Chau, H.T.B.; Anwar, C.; Wani, H.M.u.d.; Pal, A.; Saini, R.; et al. Algae as an emerging source of bioactive pigments. Bioresour. Technol. 2022, 351, 126910. [Google Scholar] [CrossRef]
- Lyu, X.; Lyu, Y.; Yu, H.; Chen, W.; Ye, L.; Yang, R. Biotechnological advances for improving natural pigment production: A state-of-the-art review. Bioresour. Biopro. 2022, 9, 8. [Google Scholar] [CrossRef]
- Shuping, Z.; Yulong, W.; Mingde, Y.; Chun, L.; Junmao, T. Pyrolysis characteristics and kinetics of the marine microalgae Dunaliella tertiolecta using thermogravimetric analyzer. Bioresour. Technol. 2010, 101, 359–365. [Google Scholar] [CrossRef]
- Sánchez, J.F.; Fernández, J.M.; Acién, F.G.; Rueda, A.; Pérez-Parra, J.; Molina, E. Influence of culture conditions on the productivity and lutein content of the new strain Scenedesmus almeriensis. Process Biochem. 2008, 43, 398–405. [Google Scholar] [CrossRef]
- Duppeti, H.; Chakraborty, S.; Das, B.S.; Mallick, N.; Kotamreddy, J.N.R. Rapid assessment of algal biomass and pigment contents using diffuse reflectance spectroscopy and chemometrics. Algal Res. 2017, 27, 274–285. [Google Scholar] [CrossRef]
- Ferruzzi, M.G.; Blakeslee, J. Digestion, absorption, and cancer preventative activity of dietary chlorophyll derivatives. Nutr. Res. 2007, 27, 1–12. [Google Scholar] [CrossRef]
- Queiroz Zepka, L.; Jacob-Lopes, E.; Roca, M. Catabolism and bioactive properties of chlorophylls. Curr. Opin. Food Sci. 2019, 26, 94–100. [Google Scholar] [CrossRef]
- Schüler, L.M.; Gangadhar, K.N.; Duarte, P.; Placines, C.; Molina-Márquez, A.M.; Léon-Bañares, R.; Sousa, V.S.; Varela, J.; Barreira, L. Improvement of carotenoid extraction from a recently isolated, robust microalga, Tetraselmis sp. CTP4 (chlorophyta). Bioprocess Biosyst. Eng. 2020, 43, 785–796. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.A.; Kang, N.; Heo, S.Y.; Oh, J.Y.; Lee, S.H.; Cha, S.H.; Kim, W.K.; Heo, S.J. Antioxidant, antiviral, and anti-inflammatory activities of lutein-enriched extract of Tetraselmis species. Mar. Drugs 2023, 21, 369. [Google Scholar] [CrossRef]
- Ibañez, E.; Cifuentes, A. Benefits of using algae as natural sources of functional ingredients. J. Sci. Food Agric. 2013, 93, 703–709. [Google Scholar] [CrossRef]
- Schalch, W.; Cohn, W.; Barker, F.M.; Köpcke, W.; Mellerio, J.; Bird, A.C.; Robson, A.G.; Fitzke, F.F.; van Kuijk, F.J.G.M. Xanthophyll accumulation in the human retina during supplementation with lutein or zeaxanthin-The LUXEA (LUtein Xanthophyll Eye Accumulation) study. Arch. Biochem. Biophys. 2007, 458, 128–135. [Google Scholar] [CrossRef]
- Schüler, L.M.; Santos, T.; Pereira, H.; Duarte, P.; Katkam, N.G.; Florindo, C.; Schulze, P.S.C.; Barreira, L.; Varela, J.C.S. Improved production of lutein and β-carotene by thermal and light intensity upshifts in the marine microalga Tetraselmis sp. CTP4. Algal Res. 2020, 45, 101732. [Google Scholar] [CrossRef]
- Chini Zittelli, G.; Rodolfi, L.; Biondi, N.; Tredici, M.R. Productivity and photosynthetic efficiency of outdoor cultures of Tetraselmis suecica in annular columns. Aquaculture 2006, 261, 932–943. [Google Scholar] [CrossRef]
- Stahl, W.; Sies, H. Photoprotection by dietary carotenoids: Concept, mechanisms, evidence and future development. Mol. Nutr. Food Res. 2012, 56, 287–295. [Google Scholar] [CrossRef]
- Lee, W.K.; Ryu, Y.K.; Choi, W.Y.; Kim, T.; Park, A.; Lee, Y.J.; Jeong, Y.; Lee, C.G.; Kang, D.H. Year-round cultivation of Tetraselmis sp. for essential lipid production in a semi-open raceway system. Mar. Drugs 2021, 19, 314. [Google Scholar] [CrossRef] [PubMed]
- Trovão, M.; Pereira, H.; Silva, J.; Páramo, J.; Quelhas, P.; Santos, T.; Silva, J.T.; Machado, A.; Gouveia, L.; Barreira, L.; et al. Growth performance, biochemical composition and sedimentation velocity of Tetraselmis sp. CTP4 under different salinities using low-cost lab- and pilot-scale systems. Heliyon 2019, 5, e01553. [Google Scholar] [CrossRef] [PubMed]
- Muller-Feuga, A.; Robert, R.; Cahu, C.; Robin, J.; Divanach, P. Uses of microalgae in aquaculture. In Live Feeds in Marine Aquaculture; Støttrup, J.G., McEvoy, L.A., Eds.; Blackwell Publishing Science Ltd.: Oxford, UK, 2003; pp. 253–299. [Google Scholar]
- Custódio, L.; Soares, F.; Pereira, H.; Barreira, L.; Vizetto-Duarte, C.; Rodrigues, M.J.; Rauter, A.P.; Alberício, F.; Varela, J. Fatty acid composition and biological activities of Isochrysis galbana T-ISO, Tetraselmis sp. and Scenedesmus sp.: Possible application in the pharmaceutical and functional food industries. J. Appl. Phycol. 2014, 26, 151–161. [Google Scholar] [CrossRef]
- Custódio, L.; Justo, T.; Silvestre, L.; Barradas, A.; Duarte, C.V.; Pereira, H.; Barreira, L.; Pilar Rauter, A.; Alberício, F.; Varela, J. Microalgae of different phyla display antioxidant, metal chelating and acetylcholinesterase inhibitory activities. Food Chem. 2012, 131, 134–140. [Google Scholar] [CrossRef]
- Sansone, C.; Galasso, C.; Orefice, I.; Nuzzo, G.; Luongo, E.; Cutignano, A.; Romano, G.; Brunet, C.; Fontana, A.; Esposito, F.; et al. The green microalga Tetraselmis suecica reduces oxidative stress and induces repairing mechanisms in human cells. Sci. Rep. 2017, 7, 41215. [Google Scholar] [CrossRef] [PubMed]
- Borowitzka, M.A.; Moheimani, N.R. Open pond culture systems. In Algae for Biofuels and Energy; Borowitzka, M.A., Moheimani, N.R., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 133–152. [Google Scholar]
- Harun, R.; Singh, M.; Forde, G.M.; Danquah, M.K. Bioprocess engineering of microalgae to produce a variety of consumer products. Renew. Sustain. Energy Rev. 2010, 14, 1037–1047. [Google Scholar] [CrossRef]
- Chen, C.Y.; Yeh, K.L.; Aisyah, R.; Lee, D.J.; Chang, J.S. Cultivation, photobioreactor design and harvesting of microalgae for biodiesel production: A critical review. Bioresour. Technol. 2011, 102, 71–81. [Google Scholar] [CrossRef]
- Molina, E.; Acién Fernández, F.G.; García Camacho, F.; Camacho Rubio, F.; Chisti, Y. Scale-up of tubular photobioreactors. J. Appl. Phycol. 2000, 12, 355–368. [Google Scholar] [CrossRef]
- Dasgupta, C.N.; Jose Gilbert, J.; Lindblad, P.; Heidorn, T.; Borgvang, S.A.; Skjanes, K.; Das, D. Recent trends on the development of photobiological processes and photobioreactors for the improvement of hydrogen production. Int. J. Hydrogen Energy 2010, 35, 10218–10238. [Google Scholar] [CrossRef]
- Singh, R.N.; Sharma, S. Development of suitable photobioreactor for algae production—A review. Renew. Sustain. Energy Rev. 2012, 16, 2347–2353. [Google Scholar] [CrossRef]
- Merchuk, J.; Ben-Zvi, S.B.; Niranjan, K. Why use bubble-column bioreactors? Trends Biotechnol. 1994, 12, 501–511. [Google Scholar] [CrossRef]
- Joshi, J.B. Computational flow modelling and design of bubble column reactors. Chem. Eng. Sci. 2001, 56, 5893–5933. [Google Scholar] [CrossRef]
- Loomba, V.; von Lieres, E.; Huber, G. How do operational and design parameters effect biomass productivity in a flat-panel photo-bioreactor? A computational analysis. Processes 2021, 9, 1387. [Google Scholar] [CrossRef]
- Yu, G.; Li, Y.; Shen, G.; Wang, W.; Lin, C.; Wu, H.; Chen, Z. A novel method using CFD to optimize the inner structure parameters of flat photobioreactors. J. Appl. Phycol. 2009, 21, 719–727. [Google Scholar] [CrossRef]
- Al-Mashhadani, M.K.H.; Wilkinson, S.J.; Zimmerman, W.B. Airlift bioreactor for biological applications with microbubble mediated transport processes. Chem. Eng. Sci. 2015, 137, 243–253. [Google Scholar] [CrossRef]
- Acién Fernández, F.G.; García Camacho, F.; Chisti, Y. Photobioreactors: Light regime, mass transfer, and scaleup. In Progress in Industrial Microbiology; Osinga, R., Tramper, J., Burgess, J.G., Wijffels, R.H., Eds.; Elsevier: Amsterdam, The Netherlands, 1999; Volume 35, pp. 231–247. [Google Scholar]
- Richmond, A. Microalgal biotechnology at the turn of the millennium: A personal view. J. Appl. Phycol. 2000, 12, 441–451. [Google Scholar] [CrossRef]
- Jeong, Y.; Choi, W.Y.; Park, A.; Lee, Y.J.; Lee, Y.; Park, G.H.; Lee, S.J.; Lee, W.K.; Ryu, Y.K.; Kang, D.H. Marine cyanobacterium Spirulina maxima as an alternate to the animal cell culture medium supplement. Sci. Rep. 2021, 11, 4906. [Google Scholar] [CrossRef]
- Patrinou, V.; Patsialou, S.; Daskalaki, A.; Economou, C.N.; Aggelis, G.; Vayenas, D.V.; Tekerlekopoulou, A.G. Laboratory- and pilot-scale cultivation of Tetraselmis striata to produce valuable metabolic compounds. Life 2023, 13, 480. [Google Scholar] [CrossRef]
- Farahin, A.W.; Natrah, I.; Nagao, N.; Katayama, T.; Imaizumi, Y.; Mamat, N.Z.; Yusoff, F.M.; Shariff, M. High intensity of light: A potential stimulus for maximizing biomass by inducing photosynthetic activity in marine microalga, Tetraselmis tetrathele. Algal Res. 2021, 60, 102523. [Google Scholar] [CrossRef]
- Alagawany, M.; Taha, A.E.; Noreldin, A.; El-Tarabily, K.A.; Abd El-Hack, M.E. Nutritional applications of species of Spirulina and Chlorella in farmed fish: A review. Aquaculture 2021, 542, 736841. [Google Scholar] [CrossRef]
- Khaleghi, E.; Arzani, K.; Moallemi, N.; Barzegar, M. Evaluation of chlorophyll content and chlorophyll fluorescence parameters and relationships between chlorophyll a, b and chlorophyll content index under water stress in Olea europaea cv. Dezful. Int. Sch. Sci. Res. Innov. 2012, 6, 636–639. [Google Scholar]
- Castrillo, M.; Díez-Montero, R.; Tejero, I. Model-based feasibility assessment of a deep solar photobioreactor for microalgae culturing. Algal Res. 2018, 29, 304–318. [Google Scholar] [CrossRef]
- Markou, G.; Dao, L.H.T.; Muylaert, K.; Beardall, J. Influence of different degrees of N limitation on photosystem II performance and heterogeneity of Chlorella vulgaris. Algal Res. 2017, 26, 84–92. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Singh, R.; Upadhyay, A.K.; Singh, D.V.; Singh, J.S.; Singh, D.P. Photosynthetic performance, nutrient status and lipid yield of microalgae Chlorella vulgaris and Chlorococcum humicola under UV-B exposure. Curr. Res. Biotechnol. 2019, 1, 65–77. [Google Scholar] [CrossRef]
- Hyka, P.; Lickova, S.; Přibyl, P.; Melzoch, K.; Kovar, K. Flow cytometry for the development of biotechnological processes with microalgae. Biotechnol. Adv. 2013, 31, 2–16. [Google Scholar] [CrossRef]
- Gupta, P.L.; Lee, S.M.; Choi, H.J. A mini review: Photobioreactors for large scale algal cultivation. World J. Microbiol. Biotechnol. 2015, 31, 1409–1417. [Google Scholar] [CrossRef]
- Zhang, K.; Miyachi, S.; Kurano, N. Evaluation of a vertical flat-plate photobioreactor for outdoor biomass production and carbon dioxide bio-fixation: Effects of reactor dimensions, irradiation and cell concentration on the biomass productivity and irradiation utilization efficiency. Appl. Microbiol. Biotechnol. 2001, 55, 428–433. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Q.; Zhao, R.; Tao, Y.; Ying, K.-z.; Mao, X.-z. Novel flat-plate photobioreactor with inclined baffles and internal structure optimization to improve light regime performance. ACS Sustain. Chem. Eng. 2021, 9, 1550–1558. [Google Scholar] [CrossRef]
- Soman, A.; Shastri, Y. Optimization of novel photobioreactor design using computational fluid dynamics. Appl. Energy 2015, 140, 246–255. [Google Scholar] [CrossRef]
- Abu-Ghosh, S.; Fixler, D.; Dubinsky, Z.; Iluz, D. Flashing light in microalgae biotechnology. Bioresour. Technol. 2016, 203, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.; Grey, D.; Stepan-Sarkissian, F.; Fowler, M.W. A flat-sided photobioreactor for culturing microalgae. Aquac. Eng. 1993, 12, 183–190. [Google Scholar] [CrossRef]
- Greve, W. The “planktonkreisel”, a new device for culturing zooplankton. Mar. Biol. 1968, 1, 201–203. [Google Scholar] [CrossRef]
- Wang, B.; Lan, C.Q.; Horsman, M. Closed photobioreactors for production of microalgal biomasses. Biotechnol. Adv. 2012, 30, 904–912. [Google Scholar] [CrossRef] [PubMed]
- Kunjapur, A.M.; Eldridge, R.B. Photobioreactor design for commercial biofuel production from microalgae. Ind. Eng. Chem. Res. 2010, 49, 3516–3526. [Google Scholar] [CrossRef]
- Norsker, N.H.; Barbosa, M.J.; Vermuë, M.H.; Wijffels, R.H. Microalgal production—A close look at the economics. Biotechnol. Adv. 2011, 29, 24–27. [Google Scholar] [CrossRef]
- Janssen, M.; Tramper, J.; Mur, L.R.; Wijffels, R.H. Enclosed outdoor photobioreactors: Light regime, photosynthetic efficiency, scale-up, and future prospects. Biotechnol. Bioeng. 2003, 81, 193–210. [Google Scholar] [CrossRef]
- Slegers, P.M.; Wijffels, R.H.; van Straten, G.; van Boxtel, A.J.B. Design scenarios for flat panel photobioreactors. Appl. Energy 2011, 88, 3342–3353. [Google Scholar] [CrossRef]
- Kim, Z.H.; Park, H.; Hong, S.-J.; Lim, S.-M.; Lee, C.-G. Development of a floating photobioreactor with internal partitions for efficient utilization of ocean wave into improved mass transfer and algal culture mixing. Bioprocess Biosyst. Eng. 2016, 39, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Guillard, R.R. Culture of phytoplankton for feeding marine invertebrates. In Culture of Marine Invertebrate Animals; Smith, W.L., Chanley, M.H., Eds.; Plenum Press: New York, NY, USA, 1975; pp. 29–60. [Google Scholar]
- Moran, R. Formulae for determination of chlorophyllous pigments extracted with N,N-dimethylformamide. Plant Physiol. 1982, 69, 1376–1381. [Google Scholar] [CrossRef] [PubMed]





| Conventional Bubble Column | ROSEMAX | |
|---|---|---|
| Laboratory scale (5 L) | ||
| Biomass concentration (g/L, DW) | 1.71 ± 0.09 a | 1.89 ± 0.14 |
| Chlorophyll-a (mg/g, DW) | 14.75 ± 1.25 a | 15.79 ± 1.17 |
| Chlorophyll-b (mg/g, DW) | 7.58 ± 0.22 Ba | 8.84 ± 0.47 Aa |
| Protochlorophyllide (mg/g, DW) | 3.02 ± 0.27 a | 2.82 ± 0.40 |
| Total carotene (mg/g, DW) | 6.08 ± 0.65 a | 6.07 ± 0.21 |
| Pilot scale (160 L) | ||
| Biomass concentration (g/L, DW) | 1.17 ± 0.11 Bb | 1.69 ± 0.11 A |
| Chlorophyll-a (mg/g, DW) | 10.67 ± 0.72 Bb | 14.60 ± 0.76 A |
| Chlorophyll-b (mg/g, DW) | 5.87 ± 0.54 Bb | 7.64 ± 0.09 Ab |
| Protochlorophyllide (mg/g, DW) | 0.84 ± 0.49 Bb | 2.28 ± 0.41 A |
| Total carotene (mg/g, DW) | 3.24 ± 0.56 Bb | 5.64 ± 0.81 A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, W.-K.; Ryu, Y.-K.; Kim, T.; Park, A.; Lee, Y.-J.; Sunwoo, I.Y.; Koh, E.-J.; Oh, C.; Choi, W.-Y.; Kang, D.-H. Enhanced Photosynthetic Pigment Production Using a Scaled-Up Continuously Circulated Bioreactor. Mar. Drugs 2023, 21, 576. https://doi.org/10.3390/md21110576
Lee W-K, Ryu Y-K, Kim T, Park A, Lee Y-J, Sunwoo IY, Koh E-J, Oh C, Choi W-Y, Kang D-H. Enhanced Photosynthetic Pigment Production Using a Scaled-Up Continuously Circulated Bioreactor. Marine Drugs. 2023; 21(11):576. https://doi.org/10.3390/md21110576
Chicago/Turabian StyleLee, Won-Kyu, Yong-Kyun Ryu, Taeho Kim, Areumi Park, Yeon-Ji Lee, In Yung Sunwoo, Eun-Jeong Koh, Chulhong Oh, Woon-Yong Choi, and Do-Hyung Kang. 2023. "Enhanced Photosynthetic Pigment Production Using a Scaled-Up Continuously Circulated Bioreactor" Marine Drugs 21, no. 11: 576. https://doi.org/10.3390/md21110576
APA StyleLee, W. -K., Ryu, Y. -K., Kim, T., Park, A., Lee, Y. -J., Sunwoo, I. Y., Koh, E. -J., Oh, C., Choi, W. -Y., & Kang, D. -H. (2023). Enhanced Photosynthetic Pigment Production Using a Scaled-Up Continuously Circulated Bioreactor. Marine Drugs, 21(11), 576. https://doi.org/10.3390/md21110576