Algae Food Products as a Healthcare Solution
Abstract
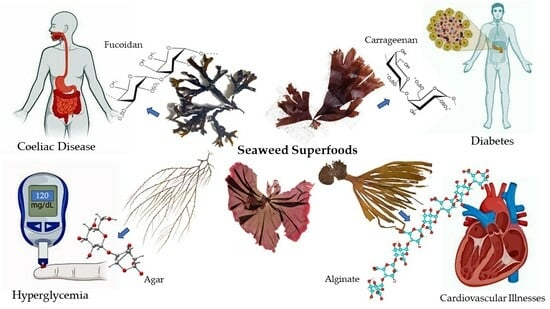
:1. Introduction
2. Diet-Related Diseases
2.1. Celiac Disease
2.2. Diabetes Mellitus (DM)
2.3. Cholesterol
3. Macroalgae
3.1. Proteins
3.2. Polysaccharides from Macroalgae
3.3. Phenolic Compounds
3.4. Pigments
3.5. Carotenoids
3.6. Fatty Acids
3.7. Mycosporyne Amino Acids
3.8. Cholesterol
4. Algae Food Products as Healthcare Solution
4.1. Red Algae (Rhodophyta)
4.2. Green Algae (Chlorophyta)
4.3. Brown Algae (Phaeophyceae)
4.4. The Use of Macroalgae in Gluten-Free Products
5. Algae Food Industry: A Possible Key Road?
5.1. Algae as Raw Source
5.2. Algae as a New Food
5.3. Food Safety
5.4. Algae as a Nutritional Source
5.5. Future Road
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Alberti, K.G.M.; Zimmet, P.; Shaw, J. The Metabolic Syndrome—A New Worldwide Definition. Lancet 2005, 366, 1059–1062. [Google Scholar] [CrossRef]
- Micha, R.; Peñalvo, J.L.; Cudhea, F.; Imamura, F.; Rehm, C.D.; Mozaffarian, D. Association Between Dietary Factors and Mortality From Heart Disease, Stroke, and Type 2 Diabetes in the United States. JAMA 2017, 317, 912. [Google Scholar] [CrossRef] [PubMed]
- Micha, R.; Khatibzadeh, S.; Shi, P.; Andrews, K.G.; Engell, R.E.; Mozaffarian, D. Global, Regional and National Consumption of Major Food Groups in 1990 and 2010: A Systematic Analysis Including 266 Country-Specific Nutrition Surveys Worldwide. BMJ Open 2015, 5, e008705. [Google Scholar] [CrossRef] [PubMed]
- Żarnowski, A.; Jankowski, M.; Gujski, M. Public Awareness of Diet-Related Diseases and Dietary Risk Factors: A 2022 Nationwide Cross-Sectional Survey among Adults in Poland. Nutrients 2022, 14, 3285. [Google Scholar] [CrossRef] [PubMed]
- Willett, W.C.; Stampfer, M.J. Current Evidence on Healthy Eating. Annu. Rev. Public Health 2013, 34, 77–95. [Google Scholar] [CrossRef]
- Leandro, A.; Pacheco, D.; Cotas, J.; Marques, J.C.; Pereira, L.; Gonçalves, A.M.M. Seaweed’s Bioactive Candidate Compounds to Food Industry and Global Food Security. Life 2020, 10, 140. [Google Scholar] [CrossRef] [PubMed]
- Segal, L.; Opie, R.S. A Nutrition Strategy to Reduce the Burden of Diet Related Disease: Access to Dietician Services Must Complement Population Health Approaches. Front. Pharmacol. 2015, 6, 160. [Google Scholar] [CrossRef]
- Vettor, R. The Right Nutrition for the Nutrition Related Diseases. Rev. Endocr. Metab. Disord. 2020, 21, 293–296. [Google Scholar] [CrossRef]
- Cascais, M.; Monteiro, P.; Pacheco, D.; Cotas, J.; Pereira, L.; Marques, J.C.; Gonçalves, A.M.M. Effects of Heat Treatment Processes: Health Benefits and Risks to the Consumer. Appl. Sci. 2021, 11, 8740. [Google Scholar] [CrossRef]
- Kılınç, B.; Cirik, S.; Turan, G.; Tekogul, H.; Koru, E.; Kılınç, B.; Cirik, S.; Turan, G.; Tekogul, H.; Koru, E. Seaweeds for Food and Industrial Applications. Chem. Age India 2013, 33, 475–482. [Google Scholar] [CrossRef]
- Lomartire, S.; Gonçalves, A.M.M. An Overview of Potential Seaweed-Derived Bioactive Compounds for Pharmaceutical Applications. Mar. Drugs 2022, 20, 141. [Google Scholar] [CrossRef] [PubMed]
- Parodi, A.; Leip, A.; De Boer, I.J.M.; Slegers, P.M.; Ziegler, F.; Temme, E.H.M.; Herrero, M.; Tuomisto, H.; Valin, H.; Van Middelaar, C.E.; et al. The Potential of Future Foods for Sustainable and Healthy Diets. Nat. Sustain. 2018, 1, 782–789. [Google Scholar] [CrossRef]
- Shannon, E.; Abu-Ghannam, N. Seaweeds as Nutraceuticals for Health and Nutrition. Phycologia 2019, 58, 563–577. [Google Scholar] [CrossRef]
- Skendi, A.; Papageorgiou, M.; Varzakas, T. High Protein Substitutes for Gluten in Gluten-Free Bread. Foods 2021, 10, 1997. [Google Scholar] [CrossRef]
- Bascuñán, K.A.; Araya, M.; Roncoroni, L.; Doneda, L.; Elli, L. Dietary Gluten as a Conditioning Factor of the Gut Microbiota in Celiac Disease. Adv. Nutr. 2020, 11, 160. [Google Scholar] [CrossRef]
- Biesiekierski, J.R. What Is Gluten? J. Gastroenterol. Hepatol. 2017, 32, 78–81. [Google Scholar] [CrossRef]
- Caio, G.; Volta, U.; Sapone, A.; Leffler, D.A.; De Giorgio, R.; Catassi, C.; Fasano, A. Celiac Disease: A Comprehensive Current Review. BMC Med. 2019, 17, 142. [Google Scholar] [CrossRef] [PubMed]
- Skendi, A.; Papageorgiou, M. Introduction in Wheat and Breadmaking. Trends Wheat Bread Mak. 2021, 1–27. [Google Scholar] [CrossRef]
- Stef, D.-S.; Rivis, A.; Trasca, T.I.; Pop, M.; Heghedus-Mîndru, G.; Stef, L.; Marcu, A. The enrichment of bread with algae species. Anim. Sci. J. 2022, 65, 558–566. [Google Scholar]
- Hager, A.S.; Wolter, A.; Czerny, M.; Bez, J.; Zannini, E.; Arendt, E.K.; Czerny, M. Investigation of Product Quality, Sensory Profile and Ultrastructure of Breads Made from a Range of Commercial Gluten-Free Flours Compared to Their Wheat Counterparts. Eur. Food Res. Technol. 2012, 235, 333–344. [Google Scholar] [CrossRef]
- Houben, A.; Höchstötter, A.; Becker, T. Possibilities to Increase the Quality in Gluten-Free Bread Production: An Overview. Eur. Food Res. Technol. 2012, 235, 195–208. [Google Scholar] [CrossRef]
- Guo, J.; Qi, M.; Chen, H.; Zhou, C.; Ruan, R.; Yan, X.; Cheng, P. Macroalgae-Derived Multifunctional Bioactive Substances: The Potential Applications for Food and Pharmaceuticals. Foods 2022, 11, 3455. [Google Scholar] [CrossRef]
- Federação Internacional de Diabetes (IDF). Available online: https://diabetesatlas.org/ (accessed on 17 July 2023).
- Abo-Shady, A.M.; Gheda, S.F.; Ismail, G.A.; Cotas, J.; Pereira, L.; Abdel-Karim, O.H. Antioxidant and Antidiabetic Activity of Algae. Life 2023, 13, 460. [Google Scholar] [CrossRef]
- Pereira, L.; Valado, A. Unlocking Nature’s Treasures: Algae-Derived Natural Products in Diabetes and Its Complications-Current Advances and Future Prospects. Life 2023, 13, 1831. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Yang, C.; Liu, B.; Lin, L.; Sarker, S.D.; Nahar, L.; Yu, H.; Cao, H.; Xiao, J. Bioactive Compounds from Marine Macroalgae and Their Hypoglycemic Benefits. Trends Food Sci. Technol. 2018, 72, 1–12. [Google Scholar] [CrossRef]
- Mudgil, D.; Barak, S. Composition, Properties and Health Benefits of Indigestible Carbohydrate Polymers as Dietary Fiber: A Review. Int. J. Biol. Macromol. 2013, 61, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Volpe, C.M.O.; Villar-Delfino, P.H.; Dos Anjos, P.M.F.; Nogueira-Machado, J.A. Cellular Death, Reactive Oxygen Species (ROS) and Diabetic Complications. Cell Death Dis. 2018, 9, 119. [Google Scholar] [CrossRef]
- Chen, N.; Karantza-Wadsworth, V. Role and Regulation of Autophagy in Cancer. Biochim. Biophys. Acta 2009, 1793, 1516. [Google Scholar] [CrossRef]
- Zhang, Y.; Peng, T.; Zhu, H.; Zheng, X.; Zhang, X.; Jiang, N.; Cheng, X.; Lai, X.; Shunnar, A.; Singh, M.; et al. Prevention of Hyperglycemia-Induced Myocardial Apoptosis by Gene Silencing of Toll-like Receptor-4. J. Transl. Med. 2010, 8, 133. [Google Scholar] [CrossRef]
- Goiris, K.; De Vreese, P.; De Cooman, L.; Muylaert, K. Rapid Screening and Guided Extraction of Antioxidants from Microalgae Using Voltammetric Methods. J. Agric. Food Chem. 2012, 60, 7359–7366. [Google Scholar] [CrossRef]
- Perego, C.; Da Dalt, L.; Pirillo, A.; Galli, A.; Catapano, A.L.; Norata, G.D. Cholesterol Metabolism, Pancreatic β-Cell Function and Diabetes. Biochim. Biophys. Acta-Mol. Basis Dis. 2019, 1865, 2149–2156. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Liu, Y.; Li, L.; Zheng, Y.; Wang, Y.; Su, J.; Yang, R.; Luo, M.; Yu, C. Correlation between the Triglyceride-to-High-Density Lipoprotein Cholesterol Ratio and Other Unconventional Lipid Parameters with the Risk of Prediabetes and Type 2 Diabetes in Patients with Coronary Heart Disease: A RCSCD-TCM Study in China. Cardiovasc. Diabetol. 2022, 21, 93. [Google Scholar] [CrossRef]
- Schofield, J.D.; Liu, Y.; Rao-Balakrishna, P.; Malik, R.A.; Soran, H. Diabetes Dyslipidemia. Diabetes Ther. 2016, 7, 203. [Google Scholar] [CrossRef]
- Bizzaro, G.; Vatland, A.K.; Pampanin, D.M. The One-Health Approach in Seaweed Food Production. Env. Int. 2022, 158, 106948. [Google Scholar] [CrossRef]
- Lähteenmäki-Uutela, A.; Rahikainen, M.; Camarena-Gómez, M.T.; Piiparinen, J.; Spilling, K.; Yang, B. European Union Legislation on Macroalgae Products. Aquac. Int. 2021, 29, 487–509. [Google Scholar] [CrossRef]
- Ashokkumar, V.; Jayashree, S.; Kumar, G.; Aruna Sharmili, S.; Gopal, M.; Dharmaraj, S.; Chen, W.-H.; Kothari, R.; Manasa, I.; Hoon Park, J.; et al. Recent Technologies in Biorefining of Macroalgae Metabolites and Their Industrial Applications—A Circular Economy Approach. Bioresour. Technol. 2022, 359, 127235. [Google Scholar] [CrossRef]
- Leandro, A.; Pereira, L.; Gonçalves, A.M.M. Diverse Applications of Marine Macroalgae. Mar. Drugs 2019, 18, 17. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, D. Extração de Agar de Algas Vermelhas do Género Gracilaria. Ph.D. Thesis, University of Coimbra, Coimbra, Portugal, 2015. [Google Scholar]
- Gomes, L.; Monteiro, P.; Cotas, J.; Gonçalves, A.M.M.; Fernandes, C.; Gonçalves, T.; Pereira, L. Seaweeds’ Pigments and Phenolic Compounds with Antimicrobial Potential. Biomol. Concepts 2022, 13, 89–102. [Google Scholar] [CrossRef]
- Michalak, I.; Chojnacka, K. Algal Extracts: Technology and Advances. Eng. Life Sci. 2014, 14, 581–591. [Google Scholar] [CrossRef]
- Dureja, H.; Kaushik, D.; Kumar, V. Developments in nutraceuticals. Indian J. Pharmacol. 2003, 35, 363–372. [Google Scholar]
- Meštrović, T. Bell’s Palsy Complications. News-Medical, 26 November 2022. [Google Scholar]
- Pacheco, D.; Cotas, J.; Leandro, A.; Poza, S.G.; Gonçalves, A.M.M.; Pereira, L. Brown Seaweed Polysaccharides—A Roadmap as Biomolecules. In Seaweed Biotechnology; Apple Academic Press: Ontario, CA, USA, 2022. [Google Scholar]
- Agregán, R.; Lorenzo, J.M.; Munekata, P.E.S.; Dominguez, R.; Carballo, J.; Franco, D. Assessment of the Antioxidant Activity of Bifurcaria Bifurcata Aqueous Extract on Canola Oil. Effect of Extract Concentration on the Oxidation Stability and Volatile Compound Generation during Oil Storage. Food Res. Int. 2017, 99, 1095–1102. [Google Scholar] [CrossRef]
- Wijesinghe, W.A.J.P.; Jeon, Y.-J. Enzyme-Assistant Extraction (EAE) of Bioactive Components: A Useful Approach for Recovery of Industrially Important Metabolites from Seaweeds: A Review. Fitoterapia 2012, 83, 6–12. [Google Scholar] [CrossRef]
- Rajapakse, N.; Kim, S.K. Nutritional and Digestive Health Benefits of Seaweed. Adv. Food Nutr. Res. 2011, 64, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.; Valado, A. The Seaweed Diet in Prevention and Treatment of the Neurodegenerative Diseases. Mar. Drugs 2021, 19, 128. [Google Scholar] [CrossRef] [PubMed]
- Parada, J.; Pérez-Correa, J.R.; Pérez-Jiménez, J. Design of Low Glycemic Response Foods Using Polyphenols from Seaweed. J. Funct. Foods 2019, 56, 33–39. [Google Scholar] [CrossRef]
- Ganesan, A.R.; Tiwari, U.; Rajauria, G. Seaweed Nutraceuticals and Their Therapeutic Role in Disease Prevention. Food Sci. Hum. Wellness 2019, 8, 252–263. [Google Scholar] [CrossRef]
- Bourgougnon, N.; Burlot, A.S.; Jacquin, A.G. Algae for Global Sustainability? Adv. Bot. Res. 2021, 100, 145–212. [Google Scholar] [CrossRef]
- Różyło, R.; Hameed Hassoon, W.; Gawlik-Dziki, U.; Siastała, M.; Dziki, D. Study on the Physical and Antioxidant Properties of Gluten-Free Bread with Brown Algae. CyTA-J. Food 2017, 15, 196–203. [Google Scholar] [CrossRef]
- Neo, Y.T.; Chia, W.Y.; Lim, S.S.; Ngan, C.L.; Kurniawan, T.A.; Chew, K.W. Smart systems in producing algae-based protein to improve functional food ingredients industries. Food Res. Int. 2023, 165, 112480. [Google Scholar] [CrossRef]
- Antunes, M. Estudo Bioquímico e Fisiológico Aplicado à Macroalga Saccorhiza Polyschides. Ph.D. Thesis, Instituto Politecnico de Leiria, Leiria, Portugal, 2021. [Google Scholar]
- Healy, L.E.; Zhu, X.; Pojić, M.; Sullivan, C.; Tiwari, U.; Curtin, J.; Tiwari, B.K. Biomolecules from Macroalgae—Nutritional Profile and Bioactives for Novel Food Product Development. Biomolecules 2023, 13, 386. [Google Scholar] [CrossRef]
- Tagliapietra, B.L.; Clerici, M.T.P.S. Brown Algae and Their Multiple Applications as Functional Ingredient in Food Production. Food Res. Int. 2023, 167, 112655. [Google Scholar] [CrossRef]
- Uchida, M.; Miyoshi, T. Algal Fermentation—The Seed for a New Fermentation Industry of Foods and Related Products. Jpn. Agric. Res. Q. JARQ 2013, 47, 53–63. [Google Scholar] [CrossRef]
- Jönsson, M.; Allahgholi, L.; Sardari, R.R.R.; Hreggviosson, G.O.; Karlsson, E.N. Extraction and Modification of Macroalgal Polysaccharides for Current and Next-Generation Applications. Molecules 2020, 25, 930. [Google Scholar] [CrossRef]
- De Aguiar, A.L.L.; Araújo, M.L.H.; Benevides, N.M.B.; Mattos, A.L.A.; da Silva Araújo, I.M.; da Silva, E.M.C. Sequential Extraction Process and Physicochemical Characterization of R-Phycoerythrin and Agar from Red Macroalgae Gracilaria Birdiae. Algal Res. 2023, 69, 102920. [Google Scholar] [CrossRef]
- Mamede, M.; Cotas, J.; Bahcevandziev, K.; Pereira, L. Seaweed Polysaccharides in Agriculture: A Next Step towards Sustainability. Appl. Sci. 2023, 13, 6594. [Google Scholar] [CrossRef]
- Menon, V.V. Seaweed Polysaccharides–Food Applications. In Handbook of Marine Macroalgae; Wiley: Hoboken, NJ, USA, 2011; pp. 541–555. [Google Scholar]
- Mohapatra, L.; Bhattamisra, S.K.; Panigrahy, R.C.; Parida, S.K. Evaluation of the Antioxidant, Hypoglycaemic and Anti-Diabetic Activities of Some Seaweed Collected From the East Coast of India. Biomed. Pharmacol. J. 2016, 9, 365–375. [Google Scholar] [CrossRef]
- Reka, P.; Banu, T.; Seethalakshmi, M. Alpha amylase and alpha glucosidase inhibition activity of selected edible seaweeds from south coast area of india. Int. J. Pharm. Pharm. Sci. 2017, 9, 64–68. [Google Scholar] [CrossRef]
- Unnikrishnan, P.S.; Suthindhiran, K.; Jayasri, M.A. Alpha-Amylase Inhibition and Antioxidant Activity of Marine Green Algae and Its Possible Role in Diabetes Management. Pharmacogn. Mag. 2015, 11, S511. [Google Scholar] [CrossRef]
- Osman, N.A.H.K.; Siam, A.A.; El-Manawy, I.M.; Jeon, Y.-J. Anti-Microbial and Anti-Diabetic Activity of Six Seaweeds Collected from the Red Sea, Egypt. Catrina Int. J. Environ. Sci. 2019, 19, 55–60. [Google Scholar] [CrossRef]
- Kim, M.S.; Kim, J.Y.; Choi, W.H.; Lee, S.S. Effects of Seaweed Supplementation on Blood Glucose Concentration, Lipid Profile, and Antioxidant Enzyme Activities in Patients with Type 2 Diabetes Mellitus. Nutr. Res. Pr. 2008, 2, 62–67. [Google Scholar] [CrossRef]
- Elangovan, M.; Noorjahan, A.; Anantharaman, P. Extraction Of Metabolites And Screening Their Antioxidant Potential From Marine Macro Algae. Int. J. Sci. Technol. Res. 2019, 8, 1059–1064. [Google Scholar]
- Al-Azzawie, H.F.; Alhamdani, M.S.S. Hypoglycemic and Antioxidant Effect of Oleuropein in Alloxan-Diabetic Rabbits. Life Sci. 2006, 78, 1371–1377. [Google Scholar] [CrossRef] [PubMed]
- Cotas, J.; Leandro, A.; Pacheco, D.; Gonçalves, A.M.M.; Pereira, L. A Comprehensive Review of the Nutraceutical and Therapeutic Applications of Red Seaweeds (Rhodophyta). Life 2020, 10, 19. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, R.Y.M.; Saber, A.A.; Hammad, H.B.I. The Possible Role of the Seaweed Ulva Fasciata on Ameliorating Hyperthyroidism-Associated Heart Inflammations in a Rat Model. Env. Sci. Pollut. Res. Int. 2021, 28, 6830–6842. [Google Scholar] [CrossRef]
- Paiva, L.; Lima, E.; Neto, A.I.; Baptista, J. Angiotensin I-Converting Enzyme (ACE) Inhibitory Activity, Antioxidant Properties, Phenolic Content and Amino Acid Profiles of Fucus Spiralis L. Protein Hydrolysate Fractions. Mar. Drugs 2017, 15, 311. [Google Scholar] [CrossRef] [PubMed]
- Cotas, J.; Leandro, A.; Monteiro, P.; Pacheco, D.; Figueirinha, A.; Goncąlves, A.M.M.; Da Silva, G.J.; Pereira, L. Seaweed Phenolics: From Extraction to Applications. Marine Drugs 2020, 18, 384. [Google Scholar] [CrossRef]
- Yan, X.; Yang, C.; Lin, G.; Chen, Y.; Miao, S.; Liu, B.; Zhao, C. Antidiabetic Potential of Green Seaweed Enteromorpha Prolifera Flavonoids Regulating Insulin Signaling Pathway and Gut Microbiota in Type 2 Diabetic Mice. J. Food Sci. 2019, 84, 165–173. [Google Scholar] [CrossRef]
- Paudel, P.; Seong, S.H.; Park, H.J.; Jung, H.A.; Choi, J.S. Anti-Diabetic Activity of 2,3,6-Tribromo-4,5-Dihydroxybenzyl Derivatives from Symphyocladia Latiuscula through PTP1B Downregulation and α-Glucosidase Inhibition. Mar. Drugs 2019, 17, 166. [Google Scholar] [CrossRef]
- Shi, D.; Xu, F.; He, J.; Li, J.; Fan, X.; Han, L. Inhibition of Bromophenols against PTP1B and Anti-Hyperglycemic Effect of Rhodomela Confervoides Extract in Diabetic Rats. Chin. Sci. Bull. 2008, 53, 2476–2479. [Google Scholar] [CrossRef]
- Choudhary, B.; Chauhan, O.P.; Mishra, A. Edible Seaweeds: A Potential Novel Source of Bioactive Metabolites and Nutraceuticals With Human Health Benefits. Front. Mar. Sci. 2021, 8, 740054. [Google Scholar] [CrossRef]
- Eggersdorfer, M.; Wyss, A. Carotenoids in Human Nutrition and Health. Arch. Biochem. Biophys. 2018, 652, 18–26. [Google Scholar] [CrossRef]
- Warnke, I.; Goralczyk, R.; Fuhrer, E.; Schwager, J. Dietary Constituents Reduce Lipid Accumulation in Murine C3H10 T1/2 Adipocytes: A Novel Fluorescent Method to Quantify Fat Droplets. Nutr. Metab. 2011, 8, 30. [Google Scholar] [CrossRef]
- Luisa Bonet, M.; Canas, J.A.; Ribot, J.; Palou, A. Carotenoids and Their Conversion Products in the Control of Adipocyte Function, Adiposity and Obesity. Arch. Biochem. Biophys. 2015, 572, 112–125. [Google Scholar] [CrossRef]
- Mofasser Hossain, A.K.M.; Brennan, M.A.; Mason, S.L.; Guo, X.; Zeng, X.A.; Brennan, C.S. The Effect of Astaxanthin-Rich Microalgae “Haematococcus Pluvialis” and Wholemeal Flours Incorporation in Improving the Physical and Functional Properties of Cookies. Foods 2017, 6, 57. [Google Scholar] [CrossRef]
- Canas, J.A.; Lochrie, A.; McGowan, A.G.; Hossain, J.; Schettino, C.; Balagopal, P.B. Effects of Mixed Carotenoids on Adipokines and Abdominal Adiposity in Children: A Pilot Study. J. Clin. Endocrinol. Metab. 2017, 102, 1983–1990. [Google Scholar] [CrossRef]
- Wells, M.L.; Potin, P.; Craigie, J.S.; Raven, J.A.; Merchant, S.S.; Helliwell, K.E.; Smith, A.G.; Camire, M.E.; Brawley, S.H. Algae as Nutritional and Functional Food Sources: Revisiting Our Understanding. J. Appl. Phycol. 2016, 29, 949–982. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Guo, F.; Liu, S.; Fang, H.; Xu, Z.; Wang, T. Recent Advances and Future Prospects of Mycosporine-like Amino Acids. Molecules 2023, 28, 5588. [Google Scholar] [CrossRef]
- Tarasuntisuk, S.; Palaga, T.; Kageyama, H.; Waditee-Sirisattha, R. Mycosporine-2-Glycine Exerts Anti-Inflammatory and Antioxidant Effects in Lipopolysaccharide (LPS)-Stimulated RAW 264.7 Macrophages. Arch. Biochem. Biophys. 2019, 662, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Wada, N.; Sakamoto, T.; Matsugo, S. Mycosporine-Like Amino Acids and Their Derivatives as Natural Antioxidants. Antioxidants 2015, 4, 603–646. [Google Scholar] [CrossRef] [PubMed]
- De La Coba, F.; Aguilera, J.; Figueroa, F.L.; De Gálvez, M.V.; Herrera, E. Antioxidant Activity of Mycosporine-like Amino Acids Isolated from Three Red Macroalgae and One Marine Lichen. J. Appl. Phycol. 2009, 21, 161–169. [Google Scholar] [CrossRef]
- de la Coba, F.; Aguilera, J.; Korbee, N.; de Gálvez, M.V.; Herrera-Ceballos, E.; Álvarez-Gómez, F.; Figueroa, F.L. UVA and UVB Photoprotective Capabilities of Topical Formulations Containing Mycosporine-like Amino Acids (MAAs) through Different Biological Effective Protection Factors (BEPFs). Mar. Drugs 2019, 17, 55. [Google Scholar] [CrossRef] [PubMed]
- Suh, S.S.; Hwang, J.; Park, M.; Seo, H.H.; Kim, H.S.; Lee, J.H.; Moh, S.H.; Lee, T.K. Anti-Inflammation Activities of Mycosporine-Like Amino Acids (MAAs) in Response to UV Radiation Suggest Potential Anti-Skin Aging Activity. Mar. Drugs 2014, 12, 5174–5187. [Google Scholar] [CrossRef] [PubMed]
- Eilam, Y.; Pintel, N.; Khattib, H.; Shagug, N.; Taha, R.; Avni, D. Regulation of Cholesterol Metabolism by Phytochemicals Derived from Algae and Edible Mushrooms in Non-Alcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2022, 23, 13667. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.T.V.; Tsou, Y.C.; Chen, Y.T.; Lu, W.J.; Hwang, P.A. Effects of Low-Molecular-Weight Fucoidan and High Stability Fucoxanthin on Glucose Homeostasis, Lipid Metabolism, and Liver Function in a Mouse Model of Type II Diabetes. Mar. Drugs 2017, 15, 113. [Google Scholar] [CrossRef]
- Miyashita, K.; Beppu, F.; Hosokawa, M.; Liu, X.; Wang, S. Nutraceutical Characteristics of the Brown Seaweed Carotenoid Fucoxanthin. Arch. Biochem. Biophys. 2020, 686, 108364. [Google Scholar] [CrossRef] [PubMed]
- Neto, R.T.; Marçal, C.; Queirós, A.S.; Abreu, H.; Silva, A.M.S.; Cardoso, S.M. Screening of Ulva Rigida, Gracilaria sp., Fucus Vesiculosus and Saccharina Latissima as Functional Ingredients. Int. J. Mol. Sci. 2018, 19, 2987. [Google Scholar] [CrossRef]
- Ha, A.W.; Kim, W.K. The Effect of Fucoxanthin Rich Power on the Lipid Metabolism in Rats with a High Fat Diet. Nutr. Res. Pr. 2013, 7, 287–293. [Google Scholar] [CrossRef]
- Ren, R.; Gong, J.; Zhao, Y.; Zhuang, X.; Ye, Y.; Lin, W. Sulfated Polysaccharides from Enteromorpha Prolifera Suppress SREBP-2 and HMG-CoA Reductase Expression and Attenuate Non-Alcoholic Fatty Liver Disease Induced by a High-Fat Diet. Food Funct. 2017, 8, 1899–1904. [Google Scholar] [CrossRef]
- Shin, H.C.; Kim, S.H.; Park, Y.; Lee, B.H.; Hwang, H.J. Effects of 12-Week Oral Supplementation of Ecklonia Cava Polyphenols on Anthropometric and Blood Lipid Parameters in Overweight Korean Individuals: A Double-Blind Randomized Clinical Trial. Phytother. Res. 2012, 26, 363–368. [Google Scholar] [CrossRef]
- Zha, X.Q.; Xiao, J.J.; Zhang, H.N.; Wang, J.H.; Pan, L.H.; Yang, X.F.; Luo, J.P. Polysaccharides in Laminaria Japonica (LP): Extraction, Physicochemical Properties and Their Hypolipidemic Activities in Diet-Induced Mouse Model of Atherosclerosis. Food Chem. 2012, 134, 244–252. [Google Scholar] [CrossRef]
- André, R.; Guedes, L.; Melo, R.; Ascensão, L.; Pacheco, R.; Vaz, P.D.; Serralheiro, M.L. Effect of Food Preparations on In Vitro Bioactivities and Chemical Components of Fucus Vesiculosus. Foods 2020, 9, 955. [Google Scholar] [CrossRef]
- Derosa, G.; Pascuzzo, M.D.; D’angelo, A.; Maffioli, P. Ascophyllum Nodosum, Fucus Vesiculosus and Chromium Picolinate Nutraceutical Composition Can Help to Treat Type 2 Diabetic Patients. Diabetes Metab. Syndr. Obes. 2019, 12, 1861–1865. [Google Scholar] [CrossRef]
- Peñalver, R.; Lorenzo, J.M.; Ros, G.; Amarowicz, R.; Pateiro, M.; Nieto, G. Seaweeds as a Functional Ingredient for a Healthy Diet. Mar. Drugs 2020, 18, 301. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, C.; Almeida, J.; Coelho, I.; Delgado, I.; Gomes, R.; Quintã, R.; Bandarra, N.M.; Afonso, C. Farming a Wild Seaweed and Changes to Its Composition, Bioactivity, and Bioaccessibility: The Saccorhiza Polyschides Case Study. Aquaculture 2023, 566, 739217. [Google Scholar] [CrossRef]
- Soares, C.; Švarc-Gajić, J.; Oliva-Teles, M.T.; Pinto, E.; Nastić, N.; Savić, S.; Almeida, A.; Delerue-Matos, C. Mineral Composition of Subcritical Water Extracts of Saccorhiza Polyschides, a Brown Seaweed Used as Fertilizer in the North of Portugal. J. Mar. Sci. Eng. 2020, 8, 244. [Google Scholar] [CrossRef]
- Bocanegra, A.; Macho-González, A.; Garcimartín, A.; Benedí, J.; Sánchez-Muniz, F.J. Whole Alga, Algal Extracts, and Compounds as Ingredients of Functional Foods: Composition and Action Mechanism Relationships in the Prevention and Treatment of Type-2 Diabetes Mellitus. Int. J. Mol. Sci. 2021, 22, 3816. [Google Scholar] [CrossRef] [PubMed]
- Allsopp, P.; Crowe, W.; Bahar, B.; Harnedy, P.A.; Brown, E.S.; Taylor, S.S.; Smyth, T.J.; Soler-Vila, A.; Magee, P.J.; Gill, C.I.R.; et al. The Effect of Consuming Palmaria Palmata-Enriched Bread on Inflammatory Markers, Antioxidant Status, Lipid Profile and Thyroid Function in a Randomised Placebo-Controlled Intervention Trial in Healthy Adults. Eur. J. Nutr. 2016, 55, 1951–1962. [Google Scholar] [CrossRef]
- Mouritsen, O.G.; Dawczynski, C.; Duelund, L.; Jahreis, G.; Vetter, W.; Schröder, M. On the Human Consumption of the Red Seaweed Dulse (Palmaria Palmata (L.) Weber & Mohr). J. Appl. Phycol. 2013, 25, 1777–1791. [Google Scholar] [CrossRef]
- Fitzgerald, C.; Gallagher, E.; Doran, L.; Auty, M.; Prieto, J.; Hayes, M. Increasing the Health Benefits of Bread: Assessment of the Physical and Sensory Qualities of Bread Formulated Using a Renin Inhibitory Palmaria Palmata Protein Hydrolysate. LWT-Food Sci. Technol. 2014, 56, 398–405. [Google Scholar] [CrossRef]
- Gunathilaka, T.L.; Samarakoon, K.; Ranasinghe, P.; Peiris, L.D.C. Antidiabetic Potential of Marine Brown Algae—A Mini Review. J. Diabetes Res. 2020, 2020, 1230218. [Google Scholar] [CrossRef]
- Senthilkumar, P.; Sellappa, S.; Prakash, S. Antidiabetic Activity of Aqueous Extract of Padina Boergesenii in Streptozotocin-Induced Diabetic Rats. Int. J. Pharm. Pharm. Sci. 2014, 6, 418–422. [Google Scholar] [CrossRef]
- Akbarzadeh, S.; Gholampour, H.; Farzadinia, P.; Daneshi, A.; Ramavandi, B.; Moazzeni, A.; Keshavarz, M.; Bargahi, A. Anti-Diabetic Effects of Sargassum Oligocystum on Streptozotocin-Induced Diabetic Rat. Iran. J. Basic. Med. Sci. 2018, 21, 342. [Google Scholar] [CrossRef]
- Gotama, T.L.; Husni, A. Ustadi Antidiabetic Activity of Sargassum Hystrix Extracts in Streptozotocin-Induced Diabetic Rats. Prev. Nutr. Food Sci. 2018, 23, 189. [Google Scholar] [CrossRef]
- Ścieszka, S.; Klewicka, E. Algae in Food: A General Review. Crit. Rev. Food Sci. Nutr. 2018, 59, 3538–3547. [Google Scholar] [CrossRef] [PubMed]
- Fradinho, P.; Raymundo, A.; Sousa, I.; Domínguez, H.; Torres, M.D. Edible Brown Seaweed in Gluten-Free Pasta: Technological and Nutritional Evaluation. Foods 2019, 8, 622. [Google Scholar] [CrossRef]
- Medvedeva, E.I.; Kalyuzhnaya, A.M.; Panchenko, A.K.; Krasil’nikova, S.V.; Petrenko, E.B. Amino Acids from Algae-Valuable Bread Additives. Khlebopekarnaya I Konditer. Promyshlennost 1969, 13, 16–17. [Google Scholar]
- Mamat, H.; Matanjun, P.; Ibrahim, S.; Siti, S.F.; Abdul Hamid, M.; Rameli, A.S. The Effect of Seaweed Composite Flour on the Textural Properties of Dough and Bread. J. Appl. Phycol. 2014, 26, 1057–1062. [Google Scholar] [CrossRef]
- Menezes, B.S.; Coelho, M.; Rivero Meza, S.; Salas-Mellado, M.; Souza, M.R.A.Z. Macroalgal Biomass as an Additional Ingredient of Bread. Int. Food Res. J. 2015, 22, 819–824. [Google Scholar]
- Passos, R.; Correia, A.P.; Pires, D.; Pires, P.; Ferreira, I.; Simões, M.; do Carmo, B.; Santos, P.; Pombo, A.; Afonso, C.; et al. Potential Use of Macroalgae Gracilaria Gracilis in Diets for European Seabass (Dicentrarchus Labrax): Health Benefits from a Sustainable Source. Fish. Shellfish Immunol. 2021, 119, 105–113. [Google Scholar] [CrossRef]
- Mendes, M.C.; Navalho, S.; Ferreira, A.; Paulino, C.; Figueiredo, D.; Silva, D.; Gao, F.; Gama, F.; Bombo, G.; Jacinto, R.; et al. Algae as Food in Europe: An Overview of Species Diversity and Their Application. Foods 2022, 11, 1871. [Google Scholar] [CrossRef]
- Pardilhó, S.; Cotas, J.; Pacheco, D.; Gonçalves, A.M.M.; Bahcevandziev, K.; Pereira, L.; Figueirinha, A.; Dias, J.M. Valorisation of Marine Macroalgae Waste Using a Cascade Biorefinery Approach: Exploratory Study. J. Clean. Prod. 2023, 385, 135672. [Google Scholar] [CrossRef]
- Valand, R.; Tanna, S.; Lawson, G.; Bengtström, L. A Review of Fourier Transform Infrared (FTIR) Spectroscopy Used in Food Adulteration and Authenticity Investigations. Food Addit. Contam. Part A 2020, 37, 19–38. [Google Scholar] [CrossRef] [PubMed]
- Rohman, A.; Ghazali, M.A.B.; Windarsih, A.; Irnawati; Riyanto, S.; Yusof, F.M.; Mustafa, S. Comprehensive Review on Application of FTIR Spectroscopy Coupled with Chemometrics for Authentication Analysis of Fats and Oils in the Food Products. Molecules 2020, 25, 5485. [Google Scholar] [CrossRef] [PubMed]
- García-Poza, S.; Leandro, A.; Cotas, C.; Cotas, J.; Marques, J.C.; Pereira, L.; Gonçalves, A.M.M. The Evolution Road of Seaweed Aquaculture: Cultivation Technologies and the Industry 4.0. Int. J. Env. Res. Public Health 2020, 17, 6528. [Google Scholar] [CrossRef]
- Castro, B.B.; Cotas, J.; Gomes, L.; Pacheco, D.; Pereira, L. Ecosystem Services Provided by Seaweeds. Hydrobiology 2023, 2, 75–96. [Google Scholar] [CrossRef]
- Ashkenazi, D.Y.; Figueroa, F.L.; Korbee, N.; García-Sánchez, M.; Vega, J.; Ben-Valid, S.; Paz, G.; Salomon, E.; Israel, Á.; Abelson, A. Enhancing Bioproducts in Seaweeds via Sustainable Aquaculture: Antioxidant and Sun-Protection Compounds. Mar. Drugs 2022, 20, 767. [Google Scholar] [CrossRef] [PubMed]
- Roohinejad, S.; Koubaa, M.; Barba, F.J.; Saljoughian, S.; Amid, M.; Greiner, R. Application of seaweeds to develop new food products with enhanced shelf-life, quality and health-related beneficial properties. Food Res. Int. 2017, 99, 1066–1083. [Google Scholar] [CrossRef]
- European Commission. Communication from the Commission to the European, the Council, the European Economic and Social Committee and the Committee of the Regions; European Commission: Brussels, Belgium, 2022.











Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tavares, J.O.; Cotas, J.; Valado, A.; Pereira, L. Algae Food Products as a Healthcare Solution. Mar. Drugs 2023, 21, 578. https://doi.org/10.3390/md21110578
Tavares JO, Cotas J, Valado A, Pereira L. Algae Food Products as a Healthcare Solution. Marine Drugs. 2023; 21(11):578. https://doi.org/10.3390/md21110578
Chicago/Turabian StyleTavares, Joana O., João Cotas, Ana Valado, and Leonel Pereira. 2023. "Algae Food Products as a Healthcare Solution" Marine Drugs 21, no. 11: 578. https://doi.org/10.3390/md21110578
APA StyleTavares, J. O., Cotas, J., Valado, A., & Pereira, L. (2023). Algae Food Products as a Healthcare Solution. Marine Drugs, 21(11), 578. https://doi.org/10.3390/md21110578