Water-Soluble Saccharina latissima Polysaccharides and Relation of Their Structural Characteristics with In Vitro Immunostimulatory and Hypocholesterolemic Activities
Abstract
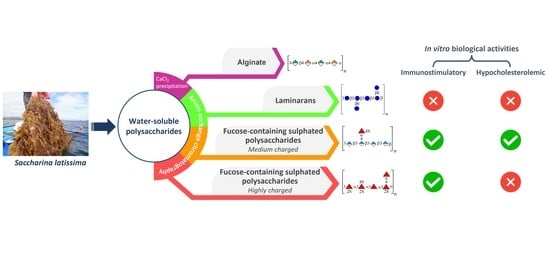
:1. Introduction
2. Results and Discussion
2.1. Fractionation and Characterization of S. latissima Polysaccharides
2.1.1. Precipitation with Calcium Chloride
2.1.2. Anion-Exchange Chromatography
2.2. In Vitro Lymphocyte Stimulatory Activity
2.3. In Vitro Hypocholesterolemic Effect
3. Materials and Methods
3.1. Extraction and Fractionation of S. latissima Polysaccharides
3.1.1. Alcohol-Insoluble Residue (AIR) Preparation
3.1.2. Hot Water Extraction
3.1.3. Precipitation with Calcium Chloride
3.1.4. Anion-Exchange Chromatography of Fraction Sn_CaCl2
3.2. Neutral Sugars Analysis and Uronic Acid Determination
3.3. Sulphate and Protein Content
3.4. In Vitro Lymphocyte Stimulatory Activity
3.5. In Vitro Assessment of Hypocholesterolemic Effect
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Choudhary, B.; Chauhan, O.P.; Mishra, A. Edible seaweeds: A potential novel source of bioactive metabolites and nutraceuticals with human health benefits. Front. Mar. Sci. 2021, 8, 740054. [Google Scholar] [CrossRef]
- Lomartire, S.; Gonçalves, A.M.M. An overview of potential seaweed-derived bioactive compounds for pharmaceutical applications. Mar. Drugs 2022, 20, 141. [Google Scholar] [CrossRef]
- Garcia-Vaquero, M.; Rajauria, G.; O’Doherty, J.V.; Sweeney, T. Polysaccharides from macroalgae: Recent advances, innovative technologies and challenges in extraction and purification. Food Res. Int. 2017, 99, 1011–1020. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zheng, Y.; Zhang, Y.; Yang, Y.; Wang, P.; Imre, B.; Wong, A.C.Y.; Hsieh, Y.S.Y.; Wang, D. Brown algae carbohydrates: Structures, pharmaceutical properties, and research challenges. Mar. Drugs 2021, 19, 620. [Google Scholar] [CrossRef]
- Liao, Y.C.; Chang, C.C.; Nagarajan, D.; Chen, C.Y.; Chang, J.S. Algae-derived hydrocolloids in foods: Applications and health-related issues. Bioengineered 2021, 12, 3787–3801. [Google Scholar] [CrossRef]
- Kang, J.; Jia, X.; Wang, N.; Xiao, M.; Song, S.; Wu, S.; Li, Z.; Wang, S.; Cui, S.W.; Guo, Q. Insights into the structure-bioactivity relationships of marine sulfated polysaccharides: A review. Food Hydrocoll. 2022, 123, 107049. [Google Scholar] [CrossRef]
- Bi, D.; Yang, X.; Yao, L.; Hu, Z.; Li, H.; Xu, X.; Lu, J. Potential food and nutraceutical applications of alginate: A review. Mar. Drugs 2022, 20, 564. [Google Scholar] [CrossRef]
- Karuppusamy, S.; Rajauria, G.; Fitzpatrick, S.; Lyons, H.; McMahon, H.; Curtin, J.; Tiwari, B.K.; O’Donnell, C. Biological properties and health-promoting functions of laminarin: A comprehensive review of preclinical and clinical studies. Mar. Drugs 2022, 20, 772. [Google Scholar] [CrossRef]
- McNaught, A.D. Nomenclature of carbohydrates. Carbohydr. Res. 1997, 297, 1–92. [Google Scholar] [CrossRef]
- Zhang, X.; Thomsen, M. Biomolecular composition and revenue explained by interactions between extrinsic factors and endogenous rhythms of Saccharina latissima. Mar. Drugs 2019, 17, 107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deniaud-Bouët, E.; Hardouin, K.; Potin, P.; Kloareg, B.; Hervé, C. A review about brown algal cell walls and fucose-containing sulfated polysaccharides: Cell wall context, biomedical properties and key research challenges. Carbohydr. Polym. 2017, 175, 395–408. [Google Scholar] [CrossRef]
- Nunes, C.; Coimbra, M.A. The potential of fucose-containing sulfated polysaccharides as scaffolds for biomedical applications. Curr. Med. Chem. 2019, 26, 6399–6411. [Google Scholar] [CrossRef]
- Bilan, M.I.; Grachev, A.A.; Shashkov, A.S.; Kelly, M.; Sanderson, C.J.; Nifantiev, N.E.; Usov, A.I. Further studies on the composition and structure of a fucoidan preparation from the brown alga Saccharina latissima. Carbohydr. Res. 2010, 345, 2038–2047. [Google Scholar] [CrossRef] [PubMed]
- Tran, V.H.N.; Nguyen, T.T.; Meier, S.; Holck, J.; Cao, H.T.T.; Van, T.T.T.; Meyer, A.S.; Mikkelsen, M.D. The endo-α(1,3)-fucoidanase Mef2 releases uniquely branched oligosaccharides from Saccharina latissima fucoidans. Mar. Drugs 2022, 20, 305. [Google Scholar] [CrossRef] [PubMed]
- Mazepa, E.; Biscaia, S.M.P.; Bellan, D.D.L.; Trindade, E.D.S.; Simas, F.F. Structural characteristics of native and chemically sulfated polysaccharides from seaweed and their antimelanoma effects. Carbohydr. Polym. 2022, 289, 119436. [Google Scholar] [CrossRef] [PubMed]
- Apostolova, E.; Lukova, P.; Baldzhieva, A.; Katsarov, P.; Nikolova, M.; Iliev, I.; Peychev, L.; Trica, B.; Oancea, F.; Delattre, C.; et al. Immunomodulatory and anti-inflammatory effects of fucoidan: A review. Polymers 2020, 12, 2338. [Google Scholar] [CrossRef]
- André, R.; Pacheco, R.; Bourbon, M.; Serralheiro, M.L. Brown algae potential as a functional food against hypercholesterolemia: Review. Foods 2021, 10, 234. [Google Scholar] [CrossRef] [PubMed]
- Morya, V.K.; Kim, J.; Kim, E.K. Algal fucoidan: Structural and size-dependent bioactivities and their perspectives. Appl. Microbiol. Biotechnol. 2012, 93, 71–82. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Mikkelsen, M.D.; Tran, V.H.N.; Trang, V.T.D.; Rhein-Knudsen, N.; Holck, J.; Rasin, A.B.; Cao, H.T.T.; Van, T.T.T.; Meyer, A.S. Enzyme-assisted fucoidan extraction from brown macroalgae Fucus distichus subsp. evanescens and Saccharina latissima. Mar. Drugs 2020, 18, 296. [Google Scholar] [CrossRef]
- Rioux, L.E.; Turgeon, S.L.; Beaulieu, M. Characterization of polysaccharides extracted from brown seaweeds. Carbohydr. Polym. 2007, 69, 530–537. [Google Scholar] [CrossRef]
- Bruhn, A.; Janicek, T.; Manns, D.; Nielsen, M.M.; Balsby, T.J.S.; Meyer, A.S.; Rasmussen, M.B.; Hou, X.; Saake, B.; Göke, C.; et al. Crude fucoidan content in two North Atlantic kelp species, Saccharina latissima and Laminaria digitata-seasonal variation and impact of environmental factors. J. Appl. Phycol. 2017, 29, 3121–3137. [Google Scholar] [CrossRef] [Green Version]
- Ehrig, K.; Alban, S. Sulfated galactofucan from the brown alga Saccharina latissima—Variability of yield, structural composition and bioactivity. Mar. Drugs 2015, 13, 76–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dobrinčić, A.; Balbino, S.; Zorić, Z.; Pedisić, S.; Kovačević, D.B.; Garofulić, I.E.; Dragović-Uzelac, V. Advanced technologies for the extraction of marine brown algal polysaccharides. Mar. Drugs 2020, 18, 168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ponce, N.M.A.; Stortz, C.A. A comprehensive and comparative analysis of the fucoidan compositional data across the Phaeophyceae. Front. Plant Sci. 2020, 11, 556312. [Google Scholar] [CrossRef]
- Mendes, M.C.; Navalho, S.; Ferreira, A.; Paulino, C.; Figueiredo, D.; Silva, D.; Gao, F.; Gama, F.; Bombo, G.; Jacinto, R.; et al. Algae as food in Europe: An overview of species diversity and their application. Foods 2022, 11, 1871. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Yang, R.; Li, M.; Zhou, Q.; Liang, X.; Elmada, Z.C. Effects of dietary fucoidan on the blood constituents, anti-oxidation and innate immunity of juvenile yellow catfish (Pelteobagrus fulvidraco). Fish Shellfish Immunol. 2014, 41, 264–270. [Google Scholar] [CrossRef]
- Ren, D.; Wang, Q.; Yang, Y.; Hu, Y.; Song, Y.; He, Y.; Liu, S.; Wu, L. Hypolipidemic effects of fucoidan fractions from Saccharina sculpera (Laminariales, Phaeophyceae). Int. J. Biol. Macromol. 2019, 140, 188–195. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, Y.; Wang, Q.; Luo, X.; He, Y.; Song, Y. Hypolipidemic effects of sulfated fucoidan from Kjellmaniella crassifolia through modulating the cholesterol and aliphatic metabolic pathways. J. Funct. Foods 2018, 51, 8–15. [Google Scholar] [CrossRef]
- An, E.-K.; Hwang, J.; Kim, S.-J.; Park, H.-B.; Zhang, W.; Ryu, J.-H.; You, S.; Jin, J.-O. Comparison of the immune activation capacities of fucoidan and laminarin extracted from Laminaria japonica. Int. J. Biol. Macromol. 2022, 208, 230–242. [Google Scholar] [CrossRef]
- Stefaniak-Vidarsson, M.M.; Gudjónsdóttir, M.; Marteinsdottir, G.; Omarsdottir, S.; Bravo, E.; Sigurjonsson, O.E.; Kristbergsson, K. Determination of bioactive properties of food grade extracts from Icelandic edible brown seaweed sugar kelp (Saccharina latissima) with in vitro human cell cultures (THP-1). Funct. Foods Health Dis. 2019, 9, 1–15. [Google Scholar] [CrossRef]
- Reed, R.H.; Davison, I.R.; Chudek, J.A.; Foster, R. The osmotic role of mannitol in the Phaeophyta: An appraisal. Phycologia 1985, 24, 35–47. [Google Scholar] [CrossRef]
- Monteiro, J.P.; Rey, F.; Melo, T.; Moreira, A.S.P.; Arbona, J.-F.; Skjermo, J.; Forbord, S.; Funderud, J.; Raposo, D.; Kerrison, P.D.; et al. The unique lipidomic signatures of Saccharina latissima can be used to pinpoint their geographic origin. Biomolecules 2020, 10, 107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martins, M.; Mesquita, L.M.d.S.; Vaz, B.M.C.; Dias, A.C.R.V.; Torres-Acosta, M.A.; Quéguineur, B.; Coutinho, J.A.P.; Ventura, S.P.M. Extraction and fractionation of pigments from Saccharina latissima (Linnaeus, 2006) using an ionic liquid + oil + water system. ACS Sustain. Chem. Eng. 2021, 9, 6599–6612. [Google Scholar] [CrossRef]
- Neupane, S.; Bittkau, K.S.; Alban, S. Size distribution and chain conformation of six different fucoidans using size-exclusion chromatography with multiple detection. J. Chromatogr. A 2020, 1612, 460658. [Google Scholar] [CrossRef]
- Bittkau, K.S.; Neupane, S.; Alban, S. Initial evaluation of six different brown algae species as source for crude bioactive fucoidans. Algal Res. 2020, 45, 101759. [Google Scholar] [CrossRef]
- Schepetkin, I.A.; Quinn, M.T. Botanical polysaccharides: Macrophage immunomodulation and therapeutic potential. Int. Immunopharmacol. 2006, 6, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Pandeirada, C.O.; Maricato, É.; Ferreira, S.S.; Correia, V.G.; Pinheiro, B.A.; Evtuguin, D.V.; Palma, A.S.; Correia, A.; Vilanova, M.; Coimbra, M.A.; et al. Structural analysis and potential immunostimulatory activity of Nannochloropsis oculata polysaccharides. Carbohydr. Polym. 2019, 222, 114962. [Google Scholar] [CrossRef]
- Borges, O.; Borchard, G.; de Sousa, A.; Junginger, H.E.; Cordeiro-da-Silva, A. Induction of lymphocytes activated marker CD69 following exposure to chitosan and alginate biopolymers. Int. J. Pharm. 2007, 337, 254–264. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, S.S.; Passos, C.P.; Madureira, P.; Vilanova, M.; Coimbra, M.A. Structure–function relationships of immunostimulatory polysaccharides: A review. Carbohydr. Polym. 2015, 132, 378–396. [Google Scholar] [CrossRef] [PubMed]
- Nunes, C.; Rocha, A.; Quitério, P.; Ferreira, S.S.; Correia, A.; Vilanova, M.; Coimbra, M.A. Salt pan brine water as a sustainable source of sulphated polysaccharides with immunostimulatory activity. Int. J. Biol. Macromol. 2019, 133, 235–242. [Google Scholar] [CrossRef]
- Ferreira, S.S.; Correia, A.; Silva, A.M.S.; Wessel, D.F.; Cardoso, S.M.; Vilanova, M.; Coimbra, M.A. Structure-function relationships of pectic polysaccharides from broccoli by-products with in vitro B lymphocyte stimulatory activity. Carbohydr. Polym. 2023, 303, 120432. [Google Scholar] [CrossRef] [PubMed]
- Yuguchi, Y.; Tran, V.T.T.; Bui, L.M.; Takebe, S.; Suzuki, S.; Nakajima, N.; Kitamura, S.; Thanh, T.T.T. Primary structure, conformation in aqueous solution, and intestinal immunomodulating activity of fucoidan from two brown seaweed species Sargassum crassifolium and Padina australis. Carbohydr. Polym. 2016, 147, 69–78. [Google Scholar] [CrossRef]
- Espinal-Ruiz, M.; Parada-Alfonso, F.; Restrepo-Sánchez, L.-P.; Narváez-Cuenca, C.-E.; McClements, D.J. Interaction of a dietary fiber (pectin) with gastrointestinal components (bile salts, calcium, and lipase): A calorimetry, electrophoresis, and turbidity study. J. Agric. Food Chem. 2014, 62, 12620–12630. [Google Scholar] [CrossRef]
- Silva, I.M.V.; Machado, F.; Moreno, M.J.; Nunes, C.; Coimbra, M.A.; Coreta-Gomes, F. Polysaccharide structures and their hypocholesterolemic potential. Molecules 2021, 26, 4559. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Chen, S.; Zhong, S. Nutritional and chemical composition of Sargassum zhangii and the physical and chemical characterization, binding bile acid, and cholesterol-lowering activity in HepG2 cells of its fucoidans. Foods 2022, 11, 1771. [Google Scholar] [CrossRef] [PubMed]
- Zárate, A.; Manuel-Apolinar, L.; Saucedo, R.; Hernández-Valencia, M.; Basurto, L. Hypercholesterolemia as a risk factor for cardiovascular disease: Current controversial therapeutic management. Arch. Med. Res. 2016, 47, 491–495. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Aveiro, S.S.; Melo, T.; Figueiredo, A.; Domingues, P.; Pereira, H.; Maia, I.B.; Silva, J.; Domingues, M.R.; Nunes, C.; Moreira, A.S.P. The polar lipidome of cultured Emiliania huxleyi: A source of bioactive lipids with relevance for biotechnological applications. Biomolecules 2020, 10, 1434. [Google Scholar] [CrossRef]
- Biancarosa, I.; Espe, M.; Bruckner, C.G.; Heesch, S.; Liland, N.; Waagbø, R.; Torstensen, B.; Lock, E.J. Amino acid composition, protein content, and nitrogen-to-protein conversion factors of 21 seaweed species from Norwegian waters. J. Appl. Phycol. 2017, 29, 1001–1009. [Google Scholar] [CrossRef]
- Ferreira, A.S.; Ferreira, S.S.; Correia, A.; Vilanova, M.; Silva, T.H.; Coimbra, M.A.; Nunes, C. Reserve, structural and extracellular polysaccharides of Chlorella vulgaris: A holistic approach. Algal Res. 2020, 45, 101757. [Google Scholar] [CrossRef]
- Coreta-Gomes, F.M.; Lopes, G.R.; Passos, C.P.; Vaz, I.M.; Machado, F.; Geraldes, C.F.G.C.; Moreno, M.J.; Nyström, L.; Coimbra, M.A. In vitro hypocholesterolemic effect of coffee compounds. Nutrients 2020, 12, 437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coreta-Gomes, F.M.; Vaz, W.L.C.; Wasielewski, E.; Geraldes, C.F.G.; Moreno, M.J. Quantification of cholesterol solubilized in dietary micelles: Dependence on human bile salt variability and the presence of dietary food ingredients. Langmuir 2016, 32, 4564–4574. [Google Scholar] [CrossRef] [PubMed]





| Sample | Yield (%, w/w) | Sugars Composition (mol%) | Total Sugars (%, w/w) | |||||
|---|---|---|---|---|---|---|---|---|
| Fuc | Xyl | Man | Gal | Glc | UA | |||
| Biomass | - | 4.2 ± 1.1 | 0.9 ± 0.4 | 29.9 ± 6.5 | 2.1 ± 0.3 | 25.6 ± 9.4 | 37.3 ± 5.9 | 42.0 ± 9.5 |
| Sn_AIR | 38.2 a | 0.2 ± 0.1 | ND | 91.2 ± 1.1 | 1.7 ± 0.2 | 6.9 ± 0.9 | ND | 24.9 ± 0.2 |
| AIR | 56.3 a | 6.8 ± 1.2 | 1.6 ± 0.8 | 3.9 ± 1.4 | 1.9 ± 0.3 | 26.0 ± 2.6 | 59.8 ± 4.8 | 53.4 ± 6.6 |
| Hot-water extraction of AIR | ||||||||
| Res_H2O | 58.7 b | 3.6 ± 0.1 | 1.1 ± 0.1 | 4.6 ± 0.8 | 1.5 ± 0.3 | 25.2 ± 0.9 | 64.1 ± 2.2 | 50.8 ± 2.8 |
| Ext_H2O d | - | 11.4 | 1.6 | 15.1 | 3.2 | 35.3 | 33.4 | 53.5 |
| Precipitation with calcium chloride of Ext_H2O | ||||||||
| Ppt_CaCl2 | 5.0 b | 3.8 ± 0.3 | ND | 1.9 ± 0.2 | ND | 0.7 ± 0.03 | 93.5 ± 0.5 | 62.1 ± 3.3 |
| Sn_CaCl2 | 5.4 b | 23.7 ± 2.1 | 2.5 ± 0.4 | 1.5 ± 0.1 | 6.7 ± 0.7 | 47.7 ± 3.4 | 17.9 ± 1.6 | 55.9 ± 5.0 |
| Anion-exchange chromatography of Sn_CaCl2 | ||||||||
| F1 | 28.6 c | ND | ND | 2.3 ± 1.9 | ND | 97.7 ± 1.9 | tr | 76.2 ± 3.4 |
| F2 | 19.3 c | 28.7 ± 0.1 | 5.7 ± 0.2 | 5.6 ± 0.2 | 8.5 ± 0.2 | 6.8 ± 0.6 | 44.7 ± 0.3 | 42.7 ± 4.8 |
| F3 | 18.4 c | 59.1 ± 2.7 | 3.0 ± 1.1 | 2.0 ± 0.6 | 20.8 ± 4.2 | 3.2 ± 1.6 | 12.0 ± 2.1 | 42.6 ± 7.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreira, A.S.P.; Gaspar, D.; Ferreira, S.S.; Correia, A.; Vilanova, M.; Perrineau, M.-M.; Kerrison, P.D.; Gachon, C.M.M.; Domingues, M.R.; Coimbra, M.A.; et al. Water-Soluble Saccharina latissima Polysaccharides and Relation of Their Structural Characteristics with In Vitro Immunostimulatory and Hypocholesterolemic Activities. Mar. Drugs 2023, 21, 183. https://doi.org/10.3390/md21030183
Moreira ASP, Gaspar D, Ferreira SS, Correia A, Vilanova M, Perrineau M-M, Kerrison PD, Gachon CMM, Domingues MR, Coimbra MA, et al. Water-Soluble Saccharina latissima Polysaccharides and Relation of Their Structural Characteristics with In Vitro Immunostimulatory and Hypocholesterolemic Activities. Marine Drugs. 2023; 21(3):183. https://doi.org/10.3390/md21030183
Chicago/Turabian StyleMoreira, Ana S. P., Diana Gaspar, Sónia S. Ferreira, Alexandra Correia, Manuel Vilanova, Marie-Mathilde Perrineau, Philip D. Kerrison, Claire M. M. Gachon, Maria Rosário Domingues, Manuel A. Coimbra, and et al. 2023. "Water-Soluble Saccharina latissima Polysaccharides and Relation of Their Structural Characteristics with In Vitro Immunostimulatory and Hypocholesterolemic Activities" Marine Drugs 21, no. 3: 183. https://doi.org/10.3390/md21030183
APA StyleMoreira, A. S. P., Gaspar, D., Ferreira, S. S., Correia, A., Vilanova, M., Perrineau, M. -M., Kerrison, P. D., Gachon, C. M. M., Domingues, M. R., Coimbra, M. A., Coreta-Gomes, F. M., & Nunes, C. (2023). Water-Soluble Saccharina latissima Polysaccharides and Relation of Their Structural Characteristics with In Vitro Immunostimulatory and Hypocholesterolemic Activities. Marine Drugs, 21(3), 183. https://doi.org/10.3390/md21030183