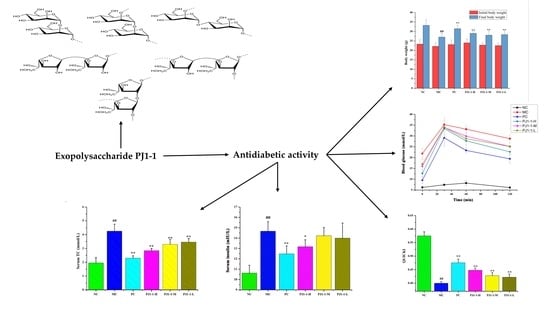
Anti-Diabetic Activity of a Novel Exopolysaccharide Produced by the Mangrove Endophytic Fungus Penicillium janthinellum N29
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structural Characteristics of the Exopolysaccharide PJ1-1
2.2. Influence of PJ1-1 on α-Glucosidase Activity In Vitro
2.3. Antidiabetic Activity In Vivo of PJ1-1
2.3.1. Effects of PJ1-1 on Body Weight and Fasting Blood Glucose Level
2.3.2. Effect of PJ1-1 on Glucose Tolerance
2.3.3. Effect of PJ1-1 on Insulin Resistance
2.3.4. Influences of PJ1-1 on Lipid Metabolism
3. Materials and Methods
3.1. Materials
3.2. Animals
3.3. Strains and Culture Conditions
3.4. Preparation of the Exopolysaccharide PJ1-1
3.5. Composition Analysis
3.6. Methylation Analysis
3.7. Spectroscopy Analysis
3.8. α-Glucosidase Inhibitory Assay
3.9. In Vivo Experiment
3.9.1. Animal Experimental Design
3.9.2. FBG and OGTT
3.9.3. Assays of Fasting Insulin Content and Related Indexes
3.9.4. Determination for Lipid Metabolic Parameter Levels
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Patel, B.K.; Patel, K.H.; Moochhala, S.M. Gut microbiota intervention strategies using active components from medicinal herbs to evaluate clinical efficacy of type 2 diabetes—A review. CTD 2023, 3, e170. [Google Scholar] [CrossRef]
- Ogurtsova, K.; da Rocha Fernandes, J.; Huang, Y.; Linnenkamp, U.; Guariguata, L.; Cho, N.H.; Cavan, D.; Shaw, J.; Makaroff, L. IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res. Clin. Pract. 2017, 128, 40–50. [Google Scholar] [CrossRef]
- Bello, N.A.; Pfeffer, M.A.; Skali, H.; McGill, J.B.; Rossert, J.; Olson, K.A.; Weinrauch, L.; Cooper, M.E.; de Zeeuw, D.; Rossing, P. Retinopathy and clinical outcomes in patients with type 2 diabetes mellitus, chronic kidney disease, and anemia. BMJ Open Diabetes Res. 2014, 2, e11. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Yi, C.; Zhang, Z.; Wen, H.; Sun, Y.; Xu, J.; Yang, T. Repair and mechanism of oligopeptide SEP-3 on oxidative stress liver injury induced by sleep deprivation in mice. Mar. Drugs 2023, 21, 139. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Li, Y.; Li, P.; Wang, M.; Wang, J.; Tang, Z.; Wang, T.; Luo, L.; Wang, C.; Zhao, B. Research progress in the relationship between type 2 diabetes mellitus and intestinal flora. Biomed. Pharmacother. 2019, 117, 109138. [Google Scholar] [CrossRef] [PubMed]
- Wińska, K.; Mączka, W.; Gabryelska, K.; Grabarczyk, M. Mushrooms of the genus Ganoderma used to treat diabetes and insulin resistance. Molecules 2019, 24, 4075. [Google Scholar] [CrossRef]
- Rosas-Ramírez, D.; Escandón-Rivera, S.; Pereda-Miranda, R. Morning glory resin glycosides as α-glucosidase inhibitors: In vitro and in silico analysis. Phytochemistry 2018, 148, 39–47. [Google Scholar] [CrossRef]
- Wang, L.; Chen, C.; Zhang, B.; Huang, Q.; Fu, X.; Li, C. Structural characterization of a novel acidic polysaccharide from Rosa roxburghii Tratt fruit and its α-glucosidase inhibitory activity. Food Funct. 2018, 9, 3974–3985. [Google Scholar] [CrossRef]
- Sun, H.; Yu, X.; Li, T.; Zhu, Z. Structure and hypoglycemic activity of a novel exopolysaccharide of Cordyceps militaris. Int. J. Biol. Macromol. 2021, 166, 496–508. [Google Scholar] [CrossRef]
- Yang, S.; Qu, Y.; Zhang, H.; Xue, Z.; Liu, T.; Yang, L.; Sun, L.; Zhou, Y.; Fan, Y. Hypoglycemic effects of polysaccharides from Gomphidiaceae rutilus fruiting bodies and their mechanisms. Food Funct. 2020, 11, 424–434. [Google Scholar] [CrossRef]
- Ye, H.; Shen, Z.; Cui, J.; Zhu, Y.; Li, Y.; Chi, Y.; Wang, J.; Wang, P. Hypoglycemic activity and mechanism of the sulfated rhamnose polysaccharides chromium (III) complex in type 2 diabetic mice. Bioorg. Chem. 2019, 88, 102942. [Google Scholar] [CrossRef]
- Shan, X.; Liu, X.; Hao, J.; Cai, C.; Fan, F.; Dun, Y.; Zhao, X.; Liu, X.; Li, C.; Yu, G. In vitro and in vivo hypoglycemic effects of brown algal fucoidans. Int. J. Biol. Macromol. 2016, 82, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Sun, X.M.; Chen, C.; Zhang, X.Y.; Chen, X.L.; Zhang, Y.Z.; Xu, F. A novel alginate lyase: Identification, characterization, and potential application in alginate trisaccharide preparation. Mar. Drugs 2022, 20, 159. [Google Scholar] [CrossRef]
- Barros, J.; Seena, S. Fungi in freshwaters: Prioritising aquatic hyphomycetes in conservation goals. Water 2022, 14, 605. [Google Scholar] [CrossRef]
- Gonçalves, M.F.; Esteves, A.C.; Alves, A. Marine fungi: Opportunities and challenges. Encyclopedia 2022, 2, 559–577. [Google Scholar] [CrossRef]
- Chen, G.; Kan, J. Characterization of a novel polysaccharide isolated from Rosa roxburghii Tratt fruit and assessment of its antioxidant in vitro and in vivo. Int. J. Biol. Macromol. 2018, 107, 166–174. [Google Scholar] [CrossRef]
- Zhou, S.; Huang, G.; Chen, G. Extraction, structural analysis, derivatization and antioxidant activity of polysaccharide from Chinese yam. Food Chem. 2021, 361, 130089. [Google Scholar] [CrossRef]
- Zhu, R.; Zhang, X.; Wang, Y.; Zhang, L.; Zhao, J.; Chen, G.; Ning, C. Characterization of polysaccharide fractions from fruit of Actinidia arguta and assessment of their antioxidant and antiglycated activities. Carbohyd. Polym. 2019, 210, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Pan, C.; Xia, W.; Zhang, W.; Wu, S. Structural characterization of the polysaccharide moiety of an aqueous glycopeptide from mannatide. Int. J. Biol. Macromol. 2014, 67, 351–359. [Google Scholar] [CrossRef]
- Ljpkind, G.M.; Shashkov, A.S.; Nifant’ev, N.E.; Kochetkov, N.K. Computer-assisted analysis of the structure of regular branched polysaccharides containing 2, 3-disubstituted rhamnopyranose and mannopyranose residues on the basis of 13C NMR data. Carbohyd. Res. 1992, 237, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Mao, W.; Yan, M.; Liu, X.; Wang, S.; Xia, Z.; Xiao, B.; Cao, S.; Yang, B.; Li, J. Purification, chemical characterization, and bioactivity of an extracellular polysaccharide produced by the marine sponge endogenous fungus Alternaria sp. SP-32. Mar. Biotechnol. 2016, 18, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xu, J.; Na, R.; Du, R.; Ping, W.; Ge, J.; Zhao, D. Purification, characterization and partial biological activities of exopolysaccharide produced by Saccharomyces cerevisiae Y3. Int. J. Biol. Macromol. 2022, 206, 777–787. [Google Scholar] [CrossRef]
- Zhou, D.; Li, P.; Dong, Z.; Wang, T.; Sun, K.; Zhao, Y.; Wang, B.; Chen, Y. Structure and immunoregulatory activity of β-d-galactofuranose-containing polysaccharides from the medicinal fungus Shiraia bambusicola. Int. J. Biol. Macromol. 2019, 129, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Jia, G.; Wang, C.; Chen, M.; Xie, F.; Nepovinnykh, N.; Goff, H.D.; Guo, Q. Structural characterisation and immunomodulatory activity of exopolysaccharides from liquid fermentation of Monascus purpureus (Hong Qu). Food Hydrocolloid. 2020, 103, 105636. [Google Scholar] [CrossRef]
- Luo, D.; Wang, Z.; Zhou, R.; Cao, S. A polysaccharide from Umbilicaria yunnana: Structural characterization and anti-inflammation effects. Int. J. Biol. Macromol. 2020, 151, 870–877. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zeng, Y.; Cui, Y.; Liu, H.; Dong, C.; Sun, Y. Structural characterization, antioxidant and immunomodulatory activities of a neutral polysaccharide from Cordyceps militaris cultivated on hull-less barley. Carbohyd. Polym. 2020, 235, 115969. [Google Scholar] [CrossRef]
- Chen, Y.; Mao, W.; Tao, H.; Zhu, W.; Yan, M.; Liu, X.; Guo, T.; Guo, T. Preparation and characterization of a novel extracellular polysaccharide with antioxidant activity, from the mangrove-associated fungus Fusarium oxysporum. Mar. Biotechnol. 2015, 17, 219–228. [Google Scholar] [CrossRef]
- Chen, Y.; Mao, W.; Tao, H.; Zhu, W.; Qi, X.; Chen, Y.; Li, H.; Zhao, C.; Yang, Y.; Hou, Y. Structural characterization and antioxidant properties of an exopolysaccharide produced by the mangrove endophytic fungus Aspergillus sp. Y16. Bioresour. Technol. 2011, 102, 8179–8184. [Google Scholar] [CrossRef]
- Prathyusha, A.; Mohana Sheela, G.; Berde, C.V.; Bramhachari, P. Current Perspectives on the Novel Structures and Antioxidant Properties of Mangrove Endophytic Fungal Exopolysaccharides, Advances in Endophytic Fungal Research; Springer: Cham, Switzerland, 2019; pp. 233–242. [Google Scholar]
- Srinivasan, K.; Viswanad, B.; Asrat, L.; Kaul, C.; Ramarao, P. Combination of high-fat diet-fed and low-dose strep tozotocin-treated rat: A model for type 2 diabetes and pharmacological screening. Pharmacol. Res. 2005, 52, 313–320. [Google Scholar] [CrossRef]
- Saltiel, A.R. Insulin signaling in health and disease. J. Clin. Investig. 2021, 131, 142241. [Google Scholar] [CrossRef]
- Vichaibun, V.; Khananurak, K.; Sophonnithiprasert, T. Comparative analysis of plasma total antioxidant capacity in patients with hyperglycemia and hyperglycemia plus dyslipidemia. Diabetes Metab. Synd. 2019, 13, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, D.; You, Y.; Zeng, S.; Hu, Y.; Duan, X.; Chen, D. Structural characterization and antidiabetic activity of a glucopyranose-rich heteropolysaccharide from Catathelasma ventricosum. Carbohyd. Polym. 2016, 149, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wu, X.; Shi, F.; Liu, Y. Comparison of antidiabetic effects of saponins and polysaccharides from Momordica charantia L. in STZ-induced type 2 diabetic mice. Biomed. Pharmacother. 2019, 109, 744–750. [Google Scholar] [CrossRef] [PubMed]
- Hsu, W.K.; Hsu, T.H.; Lin, F.Y.; Cheng, Y.K.; Yang, J.P. Separation, purification, and α-glucosidase inhibition of polysaccharides from Coriolus versicolor LH1 mycelia. Carbohyd. Polym. 2013, 92, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Ding, L.; Liu, R.; Zheng, X.; Xia, X.; Wang, F.; Qiu, Y. Characterization of Anoectochilus roxburghii polysaccharide and its therapeutic effect on type 2 diabetic mice. Int. J. Biol. Macromol. 2021, 179, 259–269. [Google Scholar] [CrossRef]
- Yan, M.; Mao, W.; Liu, X.; Wang, S.; Xia, Z.; Cao, S.; Li, J.; Qin, L.; Xian, H. Extracellular polysaccharide with novel structure and antioxidant property produced by the deep-sea fungus Aspergillus versicolor N2bc. Carbohydr. Polym. 2016, 147, 272–281. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.T.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Glyk, A.; Heinisch, S.L.; Scheper, T.; Beutel, S. Comparison of colorimetric methods for the quantification of model proteins in aqueous two-phase systems. Anal. Biochem. 2015, 477, 35–37. [Google Scholar] [CrossRef] [PubMed]
- Mao, W.; Li, H.; Li, Y.; Zhang, H.; Qi, X.; Sun, H.; Chen, Y.; Guo, S. Chemical characteristic and anticoagulant activity of the sulfated polysaccharide isolated from Monostroma latissimum (Chlorophyta). Int. J. Biol. Macromol. 2009, 44, 70–74. [Google Scholar] [CrossRef]
- Qin, L.; He, M.; Yang, Y.; Fu, Z.; Tang, C.; Shao, Z.; Zhang, J.; Mao, W. Anticoagulant-active sulfated arabinogalac tan from Chaetomorpha linum: Structural characterization and action on coagulation factors. Carbohydr. Polym. 2020, 242, 116394. [Google Scholar] [CrossRef]
- Tanaka, T.; Nakashima, T.; Ueda, T.; Tomii, K.; Kouno, I. Facile discrimination of aldose enantiomers by reversed-phase HPLC. Chem. Pharm. Bull. 2007, 55, 899–901. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.J.; Henry, R.J.; Blakeney, A.B.; Stone, B.A. An improved procedure for the methylation analysis of oli gosaccharides and polysaccharides. Carbohydr. Res. 1984, 127, 59–73. [Google Scholar] [CrossRef]
- Ni, M.; Hu, X.; Gong, D.; Zhang, G. Inhibitory mechanism of vitexin on α-glucosidase and its synergy with acarbose. Food Hydrocolloid. 2020, 105, 105824. [Google Scholar] [CrossRef]
- Usoltseva, R.V.; Anastyuk, S.D.; Shevchenko, N.M.; Surits, V.V.; Silchenko, A.S.; Isakov, V.V.; Zvyagintseva, T.N.; Thinh, P.D.; Ermakova, S.P. Polysaccharides from brown algae Sargassum duplicatum: The structure and anticancer activity in vitro. Carbohyd. Polym. 2017, 175, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Arasteh, A.; Aliyev, A.; Khamnei, S.; Delazar, A.; Mesgari, M.; Mehmannavaz, Y. Crocus sativus on serum glucose, insulin and cholesterol levels in healthy male rats. J. Med. Plants Res. 2010, 4, 397–402. [Google Scholar]
- Wu, L.; Yuan, A.; Tian, X.; Cao, J.; Qi, X.; Wei, Y.; Shen, S. Cell-membrane-coated cationic nanoparticles disguised as macrophages for the prevention and treatment of type 2 diabetes mellitus. ACS Appl. Mater. Inter. 2022, 14, 50499–50506. [Google Scholar] [CrossRef]
- Xiong, W.; Gu, L.; Wang, C.; Sun, H.; Liu, X. Anti-hyperglycemic and hypolipidemic effects of Cistanche tubulosa in type 2 diabetic db/db mice. J. Ethnopharmacol. 2013, 150, 935–945. [Google Scholar] [CrossRef]












| Methylated Alditol Acetate | Molar Percent Ratio | Linkage Pattern |
|---|---|---|
| 1,5-Di-O-acetyl-2,3,4,6-tetra-O-methyl mannitol | 8.11 | Manp-(1→ |
| 1,4-Di-O-acetyl-2,3,5,6-tri-O-methyl galactitol | 7.50 | Galf-(1→ |
| 1,2,5-Tri-O-acetyl-3,4,6-tri-O-methyl mannitol | 29.45 | →2)-Manp-(1→ |
| 1,4,5-Tri-O-acetyl-2,3,6-tri-O-methyl mannitol | 15.15 | →4)-Manp-(1→ |
| 1,2,3,4-Tetra-O-acetyl-5,6-O-methyl galactitol | 11.48 | →2,3)-Galf (1→ |
| 1,2,4-Tri-O-acetyl-3,5,6-di-O-methyl galactitol | 13.01 | →2)-Galf-(1→ |
| 1,3,4-Tri-O-acetyl-2,5,6-di-O-methyl galactitol | 15.30 | →3)-Galf-(1→ |
| Sugar Residues | Chemical Shifts (ppm) a | |||||
|---|---|---|---|---|---|---|
| H1/C1 | H2/C2 | H3/C3 | H4/C4 | H5/C5 | H6/C6 | |
| →2)-α-D-Manp-(1→ | 5.29/102.19 | 4.15/79.67 | 4.01/71.64 | 3.71/71.39 | 3.66/68.35 | 3.76/62.48 |
| →2,3)-β-D-Galf-(1→ | 5.25/108.67 | 4.17/89.53 | 4.10/75.87 | 3.87/81.42 | 3.98/61.98 | -/- |
| →2)-β-D-Galf-(1→ | 5.23/106.25 | 4.17/89.53 | 4.03/71.64 | 3.87/83.23 | 3.78/61.98 | -/- |
| β-D-Galf-(1→ | 5.20/107.87 | 4.15/79.90 | 4.01/71.56 | 3.73/82.30 | -/- | -/- |
| →4)-α-D-Manp-(1→ | 5.16/99.76 | 4.10/71.56 | 3.87/71.97 | 3.82/74.78 | 3.72/68.47 | 3.65/64.28 |
| α-D-Manp-(1→ | 5.12/103.79 | 3.92/71.56 | 4.01/71.64 | 3.75/67.96 | 3.72/64.30 | 3.92/62.58 |
| →3)-β-D-Galf-(1→ | 5.08/109.31 | 4.18/82.61 | 4.07/79.78 | 3.77/83.67 | 3.72/64.30 | -/- |
| Fasting Blood Glucose Level (mmol/L) a | ||||||
|---|---|---|---|---|---|---|
| NC | MC | PC | PJ1-1-H | PJ1-1-M | PJ1-1-L | |
| 0 week | 5.25 ± 0.18 | 18.21 ± 0.77 ## | 20.15 ± 2.15 ## | 20.67 ± 2.08 ## | 21.02 ± 2.66 ## | 22.14 ± 1.92 ## |
| 1 week | 5.19 ± 0.18 | 21.47 ± 1.10 ## | 21.18 ± 2.15 | 21.28 ± 2.11 | 21.06 ± 2.21 | 20.89 ± 1.51 |
| 2 week | 5.27 ± 0.32 | 21.50 ± 1.10 ## | 21.12 ± 1.60 | 18.62 ± 1.04 ** | 19.59 ± 1.73 | 20.12 ± 1.54 |
| 3 week | 5.19 ± 0.18 | 21.77 ± 0.89 ## | 18.18 ± 0.99 ** | 16.60 ± 1.62 ** | 16.33 ± 1.75 ** | 18.82 ± 1.37 ** |
| 4 week | 5.10 ± 0.25 | 22.13 ± 0.76 ## | 14.40 ± 0.90 ** | 15.32 ± 1.44 ** | 16.77 ± 1.47 ** | 17.62 ± 0.97 ** |
| 5 week | 5.19 ± 0.32 | 21.75 ± 1.36 ## | 9.75 ± 1.34 ** | 12.58 ± 0.77 ** | 15.42 ± 1.58 ** | 16.91 ± 1.71 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, Z.; Tian, Y.; Liu, S.; Chu, X.; Mao, W. Anti-Diabetic Activity of a Novel Exopolysaccharide Produced by the Mangrove Endophytic Fungus Penicillium janthinellum N29. Mar. Drugs 2023, 21, 270. https://doi.org/10.3390/md21050270
Shao Z, Tian Y, Liu S, Chu X, Mao W. Anti-Diabetic Activity of a Novel Exopolysaccharide Produced by the Mangrove Endophytic Fungus Penicillium janthinellum N29. Marine Drugs. 2023; 21(5):270. https://doi.org/10.3390/md21050270
Chicago/Turabian StyleShao, Zhuling, Yingying Tian, Shan Liu, Xiao Chu, and Wenjun Mao. 2023. "Anti-Diabetic Activity of a Novel Exopolysaccharide Produced by the Mangrove Endophytic Fungus Penicillium janthinellum N29" Marine Drugs 21, no. 5: 270. https://doi.org/10.3390/md21050270
APA StyleShao, Z., Tian, Y., Liu, S., Chu, X., & Mao, W. (2023). Anti-Diabetic Activity of a Novel Exopolysaccharide Produced by the Mangrove Endophytic Fungus Penicillium janthinellum N29. Marine Drugs, 21(5), 270. https://doi.org/10.3390/md21050270