Microalgae, Seaweeds and Aquatic Bacteria, Archaea, and Yeasts: Sources of Carotenoids with Potential Antioxidant and Anti-Inflammatory Health-Promoting Actions in the Sustainability Era
Abstract
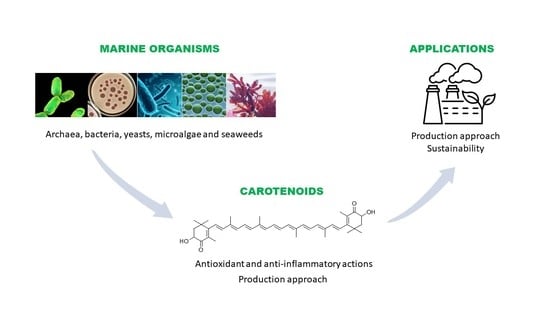
:1. Introduction: Sustainability, the Blue Economy, and the Versatility of Carotenoids in Agro-Food and Health
2. Main Marine Organisms Containing Carotenoids
2.1. Archaea
2.1.1. Ecological Importance
2.1.2. Sustainability Pros and Cons
2.1.3. Main Species (and Compounds) in the Context of Agro-Food and Health
2.1.4. Production Approaches and Production Data in Different Continents
2.1.5. Current and Prospective Applications
2.1.6. Research Needs
2.2. Bacteria
2.2.1. Ecological Importance
2.2.2. Sustainability Pros and Cons
2.2.3. Main Species (and Compounds) in the Context of Agro-Food and Health
| Organisms | Species | Carotenoids | Collection Sites | Experimental Conditions | Quantification/Identification Methodologies | Contents | References |
|---|---|---|---|---|---|---|---|
| Bacteria | Rubritalea squalenifaciens (DSM 18772T) | Diapolycopenedioc acid xylosylesters | Miura Peninsula, Kanagawa, Japan | Liquid culture (1.0% starch, 0.4% yeast extract, and 0.2% peptone in seawater), 30 °C, 120 rpm, 2 days | Carotenoids purification by chromatography and identification of the structures by HRESI-MS and spectroscopic analyses | 2.2–10.2 mg purified from cells in a 42 L culture | [81] |
| Planococcus maritimus strain iso-3 | Methyl 5-glucosyl-5,6-dihydro-apo-4,4′-lycopenoate | Clyde estuary, UK | Marine broth 2216), 30 °C, 120 rpm, 1 day | Carotenoids purification by chromatography and identification of the structures by HRESI-MS and spectroscopic analyses | 2.5 mg purified from cells in an 18 L culture | [81] | |
| 04OKA-13-27 (MBIC08261) | (3R)-saproxanthin | Coast of Okinawa Prefecture, Japan | Marine Broth 2216, 30 °C, 100 rpm, 1 day | Carotenoids purification by chromatography and identification of the structures by MS, 1H-NMR, and CD analyses | 0.3 mg purified from cells in a 2 L culture | [81] | |
| YM6-073 (MBIC06409) | (3R,2′S)-myxol | Coast of Okinawa Prefecture, Japan | Marine broth, 30 °C, 100 rpm, 1 day | Carotenoids purification by chromatography and identification of the structures by MS, 1H-NMR, and CD analyses | 0.5 mg purified from cells in a 2 L culture | [81] | |
| Erythrobacter rubeus sp. nov. | Keto-spirilloxanthin | Hamdeok Beach, Jeju Island, Republic of Korea | Marine agar 2216, 25 °C | - | - | [82] | |
| Flavobacterium sp. P8 | Zeaxanthin | King George Island, Antarctica | 2 L medium (7 g/L peptone, 7 g/L yeast extract, and 15 g/L NaCl) in a 5 L-bioreactor, 20 °C, pH 7, 1 vvm airflow, 20% pO2, 72 h | HPLC | 2.15 ± 0.15 mg/L, DW | [83] | |
| Brevundimonas scallop | Astaxanthin, 2,2′-dihydroxy-astaxanthin, and 2-hydroxy-astaxanthin | Nanao Island of Guangdong Province, China | 100 mL LB medium, 25 °C, 150 rpm, 3 days | Spectrophotometry | 104.29 ± 4.98 µg/g astaxanthin, 1068.46 ± 52.42 µg/g 2,2′-dihydroxy-astaxanthin, and 130.36 ± 6.11 µg/g 2-hydroxy-astaxanthin, DW | [84] | |
| Erythrobacter citreus LAMA 915 | Adonixanthin, caloxanthin, canthaxanthin, erythroxanthin sulfate, nostoxanthin, and zeaxanthin | Atlantic Ocean (30° S, 36° W) | Soluble fish hydrolysate 0.5% | Spectrophotometry | 1.5 mg/L total carotenoids, FW | [85] | |
| Erythrobacter flavus KJ5 | Caloxanthin, caloxanthin sulfate, caloxanthin sulfate isomer, β-carotene, β-carotene cis isomer, β-cryptoxanthin, nostoxanthin sulfate, nostoxanthin isomer, zeaxanthin, zeaxanthin isomer, zeaxanthin sulfate, and zeaxanthin sulfate cis isomer | Hard coral Acropora nasuta, Karimunjawa Islands, Indonesia | Shioi broth, 28 °C, under dark conditions, 48 h | - | - | [86] | |
| Planococcus sp. Eg-Natrun | Astaxanthin and β-carotene | Umm Risha Lake, Wadi El-Natrun, south of Al-Buhayrah province, Egypt | E1 rich marine medium (C/N~3.95), 37 °C, 150 rpm, 3 days | Spectrophotometry | 1024 ± 53 µg/g, DW | [87] | |
| Brevundimonas sp. strain N-5 | Astaxanthin | Shimoda Port, Shizuoka Prefecture on the Pacific coast of Middle Japan | NB broth, 150 rpm, 2 days | HPLC | 601.2 μg/g total carotenoids, 364.6 μg/g astaxanthin (3S, 3′S), DW | [88] | |
| Paracoccus sp. strain N-81106 | Astaxanthin | Iwate, Japan | OEG medium, 25 °C, 150 rpm, 18 h | HPLC | 0.9 ± 0.1 mg/L astaxanthin,3.1 ± 0.2 mg/L total carotenoids, FW | [89] | |
| Vitellibacter sp. BW | Zeaxanthin and β-carotene | East and West coast of India (Tuticorin, Mandappam, Rameshwaram, and Mangalore) | Marine broth, 37 °C, 200 rpm, 5 days | HPLC | 115.7 ± 5.0 mg/g total carotenoid, FW | [90] | |
| Arenibacter sp. 4W | Zeaxanthin and β-carotene | East and West coast of India (Tuticorin, Mandappam, Rameshwaram, and Mangalore) | Marine broth, 37 °C, 200 rpm, 5 days | HPLC | 99.3 ± 21.9 mg/g total carotenoid, FW | [90] | |
| Paracoccus sp. LL1 (KP288668) | Astaxanthin | Lonar lake, India | Fed-batch culture in minimal medium supplemented with 20 g/L of glucose. Fermenter with a membrane of cell filtration, 1.5 vvm airflow, 300 rpm, DO > 20% | HPLC | 8.51 ± 0.20 mg/L intracellular astaxanthin, 10.2 ± 0.24 mg/L extracellular astaxanthin, FW | [91] | |
| Paracoccus haeundaensis KCCM 10460 | Astaxanthin | - | Co-culture with lactic acid bacteria. PMF medium, 25 °C, 160 rpm, 1 vvm airflow, 72 h | HPLC | 821.09 ± 30.98 µg/g, DW | [92] | |
| Paracoccus zeaxanthinifaciens ATCC 21588 | Zeaxanthin | Purchased from BCCM/LMG, Ghent University, Belgium | Culture media, 30 °C, 180 rpm, 72 h | TLC and spectrophotometry | 11.63 mg/L, FW | [93] | |
| Paracoccus zeaxanthinifaciens ATCC 21588 | Zeaxanthin | Purchased from BCCM/LMG, Ghent University, Belgium | Culture media in a bubble column reactor, 30 °C, 80 h | HPLC | 13.76 ± 0.14 mg/L, FW | [94] | |
| Cyanobacteria | Anabaena sp. PCC 7120 | Canthaxanthin, β-carotene, echinenone, ketomyxol 2′-fucoside, and myxol 2′-fucoside | A gift from Waseda University, Japan | BG-11 medium, 26–28 °C, 110 rpm, white fluorescent light (30–40 µE m−2 s−1), 2 weeks | HPLC | Relative molecular masses (%): 1 canthaxanthin, 62 β-carotene, 25 echinenone, 4 ketomyxol 2′-fucoside, and 8 myxol 2′-fucoside | [95] |
| Anabaena variabilis IAM M-3 (=PCC 7118, ATCC 27893) | Canthaxanthin, β-carotene, echinenone, ketomyxol 2′-fucoside, 3′-hydroxyechinenone, and myxol 2′-fucoside | A gift from Waseda University, Japan | BG-11 medium, 26–28 °C, 110 rpm, white fluorescent light (30–40 µE m−2 s−1), 2 weeks | HPLC | Relative molecular masses (%): 4 canthaxanthin, 38 β-carotene, 33 echinenone, 13 ketomyxol 2′-fucoside, 1 3′-hydroxyechinenone, and 11 myxol 2′-fucoside | [95] | |
| Nostoc punctiforme PCC 73102 (=ATCC 29133) | Canthaxanthin, β-carotene, echinenone, ketomyxol 2′-fucoside, and myxol 2′-fucoside | A gift from Kanazawa University, Japan | BG-11 medium, 26-28 °C, 110 rpm, white fluorescent light (30–40 µE m−2 s−1), 2 weeks | HPLC | Relative molecular masses (%): 13 canthaxanthin, 45 β-carotene, 17 echinenone, 13 ketomyxol 2′-fucoside, and 11 myxol 2′-fucoside | [95] | |
| Anabaena variabilis ATCC 29413 (=IAM M204) | Canthaxanthin, β-carotene, echinenone, 4-hydroxymyxol, and myxol | A gift from Waseda University, Japan | BG-11 medium, 26–28 °C, 110 rpm, white fluorescent light (30–40 µE m−2 s−1), 2 weeks | HPLC | Relative molecular masses (%): 22 canthaxanthin, 51 β-carotene, 20 echinenone, 2 4-hydroxymyxol, and 5 myxol | [96] | |
| Synechocystis sp. PCC 6803 | β-carotene, deoxymyxol 2′-dimethyl-fucoside, echinenone, 3′-hydroxyechinenone, myxol 2′-dimethyl-fucoside, and zeaxanthin | A gift from Waseda University, Japan | BG-11 medium supplemented with 10 mM HEPES buffer (pH 7.5) in 10 L carboys, 30 °C with aeration and irradiation (40–100 E/m2·s) | HPLC | Relative molecular masses (%): 26 β-carotene, 1 deoxymyxol 2′-dimethyl-fucoside, 18 echinenone, 4 3′-hydroxyechinenone, 36 myxol 2′-dimethyl-fucoside, and 14 zeaxanthin | [97] |
2.2.4. Production Approaches and Production Data in Different Continents
2.2.5. Current and Prospective Applications
2.2.6. Research Needs
2.3. Macroalgae
2.3.1. Ecological Importance
2.3.2. Sustainability Pros and Cons
2.3.3. Main Species (and Compounds) in the Context of Agro-Food and Health
| Organisms | Species | Carotenoids | Collection Sites | Experimental Conditions | Quantification/Identification Methodologies | Contents | References |
|---|---|---|---|---|---|---|---|
| Macroalga (brown) | Sargassum horneri | Fucoxanthin | Nesaki, Hokkaido (41°45′ N, 140°49′ E) and Matsushima, Miyagi (38°23′ N, 141°04′ E), northern seashore of Japan | Mariculture of collected thalli in Usujiri, Japan | HPLC | 1.35–4.5 mg/g DW | [147] |
| Cystoseira hakodatensis | Fucoxanthin | Usujiri, Hokkaido (41°56′ N, 140°57′ E), Japan | Natural growth in Usujiri, Japan | HPLC | 0.6–4.1 mg/g DW | [147] | |
| 15 species of brown seaweed | Fucoxanthin | Wild harvest in intertidal zones in Shinori and Nesaki, Japan | Natural growth | HPLC | 0.1–3.7 mg/g DW | [148] | |
| Macroalga (green) | Ulva spp. | Total carotenoids | Wild harvest in Inter- and subtidal zones in India, Egypt, China, Spain, and Chile | Natural growth | Spectrophotometry | 1.25–4.6 mg/g DW | [132] |
| Ulva compressa | Lutein, neoxanthin, violaxanthin, and zeaxanthin | Coasts of Mangaluru (12°45′31.7″ N 74°51′53.2″ E to 13°06′25.9″ N 74°46′ 03.6″ E), India | Natural growth | HPLC | 4.7 μg/g lutein, 3.8 μg/g neoxanthin, 4.0 μg/g violaxanthin, and 3.9 μg/g zeaxanthin, DW | [149] * | |
| Chaetomorpha antennia | Lutein, neoxanthin, violaxanthin, and zeaxanthin | Coasts of Mangaluru (12°45′31.7″ N 74°51′53.2″ E to 13°06′25.9″ N 74°46′ 03.6″ E), India | Natural growth | HPLC | 141.3 μg/g lutein, 33.3 μg/g neoxanthin, 33.7 μg/g violaxanthin, and 34.6 μg/g zeaxanthin, DW | [149] | |
| Macroalga (red) | Grateloupia sp. | Lutein and zeaxanthin | Coasts of Mangaluru (12°45′31.7″ N 74°51′53.2″ E to 13°06′25.9″ N 74°46′ 03.6″ E), India | Natural growth | HPLC | 166.6 μg/g lutein and 36.3 μg/g zeaxanthin, DW | [149] |
| Pyropia yezoensis | α-carotene, β-carotene, lutein, zeaxanthin | Thalli of P. yezoensis strain U-51, maricultured at Shichigahama, Miyagi, Japan | Mariculture | HPLC | 0.7 mg/g α-carotene, 1.8 mg/g β-carotene, 1.4 mg/g lutein, and 0.15 mg/g zeaxanthin, DW | [150] |
2.3.4. Production Approaches and Production Data in Different Continents
2.3.5. Current and Prospective Applications
2.3.6. Research Needs
2.4. Microalgae
2.4.1. Ecological Importance
2.4.2. Sustainability Pros and Cons
2.4.3. Main Species (and Compounds) in the Context of Agro-Food and Health
| Species | Carotenoids | Collection Sites | Experimental Conditions | Quantification/Identification Methodologies | Contents | References |
|---|---|---|---|---|---|---|
| Chlorella vulgaris 211/52 | β-carotene, lutein | Grüental campus of the Zurich University of Applied Sciences, Wädenswil, Switzerland | Open thin-layer bioreactor | HPLC | 50 μg/g β-carotene, 90 μg/g lutein, DW | [201] |
| Dunaliella sp. FACHB-558 | β-carotene | Institute of Hydrobiology, Chinese Academy of Sciences, China | Two-step cultivation in anaerobically digested poultry litter wastewater | Spectrophotometry | 7.26 mg/L, FW | [193] |
| Isochrysis zhangjiangensis | Fucoxanthin | Chinese Academy of Sciences, China | Photo-autotrophically cultured at 23 °C in a bubble column photobioreactors, supplemented with f/2 medium (nutrient, trace metal, and vitamin solutions) | HPLC | 23 μg/g, DW | [202] |
| Haematococcus plivualis | Astaxanthin | Umeå University, Sweden | First step: inoculum in Bold’s basal medium, OD750 of 0.1, 20 °C, cool white LED, 1 L/min of air, 7 days. Second step: grown in a multi-cultivator with cool white LED | HPLC | 19.1 mg/g, DW | [203] |
| Desmodesmus sp. | Lutein and zeaxanthin | Isolated from a wastewater treatment system, Kalundborg Kommune, Copenhagen, Denmark | Industrial wastewater in Schott bottles, stirred, aerated (2% CO2), and with fluorescent lights (200 µmol photon/m2·s) | HPLC | 5.11 mg/g lutein and 0.28 mg/g zeaxanthin, DW | [204] |
| Nannochloropsis salina 40.85 | β-carotene and violaxanthin | Algae culture collection (SAG), University of Gottingen, Germany | Industrial wastewater in Schott bottles, stirred, aerated (2% CO2), and with fluorescent lights (200 µmol photon/m2·s) | HPLC | 2.22 mg/g β-carotene and 1.68 mg/g violaxanthin, DW | [204] |
| Phaeodactylum tricornutum | Diadinoxanthin and diatoxanthin | - | Industrial wastewater in Schott bottles, stirred, aerated (2% CO2), and with fluorescent lights (300 µmol photon/m2·s) | HPLC | 2.17 μg/g diadinoxanthin and 1.55 μg/g diatoxanthin, DW | [204] |
| Chlorella C.S1 | β-carotene and lutein | - | Industrial wastewater in flat panel reactors with fluorescent lights (2000 µmol photon/m2·s) | HPLC | 1.4 mg/g β-carotene and 3.22 mg/g lutein, DW | [204] |
| Nannochloropsis limnetica 18.99 | Neoxanthin and violaxanthin | Algal culture collection (SAG), University of Gottingen, Germany | Industrial wastewater in Schott bottles, stirred, aerated (2% CO2), and with fluorescent lights (200 µmol photon/m2·s) | HPLC | 0.42 mg/g neoxanthin and 1.22 mg/g violaxanthin, DW | [204] |
| Chlorococcum sp. | Lutein | Umeå University, Sweden | Multi-cultivator with high light/cold stress in BG11 | HPLC | 15.5 mg/g, DW | [205] |
| Scenedesmus sp. | Lutein | Umeå University, Sweden | Multi-cultivator with high light/cold stress in BG11 | HPLC | 10.7 mg/g, DW | [205] |
2.4.4. Production Approaches and Production Data in Different Continents
2.4.5. Current and Prospective Applications
2.4.6. Research Needs
2.5. Yeast
2.5.1. Ecological Importance
2.5.2. Sustainability Pros and Cons
2.5.3. Main Species (and Compounds) in the Context of Agro-Food and Health
| Species | Carotenoids | Collection Sites | Experimental Conditions | Quantification/Identification Methodologies | Contents | References |
|---|---|---|---|---|---|---|
| Sporidiobolus salmonicolor | β-carotene, 2,3 dihydroxy-γ-carotene, 4-ketotorulene, and torulene | Union Glacier, Antarctic | 30 mL YM broth in 300-mL baffled flasks, 150 rpm, 20 °C, 5 days | HPLC | 2,3-dihydroxy-γ-carotene was the main carotenoid | [247] |
| Sporidiobolus metaroseus | β-carotene, β-cryptoxanthin, 4-ketotorulene, and spirilloxanthin | Union Glacier, Antarctic | 30 mL YM broth in 300-mL baffled flasks, 150 rpm, 20 °C, 5 days | HPLC | β-carotene and 4-ketotorulene were the main carotenoids | [247] |
| Rhodotorula mucilaginosa | Astaxanthin, β-carotene, and lycopene | Chiloe, 30 km southeast of Puerto Montt, Chile | 4 g yeast/L seaweed (25% v/v), 150 rpm, 25 °C, 6 days | HPLC | 1.84 ± 0.03 mg/L total carotenoids. Carotenoids proportion: 1.8 ± 0.3% astaxanthin, 21.8 ± 1.5% β-carotene, and 38.4 ± 9.4% lycopene | [248] |
| Rhodosporidium babjevae | β-carotene, γ-carotene, torularhodin, and torulene | Grøtsundet, Northern Norway | 10 g/L Difco Marine broth 2216 (10 g/LDifco Bacto-peptone, 10 g/L glucose, 15 g/L NaCl, and 15 g/L agar), 6 °C, 140 h | HPLC | Torularhodin > torulene > β-carotene > γ-carotene | [249] |
| Rhodotorula mucilaginosa | β-carotene, torularhodin, and torulene | Escondido lake, North-western Patagonia, Argentina | 20 mL of minimal medium salt broth (MMS), 25 °C, 250 rpm, 24 h | TLC (for pigment profile), spectrophotometry, and HPLC | 205 ± 15 µg/g total carotenoids, DW. Carotenoid proportions: 10.8% β-carotene, 83.4% torularhodin, and 5.7% torulene | [250] |
| Rhodotorula dairenensis | β-carotene, γ-carotene, torularhodin, and torulene | Freshwater in the middle of the Sagamigawa River, Japan | 20 mL of YPD liquid medium (2% glucose, 2% peptone, and 1% yeast extract), 25 °C, 120 rpm, 36 h | HPLC | 267 µg/g total carotenoids, DW | [225] |
2.5.4. Production Approaches and Production Data in Different Continents
2.5.5. Current and Prospective Applications
2.5.6. Research Needs
3. Roles of Carotenoids in Marine Organisms
3.1. Photosynthesis
3.2. Secondary Antenna in Retinal Proteins
3.3. Oxidative Stress
3.4. Salt Stress
3.5. Low Temperatures
3.6. High Temperatures
3.7. Roles in Marine Animals
4. Carotenoids: Versatile Compounds with Health-Promoting for Foods, Cosmetics and Other Products
5. Potential Health-Promoting Actions of Carotenoids from Aquatic Organisms
6. Advantages and Disadvantages of Using Marine Organisms as a Source of Carotenoids over Chemical Synthesis
7. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Willett, W.; Rockström, J.; Loken, B.; Springmann, M.; Lang, T.; Vermeulen, S.; Garnett, T.; Tilman, D.; DeClerck, F.; Wood, A.; et al. Food in the Anthropocene: The EAT—Lancet Commission on Healthy Diets from Sustainable Food Systems. Lancet 2019, 393, 447–492. [Google Scholar] [CrossRef]
- European Commission. Blue Economy Report 2022; European Union: Brussels, Belgium, 2022. [Google Scholar]
- Meléndez-Martínez, A.J.; Mandić, A.I.; Bantis, F.; Böhm, V.; Borge, G.I.A.; Brnčić, M.; Bysted, A.; Cano, M.P.; Dias, M.G.; Elgersma, A.; et al. A Comprehensive Review on Carotenoids in Foods and Feeds: Status Quo, Applications, Patents, and Research Needs. Crit. Rev. Food Sci. Nutr. 2021, 62, 1999–2049. [Google Scholar] [CrossRef]
- Sosa-Hernández, J.; Romero-Castillo, K.; Parra-Arroyo, L.; Aguilar-Aguila-Isaías, M.; García-Reyes, I.; Ahmed, I.; Parra-Saldivar, R.; Bilal, M.; Iqbal, H. Mexican Microalgae Biodiversity and State-Of-The-Art Extraction Strategies to Meet Sustainable Circular Economy Challenges: High-Value Compounds and Their Applied Perspectives. Mar. Drugs 2019, 17, 174. [Google Scholar] [CrossRef]
- Cotas, J.; Gomes, L.; Pacheco, D.; Pereira, L. Ecosystem Services Provided by Seaweeds. Hydrobiology 2023, 2, 75–96. [Google Scholar] [CrossRef]
- Caporgno, M.P.; Mathys, A. Trends in Microalgae Incorporation Into Innovative Food Products with Potential Health Benefits. Front. Nutr. 2018, 5, 1–10. [Google Scholar] [CrossRef]
- Krause-Jensen, D.; Duarte, C.M. Substantial Role of Macroalgae in Marine Carbon Sequestration. Nat. Geosci. 2016, 9, 737–742. [Google Scholar] [CrossRef]
- Enzing, C.; Ploeg, M.; Barbosa, M.; Sijtsma, L. Microalgae-Based Products for the Food and Feed Sector: An Outlook for Europe; Vigani, M., Parisi, C., Rodriguez Cerezo, E., Eds.; Publications Office of the European Union: Luxembourg, 2014; ISBN 9789279340376.
- Ferdouse, F.; Løvstad Holdt, S.; Smith, R.; Murúa, P.; Yang, L. The Global Status of Seaweed Production. Trade and Utilization, Globefish Research Program. FAO 2018, 124, 1–120. [Google Scholar]
- European Market Observatory for Fisheries and Aquaculture (EUMOFA). Blue Bioeconomy: Situation Report and Perspectives; EUMOFA: Luxembourg, 2018. [Google Scholar]
- Nova, P.; Martins, A.P.; Teixeira, C.; Abreu, H.; Silva, J.G.; Silva, A.M.; Freitas, A.C.; Gomes, A.M. Foods with Microalgae and Seaweeds Fostering Consumers Health: A Review on Scientific and Market Innovations. J. Appl. Phycol. 2020, 32, 1789–1802. [Google Scholar] [CrossRef]
- Venkata Mohan, S.; Nikhil, G.N.; Chiranjeevi, P.; Nagendranatha Reddy, C.; Rohit, M.V.; Kumar, A.N.; Sarkar, O. Waste Biorefinery Models towards Sustainable Circular Bioeconomy: Critical Review and Future Perspectives. Bioresour. Technol. 2016, 215, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Venil, C.K.; Aruldass, C.A.; Dufossé, L.; Zakaria, Z.A.; Ahmad, W.A. Current Perspective on Bacterial Pigments: Emerging Sustainable Compounds with Coloring and Biological Properties for the Industry—An Incisive Evaluation. RSC Adv. 2014, 4, 39523. [Google Scholar] [CrossRef]
- Bourdichon, F.; Casaregola, S.; Farrokh, C.; Frisvad, J.C.; Gerds, M.L.; Hammes, W.P.; Harnett, J.; Huys, G.; Laulund, S.; Ouwehand, A.; et al. Food Fermentations: Microorganisms with Technological Beneficial Use. Int. J. Food Microbiol. 2012, 154, 87–97. [Google Scholar] [CrossRef]
- Gerhauser, C. Impact of Dietary Gut Microbial Metabolites on the Epigenome. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373, 20170359. [Google Scholar] [CrossRef] [PubMed]
- Suez, J.; Zmora, N.; Segal, E.; Elinav, E. The Pros, Cons, and Many Unknowns of Probiotics. Nat. Med. 2019, 25, 716–729. [Google Scholar] [CrossRef] [PubMed]
- Hadrich, D. Microbiome Research Is Becoming the Key to Better Understanding Health and Nutrition. Front. Genet. 2018, 9, 212. [Google Scholar] [CrossRef]
- Nevejan, N.; De Schryver, P.; Wille, M.; Dierckens, K.; Baruah, K.; Van Stappen, G. Bacteria as Food in Aquaculture: Do They Make a Difference? Rev. Aquac. 2018, 10, 180–212. [Google Scholar] [CrossRef]
- Grivard, A.; Goubet, I.; de Souza Duarte Filho, L.M.; Thiéry, V.; Chevalier, S.; de Oliveira-Junior, R.G.; El Aouad, N.; Guedes da Silva Almeida, J.R.; Sitarek, P.; Quintans-Junior, L.J.; et al. Archaea Carotenoids: Natural Pigments with Unexplored Innovative Potential. Mar. Drugs 2022, 20, 524. [Google Scholar] [CrossRef] [PubMed]
- Kutty, S.N.; Philip, R. Marine Yeasts-a Review. Yeast 2008, 25, 465–483. [Google Scholar] [CrossRef] [PubMed]
- Chi, Z.; Liu, G.-L.; Lu, Y.; Jiang, H.; Chi, Z.-M. Bio-Products Produced by Marine Yeasts and Their Potential Applications. Bioresour. Technol. 2016, 202, 244–252. [Google Scholar] [CrossRef]
- Carlozzi, P.; Touloupakis, E.; Di Lorenzo, T.; Giovannelli, A.; Seggiani, M.; Cinelli, P.; Lazzeri, A. Whey and Molasses as Inexpensive Raw Materials for Parallel Production of Biohydrogen and Polyesters via a Two-Stage Bioprocess: New Routes towards a Circular Bioeconomy. J. Biotechnol. 2019, 303, 37–45. [Google Scholar] [CrossRef]
- Gupta, V.; Goel, R. Managing Dissolved Methane Gas in Anaerobic Effluents Using Microbial Resource Management-Based Strategies. Bioresour. Technol. 2019, 289, 121601. [Google Scholar] [CrossRef]
- Sarsaiya, S.; Jain, A.; Kumar Awasthi, S.; Duan, Y.; Kumar Awasthi, M.; Shi, J. Microbial Dynamics for Lignocellulosic Waste Bioconversion and Its Importance with Modern Circular Economy, Challenges and Future Perspectives. Bioresour. Technol. 2019, 291, 121905. [Google Scholar] [CrossRef]
- Tao, Y.; Ersahin, M.E.; Ghasimi, D.S.M.; Ozgun, H.; Wang, H.; Zhang, X.; Guo, M.; Yang, Y.; Stuckey, D.C.; van Lier, J.B. Biogas Productivity of Anaerobic Digestion Process Is Governed by a Core Bacterial Microbiota. Chem. Eng. J. 2020, 380, 122425. [Google Scholar] [CrossRef]
- López, A.R.; Rodríguez, S.B.; Vallejo, R.A.; García, P.G.; Macías-Sánchez, M.D.; Díaz, M.G.; Librán, R.G.; Acero, F.J.F. Sustainable Cultivation of Nannochloropsis gaditana Microalgae in Outdoor Raceways Using Flue Gases for a Complete 2-Year Cycle: A Circular Economy Challenge. J. Appl. Phycol. 2019, 31, 1515–1523. [Google Scholar] [CrossRef]
- Stiles, W.A.V.; Styles, D.; Chapman, S.P.; Esteves, S.; Bywater, A.; Melville, L.; Silkina, A.; Lupatsch, I.; Fuentes Grünewald, C.; Lovitt, R.; et al. Using Microalgae in the Circular Economy to Valorise Anaerobic Digestate: Challenges and Opportunities. Bioresour. Technol. 2018, 267, 732–742. [Google Scholar] [CrossRef]
- Rodríguez, P.D.; Arce Bastias, F.; Arena, A.P. Modeling and Environmental Evaluation of a System Linking a Fishmeal Facility with a Microalgae Plant within a Circular Economy Context. Sustain. Prod. Consum. 2019, 20, 356–364. [Google Scholar] [CrossRef]
- Mata-Gómez, L.C.; Montañez, J.C.; Méndez-Zavala, A.; Aguilar, C.N. Biotechnological Production of Carotenoids by Yeasts: An Overview. Microb. Cell Fact. 2014, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Liu, Y. Adsorption of CO2 from Flue Gas by Novel Seaweed-Based KOH-Activated Porous Biochars. Fuel 2020, 260, 116382. [Google Scholar] [CrossRef]
- Israel, A.; Gavrieli, J.; Glazer, A.; Friedlander, M. Utilization of Flue Gas from a Power Plant for Tank Cultivation of the Red Seaweed Gracilaria cornea. Aquaculture 2005, 249, 311–316. [Google Scholar] [CrossRef]
- Batista, A.P.; Raymundo, A.; Sousa, I.; Empis, J. Rheological Characterization of Coloured Oil-in-Water Food Emulsions with Lutein and Phycocyanin Added to the Oil and Aqueous Phases. Food Hydrocoll. 2006, 20, 44–52. [Google Scholar] [CrossRef]
- Meléndez-Martínez, A.J. An Overview of Carotenoids, Apocarotenoids and Vitamin A in Agro-Food, Nutrition, Health and Disease. Mol. Nutr. Food Res. 2019, 63, 1801045. [Google Scholar] [CrossRef]
- Meléndez-Martínez, A.J.; Stinco, C.M.; Mapelli-Brahm, P. Skin Carotenoids in Public Health and Nutricosmetics: The Emerging Roles and Applications of the UV Radiation-Absorbing Colourless Carotenoids Phytoene and Phytofluene. Nutrients 2019, 11, 1093. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Concepcion, M.; Avalos, J.; Bonet, M.L.; Boronat, A.; Gomez-Gomez, L.; Hornero-Mendez, D.; Carmen Limon, M.; Meléndez-Martínez, A.J.; Olmedilla-Alonso, B.; Palou, A.; et al. A Global Perspective on Carotenoids: Metabolism, Biotechnology, and Benefits for Nutrition and Health. Prog. Lipid Res. 2018, 70, 62–93. [Google Scholar] [CrossRef] [PubMed]
- Stachowiak, B.; Szulc, P. Astaxanthin for the Food Industry. Molecules 2021, 26, 2666. [Google Scholar] [CrossRef] [PubMed]
- Rapoport, A.; Guzhova, I.; Bernetti, L.; Buzzini, P.; Kieliszek, M.; Kot, A.M. Carotenoids and Some Other Pigments from Fungi and Yeasts. Metabolites 2021, 11, 92. [Google Scholar] [CrossRef]
- Woese, C.R.; Kandler, O.; Wheelis, M.L. Towards a Natural System of Organisms: Proposal for the Domains Archaea, Bacteria, and Eucarya. Proc. Natl. Acad. Sci. USA 1990, 87, 4576–4579. [Google Scholar] [CrossRef] [PubMed]
- DeLong, E.F. Exploring Marine Planktonic Archaea: Then and Now. Front. Microbiol. 2021, 11, 616086. [Google Scholar] [CrossRef]
- Heal, K.R.; Qin, W.; Ribalet, F.; Bertagnolli, A.D.; Coyote-Maestas, W.; Hmelo, L.R.; Moffett, J.W.; Devol, A.H.; Armbrust, E.V.; Stahl, D.A.; et al. Two Distinct Pools of B 12 Analogs Reveal Community Interdependencies in the Ocean. Proc. Natl. Acad. Sci. USA 2017, 114, 364–369. [Google Scholar] [CrossRef]
- Santoro, A.E.; Richter, R.A.; Dupont, C.L. Planktonic Marine Archaea. Ann. Rev. Mar. Sci. 2019, 11, 131–158. [Google Scholar] [CrossRef]
- Bomberg, M.; Montonen, L.; Münster, U.; Jurgens, G. Diversity and Function of Archaea in Freshwater Habitats. Curr. Trends Microbiol. 2008, 4, 61–89. [Google Scholar]
- Juottonen, H.; Fontaine, L.; Wurzbacher, C.; Drakare, S.; Peura, S.; Eiler, A. Archaea in Boreal Swedish Lakes Are Diverse, Dominated by Woesearchaeota and Follow Deterministic Community Assembly. Environ. Microbiol. 2020, 22, 3158–3171. [Google Scholar] [CrossRef]
- Chaban, B.; Ng, S.Y.M.; Jarrell, K.F. Archaeal Habitats—From the Extreme to the Ordinary. Can. J. Microbiol. 2006, 52, 73–116. [Google Scholar] [CrossRef]
- Miralles-Robledillo, J.M.; Bernabeu, E.; Giani, M.; Martínez-Serna, E.; Martínez-Espinosa, R.M.; Pire, C. Distribution of Denitrification among Haloarchaea: A Comprehensive Study. Microorganisms 2021, 9, 1669. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, K.; Ergal, İ.; Koller, M.; Basen, M.; Schuster, B.; Rittmann, S.K.-M.R. Archaea Biotechnology. Biotechnol. Adv. 2021, 47, 107668. [Google Scholar] [CrossRef] [PubMed]
- Halorubin. Available online: https://cosmetics.specialchem.com/product/i-adeka-halorubin (accessed on 30 May 2023).
- Abbes, M.; Baati, H.; Guermazi, S.; Messina, C.; Santulli, A.; Gharsallah, N.; Ammar, E. Biological Properties of Carotenoids Extracted from Halobacterium halobium Isolated from a Tunisian Solar Saltern. BMC Complement. Altern. Med. 2013, 13, 255. [Google Scholar] [CrossRef]
- Yatsunami, R.; Ando, A.; Yang, Y.; Takaichi, S.; Kohno, M.; Matsumura, Y.; Ikeda, H.; Fukui, T.; Nakasone, K.; Fujita, N.; et al. Identification of Carotenoids from the Extremely Halophilic Archaeon Haloarcula japonica. Front. Microbiol. 2014, 5, 1–5. [Google Scholar] [CrossRef] [PubMed]
- de la Vega, M.; Sayago, A.; Ariza, J.; Barneto, A.G.; León, R. Characterization of a Bacterioruberin-Producing Haloarchaea Isolated from the Marshlands of the Odiel River in the Southwest of Spain. Biotechnol. Prog. 2016, 32, 592–600. [Google Scholar] [CrossRef]
- Squillaci, G.; Parrella, R.; Carbone, V.; Minasi, P.; La Cara, F.; Morana, A. Carotenoids from the Extreme Halophilic Archaeon Haloterrigena turkmenica: Identification and Antioxidant Activity. Extremophiles 2017, 21, 933–945. [Google Scholar] [CrossRef]
- Sahli, K.; Gomri, M.A.; Esclapez, J.; Gómez-Villegas, P.; Ghennai, O.; Bonete, M.-J.; León, R.; Kharroub, K. Bioprospecting and Characterization of Pigmented Halophilic Archaeal Strains from Algerian Hypersaline Environments with Analysis of Carotenoids Produced by Halorubrum sp. BS2. J. Basic Microbiol. 2020, 60, 624–638. [Google Scholar] [CrossRef]
- Lizama, C.; Romero-Parra, J.; Andrade, D.; Riveros, F.; Bórquez, J.; Ahmed, S.; Venegas-Salas, L.; Cabalín, C.; Simirgiotis, M.J. Analysis of Carotenoids in Haloarchaea Species from Atacama Saline Lakes by High Resolution UHPLC-Q-Orbitrap-Mass Spectrometry: Antioxidant Potential and Biological Effect on Cell Viability. Antioxidants 2021, 10, 1230. [Google Scholar] [CrossRef]
- Giani, M.; Gervasi, L.; Loizzo, M.R.; Martínez-Espinosa, R.M. Carbon Source Influences Antioxidant, Antiglycemic, and Antilipidemic Activities of Haloferax Mediterranei Carotenoid Extracts. Mar. Drugs 2022, 20, 659. [Google Scholar] [CrossRef]
- Yang, Y.; Yatsunami, R.; Ando, A.; Miyoko, N.; Fukui, T.; Takaichi, S.; Nakamura, S. Complete Biosynthetic Pathway of the C 50 Carotenoid Bacterioruberin from Lycopene in the Extremely Halophilic Archaeon Haloarcula japonica. J. Bacteriol. 2015, 197, 1614–1623. [Google Scholar] [CrossRef]
- Alvares, J.J.; Furtado, I.J. Characterization of Multicomponent Antioxidants from Haloferax alexandrinus GUSF-1 (KF796625). 3 Biotech 2021, 11, 1–12. [Google Scholar] [CrossRef]
- Hou, J.; Cui, H.-L. In Vitro Antioxidant, Antihemolytic, and Anticancer Activity of the Carotenoids from Halophilic Archaea. Curr. Microbiol. 2018, 75, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, G.E.; Abu-Serie, M.M.; Abo-Elela, G.M.; Ghozlan, H.; Sabry, S.A.; Soliman, N.A.; Abdel-Fattah, Y.R. In Vitro Dual (Anticancer and Antiviral) Activity of the Carotenoids Produced by Haloalkaliphilic Archaeon Natrialba sp. M6. Sci. Rep. 2020, 10, 5986. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Villegas, P.; Vigara, J.; Vila, M.; Varela, J.; Barreira, L.; Léon, R. Antioxidant, Antimicrobial, and Bioactive Potential of Two New Haloarchaeal Strains Isolated from Odiel Salterns (Southwest Spain). Biology 2020, 9, 298. [Google Scholar] [CrossRef]
- Zalazar, L.; Pagola, P.; Miró, M.V.; Churio, M.S.; Cerletti, M.; Martínez, C.; Iniesta-Cuerda, M.; Soler, A.J.; Cesari, A.; De Castro, R. Bacterioruberin Extracts from a Genetically Modified Hyperpigmented Haloferax volcanii Strain: Antioxidant Activity and Bioactive Properties on Sperm Cells. J. Appl. Microbiol. 2019, 126, 796–810. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, S.E.; Altekar, W.; D’Souza, S.F. Adaptive Response of Haloferax Mediterranei to Low Concentrations of NaCl (<20%) in the Growth Medium. Arch. Microbiol. 1997, 168, 68–71. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.-J.; Ku, K.-L.; Lee, M.-H.; Su, N.-W. Influence of Nutritive Factors on C50 Carotenoids Production by Haloferax Mediterranei ATCC 33500 with Two-Stage Cultivation. Bioresour. Technol. 2010, 101, 6487–6493. [Google Scholar] [CrossRef]
- Calegari-Santos, R.; Diogo, R.A.; Fontana, J.D.; Bonfim, T.M.B. Carotenoid Production by Halophilic Archaea under Different Culture Conditions. Curr. Microbiol. 2016, 72, 641–651. [Google Scholar] [CrossRef]
- Tatiana, A.; Yasynska, O.; Rivas, P.C.; Lilia, E.; Jose, M. Journal of Drug Delivery Science and Technology Improved Stability and Biological Activity of Bacterioruberin in Nanovesicles. J. Drug Deliv. Sci. Technol. 2022, 77, 103896. [Google Scholar] [CrossRef]
- Chaari, M.; Theochari, I.; Papadimitriou, V.; Xenakis, A.; Ammar, E. Encapsulation of Carotenoids Extracted from Halophilic Archaea in Oil-in-Water (O/W) Micro- and Nano-Emulsions. Colloids Surf. B Biointerfaces 2018, 161, 219–227. [Google Scholar] [CrossRef]
- Pagels, F.; Vasconcelos, V.; Guedes, A.C. Carotenoids from Cyanobacteria: Biotechnological Potential and Optimization Strategies. Biomolecules 2021, 11, 735. [Google Scholar] [CrossRef]
- George, D.M.; Vincent, A.S.; Mackey, H.R. An Overview of Anoxygenic Phototrophic Bacteria and Their Applications in Environmental Biotechnology for Sustainable Resource Recovery. Biotechnol. Rep. 2020, 28, e00563. [Google Scholar] [CrossRef]
- Sandmann, G. Diversity and Origin of Carotenoid Biosynthesis: Its History of Coevolution towards Plant Photosynthesis. N. Phytol. 2021, 232, 479–493. [Google Scholar] [CrossRef]
- Béjà, O.; Suzuki, M.T. Photoheterotrophic Marine Prokaryotes. In Microbial Ecology of the Oceans; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008; pp. 131–157. [Google Scholar]
- Imhoff, J. True Marine and Halophilic Anoxygenic Phototrophic Bacteria. Arch. Microbiol. 2001, 176, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Hubas, C.; Jesus, B.; Passarelli, C.; Jeanthon, C. Tools Providing New Insight into Coastal Anoxygenic Purple Bacterial Mats: Review and Perspectives. Res. Microbiol. 2011, 162, 858–868. [Google Scholar] [CrossRef] [PubMed]
- Hess, W.R.; Garczarek, L.; Pfreundt, U.; Partensky, F. Phototrophic Microorganisms: The Basis of the Marine Food Web. In The Marine Microbiome; Springer International Publishing: Berlin, Germany, 2016; pp. 57–97. [Google Scholar]
- Gómez-Consarnau, L.; Akram, N.; Lindell, K.; Pedersen, A.; Neutze, R.; Milton, D.L.; González, J.M.; Pinhassi, J. Proteorhodopsin Phototrophy Promotes Survival of Marine Bacteria during Starvation. PLoS Biol. 2010, 8, e1000358. [Google Scholar] [CrossRef]
- Béjà, O.; Spudich, E.N.; Spudich, J.L.; Leclerc, M.; DeLong, E.F. Proteorhodopsin Phototrophy in the Ocean. Nature 2001, 411, 786–789. [Google Scholar] [CrossRef]
- Armstrong, G.A. Genetics of Eubacterial Carotenoids Biosynthesis: A Colorful Tale. Annu. Rev. Microbiol. 1997, 51, 629–659. [Google Scholar] [CrossRef]
- Stafsnes, M.H.; Josefsen, K.D.; Kildahl-Andersen, G.; Valla, S.; Ellingsen, T.E.; Bruheim, P. Isolation and Characterization of Marine Pigmented Bacteria from Norwegian Coastal Waters and Screening for Carotenoids with UVA-Blue Light Absorbing Properties. J. Microbiol. 2010, 48, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Dieser, M.; Greenwood, M.; Foreman, C.M. Carotenoid Pigmentation in Antarctic Heterotrophic Bacteria as a Strategy to Withstand Environmental Stresses. Arct. Antarct. Alp. Res. 2010, 42, 396–405. [Google Scholar] [CrossRef]
- Ram, S.; Mitra, M.; Shah, F.; Tirkey, S.R.; Mishra, S. Bacteria as an Alternate Biofactory for Carotenoid Production: A Review of Its Applications, Opportunities and Challenges. J. Funct. Foods 2020, 67, 103867. [Google Scholar] [CrossRef]
- Kristjansdottir, T.; Ron, E.Y.C.; Molins-Delgado, D.; Fridjonsson, O.H.; Turner, C.; Bjornsdottir, S.H.; Gudmundsson, S.; van Niel, E.W.J.; Karlsson, E.N.; Hreggvidsson, G.O. Engineering the Carotenoid Biosynthetic Pathway in Rhodothermus marinus for Lycopene Production. Metab. Eng. Commun. 2020, 11, e00140. [Google Scholar] [CrossRef]
- Park, S.; Cho, S.W.; Lee, Y.; Choi, M.; Yang, J.; Lee, H.; Seo, S.W. Engineering Vibrio sp. SP1 for the Production of Carotenoids Directly from Brown Macroalgae. Comput. Struct. Biotechnol. J. 2021, 19, 1531–1540. [Google Scholar] [CrossRef]
- Shindo, K.; Misawa, N. New and Rare Carotenoids Isolated from Marine Bacteria and Their Antioxidant Activities. Mar. Drugs 2014, 12, 1690–1698. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Lee, E.-Y.; Nam, S.-J. Erythrobacter Rubeus sp. Nov., a Carotenoid-Producing Alphaproteobacterium Isolated from Coastal Seawater. Arch. Microbiol. 2022, 204, 125. [Google Scholar] [CrossRef]
- Vila, E.; Hornero-Méndez, D.; Lareo, C.; Saravia, V. Biotechnological Production of Zeaxanthin by an Antarctic Flavobacterium: Evaluation of Culture Conditions. J. Biotechnol. 2020, 319, 54–60. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, C.; Zhang, X.; Tan, K.; Zhang, H.; Cheng, D.; Ye, T.; Li, S.; Ma, H.; Zheng, H. A Novel Carotenoids-Producing Marine Bacterium from Noble Scallop Chlamys nobilis and Antioxidant Activities of Its Carotenoid Compositions. Food Chem. 2020, 320, 126629. [Google Scholar] [CrossRef]
- Niero, H.; da Silva, M.A.C.; de Felicio, R.; Trivella, D.B.B.; Lima, A.O.d.S. Carotenoids Produced by the Deep-Sea Bacterium Erythrobacter citreus LAMA 915: Detection and Proposal of Their Biosynthetic Pathway. Folia Microbiol. 2021, 66, 441–456. [Google Scholar] [CrossRef]
- Setiyono, E.; Heriyanto; Pringgenies, D.; Shioi, Y.; Kanesaki, Y.; Awai, K.; Brotosudarmo, T.H.P. Sulfur-Containing Carotenoids from A Marine Coral Symbiont Erythrobacter flavus Strain KJ5. Mar. Drugs 2019, 17, 349. [Google Scholar] [CrossRef]
- Sayed, A.; Elbalasy, I.; Mohamed, M.S. Novel β-Carotene and Astaxanthin-Producing Marine Planococcus sp.: Insights into Carotenogenesis Regulation and Genetic Aspects. Appl. Biochem. Biotechnol. 2022, 195, 217–235. [Google Scholar] [CrossRef] [PubMed]
- Asker, D. Isolation and Characterization of a Novel, Highly Selective Astaxanthin-Producing Marine Bacterium. J. Agric. Food Chem. 2017, 65, 9101–9109. [Google Scholar] [CrossRef]
- Ide, T.; Hoya, M.; Tanaka, T.; Harayama, S. Enhanced Production of Astaxanthin in Paracoccus sp. Strain N-81106 by Using Random Mutagenesis and Genetic Engineering. Biochem. Eng. J. 2012, 65, 37–43. [Google Scholar] [CrossRef]
- Sowmya, R.; Sachindra, N.M. Biochemical and Molecular Characterization of Carotenogenic Flavobacterial Isolates from Marine Waters. Pol. J. Microbiol. 2016, 65, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Khomlaem, C.; Aloui, H.; Oh, W.-G.; Kim, B.S. High Cell Density Culture of Paracoccus sp. LL1 in Membrane Bioreactor for Enhanced Co-Production of Polyhydroxyalkanoates and Astaxanthin. Int. J. Biol. Macromol. 2021, 192, 289–297. [Google Scholar] [CrossRef]
- Choi, S.S.; Seo, Y.B.; Nam, S.-W.; Kim, G.-D. Enhanced Production of Astaxanthin by Co-Culture of Paracoccus haeundaensis and Lactic Acid Bacteria. Front. Mar. Sci. 2021, 7, 597553. [Google Scholar] [CrossRef]
- Joshi, C.; Singhal, R.S. Modelling and Optimization of Zeaxanthin Production by Paracoccus zeaxanthinifaciens ATCC 21588 Using Hybrid Genetic Algorithm Techniques. Biocatal. Agric. Biotechnol. 2016, 8, 228–235. [Google Scholar] [CrossRef]
- Joshi, C.; Singhal, R.S. Zeaxanthin Production by Paracoccus Zeaxanthinifaciens ATCC 21588 in a Lab-Scale Bubble Column Reactor: Artificial Intelligence Modelling for Determination of Optimal Operational Parameters and Energy Requirements. Korean J. Chem. Eng. 2018, 35, 195–203. [Google Scholar] [CrossRef]
- Takaichi, S.; Mochimaru, M.; Maoka, T.; Katoh, H. Myxol and 4-Ketomyxol 2′-Fucosides, Not Rhamnosides, from Anabaena sp. PCC 7120 and Nostoc Punctiforme PCC 73102, and Proposal for the Biosynthetic Pathway of Carotenoids. Plant Cell Physiol. 2005, 46, 497–504. [Google Scholar] [CrossRef]
- Takaichi, S.; Mochimaru, M.; Maoka, T. Presence of Free Myxol and 4-Hydroxymyxol and Absence of Myxol Glycosides in Anabaena variabilis ATCC 29413, and Proposal of a Biosynthetic Pathway of Carotenoids. Plant Cell Physiol. 2006, 47, 211–216. [Google Scholar] [CrossRef]
- Takaichi, S.; Maoka, T.; Masamoto, K. Myxoxanthophyll in Synechocystis sp. PCC 6803 Is Myxol 2′-Dimethyl-Fucoside, (3R,2′S)-Myxol 2′-(2,4-Di-O-Methyl-α-l-Fucoside), Not Rhamnoside. Plant Cell Physiol. 2001, 42, 756–762. [Google Scholar] [CrossRef] [PubMed]
- Britton, G.; Brown, D.J.; Goodwin, T.W.; Leuenberger, F.J.; Schocher, A.J. The Carotenoids of Flavobacterium Strain R1560. Arch. Microbiol. 1977, 113, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Sowmya, R.; Sachindra, N.M. Carotenoid Production by Formosa sp. KMW, a Marine Bacteria of Flavobacteriaceae Family: Influence of Culture Conditions and Nutrient Composition. Biocatal. Agric. Biotechnol. 2015, 4, 559–567. [Google Scholar] [CrossRef]
- Vila, E.; Hornero-Méndez, D.; Azziz, G.; Lareo, C.; Saravia, V. Carotenoids from Heterotrophic Bacteria Isolated from Fildes Peninsula, King George Island, Antarctica. Biotechnol. Rep. 2019, 21, e00306. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, Y.S.; Choi, T.-J.; Lee, W.J.; Kim, Y.T. Paracoccus Haeundaensis sp. Nov., a Gram-Negative, Halophilic, Astaxanthin-Producing Bacterium. Int. J. Syst. Evol. Microbiol. 2004, 54, 1699–1702. [Google Scholar] [CrossRef]
- Berry, A.; Janssens, D.; Hümbelin, M.; Jore, J.P.M.; Hoste, B.; Cleenwerck, I.; Vancanneyt, M.; Bretzel, W.; Mayer, A.F.; Lopez-Ulibarri, R.; et al. Paracoccus zeaxanthinifaciens sp. Nov., a Zeaxanthin-Producing Bacterium. Int. J. Syst. Evol. Microbiol. 2003, 53, 231–238. [Google Scholar] [CrossRef]
- Takaichi, S.; Mochimaru, M. Carotenoids and Carotenogenesis in Cyanobacteria: Unique Ketocarotenoids and Carotenoid Glycosides. Cell. Mol. Life Sci. 2007, 64, 2607–2619. [Google Scholar] [CrossRef]
- Sarnaik, A.; Nambissan, V.; Pandit, R.; Lali, A. Recombinant Synechococcus elongatus PCC 7942 for Improved Zeaxanthin Production under Natural Light Conditions. Algal Res. 2018, 36, 139–151. [Google Scholar] [CrossRef]
- Shimada, N.; Okuda, Y.; Maeda, K.; Umeno, D.; Takaichi, S.; Ikeuchi, M. Astaxanthin Production in a Model Cyanobacterium Synechocystis sp. PCC 6803. J. Gen. Appl. Microbiol. 2020, 66, 116–120. [Google Scholar] [CrossRef]
- Sandmann, G.; Albrecht, M.; Schnurr, G.; Knörzer, O.; Böger, P. The Biotechnological Potential and Design of Novel Carotenoids by Gene Combination in Escherichia coli. Trends Biotechnol. 1999, 17, 233–237. [Google Scholar] [CrossRef]
- Schmidt-Dannert, C. Engineering Novel Carotenoids in Microorganisms. Curr. Opin. Biotechnol. 2000, 11, 255–261. [Google Scholar] [CrossRef]
- Henke, N.; Heider, S.; Peters-Wendisch, P.; Wendisch, V. Production of the Marine Carotenoid Astaxanthin by Metabolically Engineered Corynebacterium glutamicum. Mar. Drugs 2016, 14, 124. [Google Scholar] [CrossRef] [PubMed]
- Buijs, Y.; Bech, P.K.; Vazquez-Albacete, D.; Bentzon-Tilia, M.; Sonnenschein, E.C.; Gram, L.; Zhang, S.-D. Marine Proteobacteria as a Source of Natural Products: Advances in Molecular Tools and Strategies. Nat. Prod. Rep. 2019, 36, 1333–1350. [Google Scholar] [CrossRef] [PubMed]
- Takemura, M.; Takagi, C.; Aikawa, M.; Araki, K.; Choi, S.-K.; Itaya, M.; Shindo, K.; Misawa, N. Heterologous Production of Novel and Rare C30-Carotenoids Using Planococcus Carotenoid Biosynthesis Genes. Microb. Cell Fact. 2021, 20, 194. [Google Scholar] [CrossRef]
- Bech, P.K.; Lysdal, K.L.; Gram, L.; Bentzon-Tilia, M.; Strube, M.L. Marine Sediments Hold an Untapped Potential for Novel Taxonomic and Bioactive Bacterial Diversity. mSystems 2020, 5, e00782-20. [Google Scholar] [CrossRef]
- Joint, I.; Mühling, M.; Querellou, J. Culturing Marine Bacteria—An Essential Prerequisite for Biodiscovery. Microb. Biotechnol. 2010, 3, 564–575. [Google Scholar] [CrossRef]
- Overmann, J.; Lepleux, C. Marine Bacteria and Archaea: Diversity, Adaptations, and Culturability. In The Marine Microbiome; Springer International Publishing: Berlin, Germany, 2016; pp. 21–55. [Google Scholar]
- Matallana-Surget, S.; Joux, F.; Lebaron, P.; Cavicchioli, R. Isolement et Caractérisation de Bactéries Marines Oligotrophes. J. Soc. Biol. 2007, 201, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Rygaard, A.M.; Thøgersen, M.S.; Nielsen, K.F.; Gram, L.; Bentzon-Tilia, M. Effects of Gelling Agent and Extracellular Signaling Molecules on the Culturability of Marine Bacteria. Appl. Environ. Microbiol. 2017, 83, e00243-17. [Google Scholar] [CrossRef]
- Stewart, E.J. Growing Unculturable Bacteria. J. Bacteriol. 2012, 194, 4151–4160. [Google Scholar] [CrossRef]
- Kato, S.; Yamagishi, A.; Daimon, S.; Kawasaki, K.; Tamaki, H.; Kitagawa, W.; Abe, A.; Tanaka, M.; Sone, T.; Asano, K.; et al. Isolation of Previously Uncultured Slow-Growing Bacteria by Using a Simple Modification in the Preparation of Agar Media. Appl. Environ. Microbiol. 2018, 84, e00807-18. [Google Scholar] [CrossRef]
- Ben-Dov, E.; Kramarsky-Winter, E.; Kushmaro, A. An in Situ Method for Cultivating Microorganisms Using a Double Encapsulation Technique. FEMS Microbiol. Ecol. 2009, 68, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Manca, F.; Mulà, C.; Gustafsson, C.; Mauri, A.; Roslin, T.; Thomas, D.N.; Benedetti-Cecchi, L.; Norkko, A.; Strona, G. Unveiling the Complexity and Ecological Function of Aquatic Macrophyte—Animal Networks in Coastal Ecosystems. Biol. Rev. 2022, 97, 1306–1324. [Google Scholar] [CrossRef] [PubMed]
- Steneck, R.S.; Graham, M.H.; Bourque, B.J.; Corbett, D.; Erlandson, J.M.; Estes, J.A.; Tegner, M.J. Kelp Forest Ecosystems: Biodiversity, Stability, Resilience and Future. Environ. Conserv. 2002, 29, 436–459. [Google Scholar] [CrossRef]
- Singh, C.L.; Huggett, M.J.; Lavery, P.S.; Säwström, C.; Hyndes, G.A. Kelp-Associated Microbes Facilitate Spatial Subsidy in a Detrital-Based Food Web in a Shoreline Ecosystem. Front. Mar. Sci. 2021, 8, 678222. [Google Scholar] [CrossRef]
- Lotze, H.K.; Milewski, I.; Fast, J.; Kay, L.; Worm, B. Ecosystem-Based Management of Seaweed Harvesting. Bot. Mar. 2019, 62, 395–409. [Google Scholar] [CrossRef]
- Tano, S.A.; Eggertsen, M.; Wikström, S.A.; Berkström, C.; Buriyo, A.S.; Halling, C. Tropical Seaweed Beds as Important Habitats for Juvenile Fish. Mar. Freshw. Res. 2017, 68, 1921. [Google Scholar] [CrossRef]
- Yong, W.T.L.; Thien, V.Y.; Rupert, R.; Rodrigues, K.F. Seaweed: A Potential Climate Change Solution. Renew. Sustain. Energy Rev. 2022, 159, 112222. [Google Scholar] [CrossRef]
- Costanza, R.; de Groot, R.; Sutton, P.; van der Ploeg, S.; Anderson, S.J.; Kubiszewski, I.; Farber, S.; Turner, R.K. Changes in the Global Value of Ecosystem Services. Glob. Environ. Chang. 2014, 26, 152–158. [Google Scholar] [CrossRef]
- McGlathery, K.; Sundbäck, K.; Anderson, I. Eutrophication in Shallow Coastal Bays and Lagoons: The Role of Plants in the Coastal Filter. Mar. Ecol. Prog. Ser. 2007, 348, 1–18. [Google Scholar] [CrossRef]
- Watanabe, K.; Yoshida, G.; Hori, M.; Umezawa, Y.; Moki, H.; Kuwae, T. Macroalgal Metabolism and Lateral Carbon Flows Can Create Significant Carbon Sinks. Biogeosciences 2020, 17, 2425–2440. [Google Scholar] [CrossRef]
- Kim, S.-K.; Pangestuti, R. Biological Activities and Potential Health Benefits of Fucoxanthin Derived from Marine Brown Algae. Adv. Food Nutr. Res. 2011, 64, 111–128. [Google Scholar] [CrossRef]
- Larkum, A.W.D.; Kühl, M. Chlorophyll d: The Puzzle Resolved. Trends Plant Sci. 2005, 10, 355–357. [Google Scholar] [CrossRef] [PubMed]
- Clark, M.; Macdiarmid, J.; Jones, A.D.; Ranganathan, J.; Herrero, M.; Fanzo, J. The Role of Healthy Diets in Environmentally Sustainable Food Systems. Food Nutr. Bull. 2020, 41, 31S–58S. [Google Scholar] [CrossRef]
- WHO; FAO. Sustainable Healthy Diets: Guiding Principles; WHO: Geneva, Switzerland, 2019; ISBN 9789241516648.
- Eismann, A.I.; Perpetuo Reis, R.; Ferreira da Silva, A.; Negrão Cavalcanti, D. Ulva spp. Carotenoids: Responses to Environmental Conditions. Algal Res. 2020, 48, 101916. [Google Scholar] [CrossRef]
- Xie, X.; Lu, X.; Wang, L.; He, L.; Wang, G. High Light Intensity Increases the Concentrations of β-Carotene and Zeaxanthin in Marine Red Macroalgae. Algal Res. 2020, 47, 101852. [Google Scholar] [CrossRef]
- Quitério, E.; Grosso, C.; Ferraz, R.; Delerue-Matos, C.; Soares, C. A Critical Comparison of the Advanced Extraction Techniques Applied to Obtain Health-Promoting Compounds from Seaweeds. Mar. Drugs 2022, 20, 677. [Google Scholar] [CrossRef]
- Hasselström, L.; Thomas, J.-B.E. A Critical Review of the Life Cycle Climate Impact in Seaweed Value Chains to Support Carbon Accounting and Blue Carbon Financing. Clean. Environ. Syst. 2022, 6, 100093. [Google Scholar] [CrossRef]
- Koesling, M.; Kvadsheim, N.P.; Halfdanarson, J.; Emblemsvåg, J.; Rebours, C. Environmental Impacts of Protein-Production from Farmed Seaweed: Comparison of Possible Scenarios in Norway. J. Clean. Prod. 2021, 307, 127301. [Google Scholar] [CrossRef]
- Hentati, F.; Tounsi, L.; Djomdi, D.; Pierre, G.; Delattre, C.; Ursu, A.V.; Fendri, I.; Abdelkafi, S.; Michaud, P. Bioactive Polysaccharides from Seaweeds. Molecules 2020, 25, 3152. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Mohamed, A.A.; Mohamed, H.I.; Ramadan, K.M.A.; Barqawi, A.A.; Mansour, A.T. Phytochemical and Potential Properties of Seaweeds and Their Recent Applications: A Review. Mar. Drugs 2022, 20, 342. [Google Scholar] [CrossRef] [PubMed]
- Din, N.A.S.; Mohd Alayudin, ‘A.S.; Sofian-Seng, N.-S.; Rahman, H.A.; Mohd Razali, N.S.; Lim, S.J.; Wan Mustapha, W.A. Brown Algae as Functional Food Source of Fucoxanthin: A Review. Foods 2022, 11, 2235. [Google Scholar] [CrossRef] [PubMed]
- Mikami, K.; Hosokawa, M. Biosynthetic Pathway and Health Benefits of Fucoxanthin, an Algae-Specific Xanthophyll in Brown Seaweeds. Int. J. Mol. Sci. 2013, 14, 13763–13781. [Google Scholar] [CrossRef] [PubMed]
- Zarekarizi, A.; Hoffmann, L.; Burritt, D. Approaches for the Sustainable Production of Fucoxanthin, a Xanthophyll with Potential Health Benefits. J. Appl. Phycol. 2019, 31, 281–299. [Google Scholar] [CrossRef]
- Shen, Y.; Li, J.; Gu, R.; Yue, L.; Wang, H.; Zhan, X.; Xing, B. Carotenoid and Superoxide Dismutase Are the Most Effective Antioxidants Participating in ROS Scavenging in Phenanthrene Accumulated Wheat Leaf. Chemosphere 2018, 197, 513–525. [Google Scholar] [CrossRef] [PubMed]
- Van den Hoek, C.; Mann, D.G.; Jahns, H.M. Algae. An Introduction to Phycology; Guiry, M.D., Ed.; Issue 2; Cambridge University Press: Cambridge, UK, 1995; ISBN 0521304199. [Google Scholar]
- Matsuno, T. Aquatic Animal Carotenoids. Fish. Sci. 2001, 67, 771–783. [Google Scholar] [CrossRef]
- Ren, Y.; Sun, H.; Deng, J.; Huang, J.; Chen, F. Carotenoid Production from Microalgae: Biosynthesis, Salinity Responses and Novel Biotechnologies. Mar. Drugs 2021, 19, 713. [Google Scholar] [CrossRef]
- Fukushima, Y.; Taguchi, C.; Kishimoto, Y.; Kondo, K. Japanese Carotenoid Database with α- and β-Carotene, β-Cryptoxanthin, Lutein, Zeaxanthin, Lycopene, and Fucoxanthin and Intake in Adult Women. Int. J. Vitam. Nutr. Res. 2023, 93, 42–53. [Google Scholar] [CrossRef]
- Nomura, M.; Kamogawa, H.; Susanto, E.; Kawagoe, C.; Yasui, H.; Saga, N.; Hosokawa, M.; Miyashita, K. Seasonal Variations of Total Lipids, Fatty Acid Composition, and Fucoxanthin Contents of Sargassum Horneri (Turner) and Cystoseira Hakodatensis (Yendo) from the Northern Seashore of Japan. J. Appl. Phycol. 2013, 25, 1159–1169. [Google Scholar] [CrossRef]
- Terasaki, M.; Hirose, A.; Narayan, B.; Baba, Y.; Kawagoe, C.; Yasui, H.; Saga, N.; Hosokawa, M.; Miyashita, K. Evaluation of Recoverable Functional Lipid Components of Several Brown Seaweeds (Phaeophyta) from Japan with Special Reference to Fucoxanthin and Fucosterol Content. J. Phycol. 2009, 45, 974–980. [Google Scholar] [CrossRef]
- Bhat, I.; Haripriya, G.; Jogi, N.; Mamatha, B.S. Carotenoid Composition of Locally Found Seaweeds of Dakshina Kannada District in India. Algal Res. 2021, 53, 102154. [Google Scholar] [CrossRef]
- Koizumi, J.; Takatani, N.; Kobayashi, N.; Mikami, K.; Miyashita, K.; Yamano, Y.; Wada, A.; Maoka, T.; Hosokawa, M. Carotenoid Profiling of a Red Seaweed Pyropia yezoensis: Insights into Biosynthetic Pathways in the Order Bangiales. Mar. Drugs 2018, 16, 426. [Google Scholar] [CrossRef] [PubMed]
- Duarte, C.M.; Holmer, M.; Olsen, Y.; Soto, D.; Marbà, N.; Guiu, J.; Black, K.; Karakassis, I. Will the Oceans Help Feed Humanity? Bioscience 2009, 59, 967–976. [Google Scholar] [CrossRef]
- Duarte, C.M.; Bruhn, A.; Krause-Jensen, D. A Seaweed Aquaculture Imperative to Meet Global Sustainability Targets. Nat. Sustain. 2022, 5, 185–193. [Google Scholar] [CrossRef]
- Rebours, C.; Marinho-Soriano, E.; Zertuche-González, J.A.; Hayashi, L.; Vásquez, J.A.; Kradolfer, P.; Soriano, G.; Ugarte, R.; Abreu, M.H.; Bay-Larsen, I.; et al. Seaweeds: An Opportunity for Wealth and Sustainable Livelihood for Coastal Communities. J. Appl. Phycol. 2014, 26, 1939–1951. [Google Scholar] [CrossRef] [PubMed]
- Barbier, M.; Araújo, R.; Rebours, C.; Jacquemin, B.; Holdt, S.L.; Charrier, B. Development and Objectives of the PHYCOMORPH European Guidelines for the Sustainable Aquaculture of Seaweeds (PEGASUS). Bot. Mar. 2020, 63, 5–16. [Google Scholar] [CrossRef]
- Smale, D.A.; Pessarrodona, A.; King, N.; Burrows, M.T.; Yunnie, A.; Vance, T.; Moore, P. Environmental Factors Influencing Primary Productivity of the Forest-Forming Kelp Laminaria Hyperborea in the Northeast Atlantic. Sci. Rep. 2020, 10, 12161. [Google Scholar] [CrossRef]
- White, L.; Loisel, S.; Sevin, L.; Davoult, D. In Situ Estimates of Kelp Forest Productivity in Macro-tidal Environments. Limnol. Oceanogr. 2021, 66, 4227–4239. [Google Scholar] [CrossRef]
- Bae, M.; Kim, M.-B.; Park, Y.-K.; Lee, J.-Y. Health Benefits of Fucoxanthin in the Prevention of Chronic Diseases. Biochim. Biophys. Acta–Mol. Cell Biol. Lipids 2020, 1865, 158618. [Google Scholar] [CrossRef]
- Hosokawa, M. Health-Promoting Functions of the Marine Carotenoid Fucoxanthin. Adv. Exp. Med. Biol. 2021, 1261, 273–284. [Google Scholar] [CrossRef]
- Liu, M.; Li, W.; Chen, Y.; Wan, X.; Wang, J. Fucoxanthin: A Promising Compound for Human Inflammation-Related Diseases. Life Sci. 2020, 255, 117850. [Google Scholar] [CrossRef]
- Olsthoorn, S.E.M.; Wang, X.; Tillema, B.; Vanmierlo, T.; Kraan, S.; Leenen, P.J.M.; Mulder, M.T. Brown Seaweed Food Supplementation: Effects on Allergy and Inflammation and Its Consequences. Nutrients 2021, 13, 2613. [Google Scholar] [CrossRef] [PubMed]
- Kong, Z.-L.; Sudirman, S.; Hsu, Y.-C.; Su, C.-Y.; Kuo, H.-P. Fucoxanthin-Rich Brown Algae Extract Improves Male Reproductive Function on Streptozotocin-Nicotinamide-Induced Diabetic Rat Model. Int. J. Mol. Sci. 2019, 20, 4485. [Google Scholar] [CrossRef]
- Kong, Z.-L.; Kao, N.-J.; Hu, J.-Y.; Wu, C.-S. Fucoxanthin-Rich Brown Algae Extract Decreases Inflammation and Attenuates Colitis-Associated Colon Cancer in Mice. J. Food Nutr. Res. 2016, 4, 137–147. [Google Scholar] [CrossRef]
- Wang, P.-T.; Sudirman, S.; Hsieh, M.-C.; Hu, J.-Y.; Kong, Z.-L. Oral Supplementation of Fucoxanthin-Rich Brown Algae Extract Ameliorates Cisplatin-Induced Testicular Damage in Hamsters. Biomed. Pharmacother. 2020, 125, 109992. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-P.; Tong, Q.-Y.; Zheng, S.-H.; Zhou, M.-D.; Zeng, Y.-M.; Zhou, T.-T. Anti-Inflammatory Effect of Fucoxanthin on Dextran Sulfate Sodium-Induced Colitis in Mice. Nat. Prod. Res. 2020, 34, 1791–1795. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, Y.; Kinoshita, Y.; Usui, M.; Tanaka, R.; Matsushita, T.; Miyata, M. The Suppressive Effect of a Marine Carotenoid, Fucoxanthin, on Mouse Ear Swelling through Regulation of Activities and MRNA Expression of Inflammation-Associated Enzymes. Food Sci. Technol. Res. 2016, 22, 227–234. [Google Scholar] [CrossRef]
- Tan, C.; Hou, Y. First Evidence for the Anti-Inflammatory Activity of Fucoxanthin in High-Fat-Diet-Induced Obesity in Mice and the Antioxidant Functions in PC12 Cells. Inflammation 2014, 37, 443–450. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, G.; Lin, Q.; Tang, Z.; Yan, Q.; Yu, X. Fucoxanthin Prevents Lipopolysaccharide-Induced Depressive-like Behavior in Mice via AMPK- NF-ΚB Pathway. Metab. Brain Dis. 2019, 34, 431–442. [Google Scholar] [CrossRef]
- Miyashita, K.; Hosokawa, M. Fucoxanthin in the Management of Obesity and Its Related Disorders. J. Funct. Foods 2017, 36, 195–202. [Google Scholar] [CrossRef]
- Hitoe, S.; Shimoda, H. Seaweed Fucoxanthin Supplementation Improves Obesity Parameters in Mild Obese Japanese Subjects. Funct. Foods Health Dis. 2017, 7, 246. [Google Scholar] [CrossRef]
- Hu, L.; Chen, W.; Tian, F.; Yuan, C.; Wang, H.; Yue, H. Neuroprotective Role of Fucoxanthin against Cerebral Ischemic/Reperfusion Injury through Activation of Nrf2/HO-1 Signaling. Biomed. Pharmacother. 2018, 106, 1484–1489. [Google Scholar] [CrossRef]
- Sun, G.; Xin, T.; Zhang, R.; Liu, C.; Pang, Q. Fucoxanthin Attenuates Behavior Deficits and Neuroinflammatory Response in 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine-Induced Parkinson’s Disease in Mice. Pharmacogn. Mag. 2020, 16, 51. [Google Scholar] [CrossRef]
- Aziz, E.; Batool, R.; Khan, M.U.; Rauf, A.; Akhtar, W.; Heydari, M.; Rehman, S.; Shahzad, T.; Malik, A.; Mosavat, S.H.; et al. An Overview on Red Algae Bioactive Compounds and Their Pharmaceutical Applications. J. Complement. Integr. Med. 2020, 17. [Google Scholar] [CrossRef]
- Ali, I.; Manzoor, Z.; Koo, J.-E.; Kim, J.-E.; Byeon, S.-H.; Yoo, E.-S.; Kang, H.-K.; Hyun, J.-W.; Lee, N.-H.; Koh, Y.-S. 3-Hydroxy-4,7-Megastigmadien-9-One, Isolated from Ulva Pertusa, Attenuates TLR9-Mediated Inflammatory Response by down-Regulating Mitogen-Activated Protein Kinase and NF-ΚB Pathways. Pharm. Biol. 2017, 55, 435–440. [Google Scholar] [CrossRef]
- Young Park, S.; Su Seo, I.; Joo Lee, S.; Pyung Lee, S. Study on the Health Benefits of Brown Algae (Sargassum muticum) in Volunteers. J. Food Nutr. Res. 2015, 3, 126–130. [Google Scholar] [CrossRef]
- Shih, P.-H.; Shiue, S.-J.; Chen, C.-N.; Cheng, S.-W.; Lin, H.-Y.; Wu, L.-W.; Wu, M.-S. Fucoidan and Fucoxanthin Attenuate Hepatic Steatosis and Inflammation of NAFLD through Modulation of Leptin/Adiponectin Axis. Mar. Drugs 2021, 19, 148. [Google Scholar] [CrossRef] [PubMed]
- Asai, A.; Sugawara, T.; Ono, H.; Nagao, A. Biotransformation of Fucoxanthinol into Amarouciaxanthin A in Mice and HepG2 Cells: Formation and Cytotoxicity of Fucoxanthin Metabolites. Drug Metab. Dispos. 2004, 32, 205–211. [Google Scholar] [CrossRef]
- Hashimoto, T.; Ozaki, Y.; Taminato, M.; Das, S.K.; Mizuno, M.; Yoshimura, K.; Maoka, T.; Kanazawa, K. The Distribution and Accumulation of Fucoxanthin and Its Metabolites after Oral Administration in Mice. Br. J. Nutr. 2009, 102, 242–248. [Google Scholar] [CrossRef]
- Hashimoto, T.; Ozaki, Y.; Mizuno, M.; Yoshida, M.; Nishitani, Y.; Azuma, T.; Komoto, A.; Maoka, T.; Tanino, Y.; Kanazawa, K. Pharmacokinetics of Fucoxanthinol in Human Plasma after the Oral Administration of Kombu Extract. Br. J. Nutr. 2012, 107, 1566–1569. [Google Scholar] [CrossRef] [PubMed]
- Mikami, N. A Protocol for Human Serum Fucoxanthinol Quantitation Using LC-MS/MS System. J. Nutr. Med. Diet Care 2016, 2, 019. [Google Scholar] [CrossRef]
- Novotny, J.A.; Kurilich, A.C.; Britz, S.J.; Clevidence, B.A. Plasma Appearance of Labeled β-Carotene, Lutein, and Retinol in Humans after Consumption of Isotopically Labeled Kale. J. Lipid Res. 2005, 46, 1896–1903. [Google Scholar] [CrossRef]
- Mohibbullah, M.; Haque, M.N.; Sohag, A.A.M.; Hossain, M.T.; Zahan, M.S.; Uddin, M.J.; Hannan, M.A.; Moon, I.S.; Choi, J.-S. A Systematic Review on Marine Algae-Derived Fucoxanthin: An Update of Pharmacological Insights. Mar. Drugs 2022, 20, 279. [Google Scholar] [CrossRef] [PubMed]
- Irisson, J.-O.; Ayata, S.-D.; Lindsay, D.J.; Karp-Boss, L.; Stemmann, L. Machine Learning for the Study of Plankton and Marine Snow from Images. Ann. Rev. Mar. Sci. 2022, 14, 277–301. [Google Scholar] [CrossRef]
- Fernández, F.G.A.; Reis, A.; Wijffels, R.H.; Barbosa, M.; Verdelho, V.; Llamas, B. The Role of Microalgae in the Bioeconomy. N. Biotechnol. 2021, 61, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Fabris, M.; Abbriano, R.M.; Pernice, M.; Sutherland, D.L.; Commault, A.S.; Hall, C.C.; Labeeuw, L.; McCauley, J.I.; Kuzhiuparambil, U.; Ray, P.; et al. Emerging Technologies in Algal Biotechnology: Toward the Establishment of a Sustainable, Algae-Based Bioeconomy. Front. Plant Sci. 2020, 11, 279. [Google Scholar] [CrossRef] [PubMed]
- Adarme-Vega, T.C.; Lim, D.K.Y.; Timmins, M.; Vernen, F.; Li, Y.; Schenk, P.M. Microalgal Biofactories: A Promising Approach towards Sustainable Omega-3 Fatty Acid Production. Microb. Cell Fact. 2012, 11, 96. [Google Scholar] [CrossRef]
- Russell, C.; Rodriguez, C.; Yaseen, M. High-Value Biochemical Products & Applications of Freshwater Eukaryotic Microalgae. Sci. Total Environ. 2022, 809, 151111. [Google Scholar] [CrossRef]
- López-Sánchez, A.; Silva-Gálvez, A.L.; Aguilar-Juárez, Ó.; Senés-Guerrero, C.; Orozco-Nunnelly, D.A.; Carrillo-Nieves, D.; Gradilla-Hernández, M.S. Microalgae-Based Livestock Wastewater Treatment (MbWT) as a Circular Bioeconomy Approach: Enhancement of Biomass Productivity, Pollutant Removal and High-Value Compound Production. J. Environ. Manag. 2022, 308, 114612. [Google Scholar] [CrossRef]
- Srimongkol, P.; Sangtanoo, P.; Songserm, P.; Watsuntorn, W.; Karnchanatat, A. Microalgae-Based Wastewater Treatment for Developing Economic and Environmental Sustainability: Current Status and Future Prospects. Front. Bioeng. Biotechnol. 2022, 10, 904046. [Google Scholar] [CrossRef]
- Verma, R.; Suthar, S.; Chand, N.; Mutiyar, P.K. Phycoremediation of Milk Processing Wastewater and Lipid-Rich Biomass Production Using Chlorella vulgaris under Continuous Batch System. Sci. Total Environ. 2022, 833, 155110. [Google Scholar] [CrossRef]
- Iliopoulou, A.; Zkeri, E.; Panara, A.; Dasenaki, M.; Fountoulakis, M.S.; Thomaidis, N.S.; Stasinakis, A.S. Treatment of Different Dairy Wastewater with Chlorella sorokiniana: Removal of Pollutants and Biomass Characterization. J. Chem. Technol. Biotechnol. 2022, 97, 3193–3201. [Google Scholar] [CrossRef]
- León-Vaz, A.; León, R.; Díaz-Santos, E.; Vigara, J.; Raposo, S. Using Agro-Industrial Wastes for Mixotrophic Growth and Lipids Production by the Green Microalga Chlorella sorokiniana. N. Biotechnol. 2019, 51, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Li, X.; Zhang, Q.; Dong, S.; Liu, H.; Liu, Y.; Chaves, A.V.; Ralph, P.J.; Ruan, R.; Wang, Q. Ecotoxicological Response of Spirulina Platensis to Coexisted Copper and Zinc in Anaerobic Digestion Effluent. Sci. Total Environ. 2022, 837, 155874. [Google Scholar] [CrossRef] [PubMed]
- Han, T.; Lu, H.; Zhao, Y.; Xu, H.; Zhang, Y.; Li, B. Two-Step Strategy for Obtaining Dunaliella sp. Biomass and β-Carotene from Anaerobically Digested Poultry Litter Wastewater. Int. Biodeterior. Biodegradation 2019, 143, 104714. [Google Scholar] [CrossRef]
- Shah, M.M.R. Astaxanthin Production by Microalgae Haematococcus pluvialis through Wastewater Treatment: Waste to Resource. In Application of Microalgae in Wastewater Treatment; Springer International Publishing: Berlin, Germany, 2019; pp. 17–39. [Google Scholar]
- Sathasivam, R.; Radhakrishnan, R.; Hashem, A.; Abd_Allah, E.F. Microalgae Metabolites: A Rich Source for Food and Medicine. Saudi J. Biol. Sci. 2019, 26, 709–722. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.K.; Tambat, V.S.; Chen, C.-W.; Chauhan, A.S.; Kumar, P.; Vadrale, A.P.; Huang, C.-Y.; Dong, C.-D.; Singhania, R.R. Recent Advancements in Astaxanthin Production from Microalgae: A Review. Bioresour. Technol. 2022, 364, 128030. [Google Scholar] [CrossRef]
- Muhammad, G.; Butler, T.O.; Chen, B.; Lv, Y.; Xiong, W.; Zhao, X.; Solovchenko, A.E.; Zhao, A.; Mofijur, M.; Xu, J.; et al. Sustainable Production of Lutein-an Underexplored Commercially Relevant Pigment from Microalgae. Biomass Convers. Biorefinery 2022, 1–22. [Google Scholar] [CrossRef]
- Gong, M.; Bassi, A. Investigation of Chlorella Vulgaris UTEX 265 Cultivation under Light and Low Temperature Stressed Conditions for Lutein Production in Flasks and the Coiled Tree Photo-Bioreactor (CTPBR). Appl. Biochem. Biotechnol. 2017, 183, 652–671. [Google Scholar] [CrossRef]
- Safafar, H.; Uldall Nørregaard, P.; Ljubic, A.; Møller, P.; Løvstad Holdt, S.; Jacobsen, C. Enhancement of Protein and Pigment Content in Two Chlorella Species Cultivated on Industrial Process Water. J. Mar. Sci. Eng. 2016, 4, 84. [Google Scholar] [CrossRef]
- Sun, H.; Yang, S.; Zhao, W.; Kong, Q.; Zhu, C.; Fu, X.; Zhang, F.; Liu, Z.; Zhan, Y.; Mou, H.; et al. Fucoxanthin from Marine Microalgae: A Promising Bioactive Compound for Industrial Production and Food Application. Crit. Rev. Food Sci. Nutr. 2022, 1–17. [Google Scholar] [CrossRef]
- Damergi, E.; Schwitzguébel, J.-P.; Refardt, D.; Sharma, S.; Holliger, C.; Ludwig, C. Extraction of Carotenoids from Chlorella vulgaris Using Green Solvents and Syngas Production from Residual Biomass. Algal Res. 2017, 25, 488–495. [Google Scholar] [CrossRef]
- Li, Y.; Sun, H.; Wu, T.; Fu, Y.; He, Y.; Mao, X.; Chen, F. Storage Carbon Metabolism of Isochrysis Zhangjiangensis under Different Light Intensities and Its Application for Co-Production of Fucoxanthin and Stearidonic Acid. Bioresour. Technol. 2019, 282, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Martínez, J.M.; Gojkovic, Z.; Ferro, L.; Maza, M.; Álvarez, I.; Raso, J.; Funk, C. Use of Pulsed Electric Field Permeabilization to Extract Astaxanthin from the Nordic Microalga Haematococcus pluvialis. Bioresour. Technol. 2019, 289, 121694. [Google Scholar] [CrossRef] [PubMed]
- Safafar, H.; Van Wagenen, J.; Møller, P.; Jacobsen, C. Carotenoids, Phenolic Compounds and Tocopherols Contribute to the Antioxidative Properties of Some Microalgae Species Grown on Industrial Wastewater. Mar. Drugs 2015, 13, 7339–7356. [Google Scholar] [CrossRef] [PubMed]
- León-Vaz, A.; León, R.; Vigara, J.; Funk, C. Exploring Nordic Microalgae as a Potential Novel Source of Antioxidant and Bioactive Compounds. N. Biotechnol. 2023, 73, 1–8. [Google Scholar] [CrossRef]
- Yusof, Z.; Khong, N.M.H.; Choo, W.S.; Foo, S.C. Opportunities for the Marine Carotenoid Value Chain from the Perspective of Fucoxanthin Degradation. Food Chem. 2022, 383, 132394. [Google Scholar] [CrossRef]
- Loke Show, P. Global Market and Economic Analysis of Microalgae Technology: Status and Perspectives. Bioresour. Technol. 2022, 357, 127329. [Google Scholar] [CrossRef]
- Jannel, S.; Caro, Y.; Bermudes, M.; Petit, T. Novel Insights into the Biotechnological Production of Haematococcus pluvialis-Derived Astaxanthin: Advances and Key Challenges to Allow Its Industrial Use as Novel Food Ingredient. J. Mar. Sci. Eng. 2020, 8, 789. [Google Scholar] [CrossRef]
- Capelli, B.; Bagchi, D.; Cysewski, G.R. Synthetic Astaxanthin Is Significantly Inferior to Algal-Based Astaxanthin as an Antioxidant and May Not Be Suitable as a Human Nutraceutical Supplement. Nutrafoods 2013, 12, 145–152. [Google Scholar] [CrossRef]
- Singh, R.V.; Sambyal, K. An Overview of β-Carotene Production: Current Status and Future Prospects. Food Biosci. 2022, 47, 101717. [Google Scholar] [CrossRef]
- Mohebi Najafabadi, M.; Naeimpoor, F. Boosting β-Carotene and Storage Materials Productivities by Two-Stage Mixed and Monochromatic Exposure Stresses on Dunaliella salina. Int. J. Phytoremediation 2022, 25, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Prieto, A.; Pedro Cañavate, J.; García-González, M. Assessment of Carotenoid Production by Dunaliella salina in Different Culture Systems and Operation Regimes. J. Biotechnol. 2011, 151, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Calijuri, M.L.; Silva, T.A.; Magalhães, I.B.; de Paula Pereira, A.S.A.; Marangon, B.B.; de Assis, L.R.; Lorentz, J.F. Bioproducts from Microalgae Biomass: Technology, Sustainability, Challenges and Opportunities. Chemosphere 2022, 305, 135508. [Google Scholar] [CrossRef]
- Poojary, M.; Barba, F.; Aliakbarian, B.; Donsì, F.; Pataro, G.; Dias, D.; Juliano, P. Innovative Alternative Technologies to Extract Carotenoids from Microalgae and Seaweeds. Mar. Drugs 2016, 14, 214. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Morón-Ortiz, Á.; Zhou, J.; Benítez-González, A.; Mapelli-Brahm, P.; Meléndez-Martínez, A.J.; Barba, F.J. Effects of Pressurized Liquid Extraction with Dimethyl Sulfoxide on the Recovery of Carotenoids and Other Dietary Valuable Compounds from the Microalgae Spirulina, chlorella and Phaeodactylum tricornutum. Food Chem. 2022, 405, 134885. [Google Scholar] [CrossRef]
- Monapathi, M.E.; Bezuidenhout, C.C.; James Rhode, O.H. Aquatic Yeasts: Diversity, Characteristics and Potential Health Implications. J. Water Health 2020, 18, 91–105. [Google Scholar] [CrossRef] [PubMed]
- Nagahama, T. Yeast Biodiversity in Freshwater, Marine and Deep-Sea Environments BT–Biodiversity and Ecophysiology of Yeasts. In Biodiversity and Ecophysiology of Yeasts; Péter, G., Rosa, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 241–262. ISBN 978-3-540-30985-7. [Google Scholar]
- Garmendia, G.; Alvarez, A.; Villarreal, R.; Martínez-Silveira, A.; Wisniewski, M.; Vero, S. Fungal Diversity in the Coastal Waters of King George Island (Maritime Antarctica). World J. Microbiol. Biotechnol. 2021, 37, 142. [Google Scholar] [CrossRef]
- Vero, S.; Garmendia, G.; Martínez-Silveira, A.; Cavello, I.; Wisniewski, M. Yeast Activities Involved in Carbon and Nitrogen Cycles in Antarctica. In The Ecological Role of Micro-Organisms in the Antarctic Environment; Castro-Sowinski, S., Ed.; Springer Polar Sciences: Berlin/Heidelberg, Germany, 2019; pp. 45–64. ISBN 978-3-030-02785-8. [Google Scholar]
- Jafari, N.; Soudi, M.R.; Kasra-Kermanshahi, R. Biodegradation Perspectives of Azo Dyes by Yeasts. Microbiology 2014, 83, 484–497. [Google Scholar] [CrossRef]
- Ruscasso, F.; Cavello, I.; Butler, M.; Loveira, E.L.; Curutchet, G.; Cavalitto, S. Biodegradation and Detoxification of Reactive Orange 16 by Candida Sake 41E. Bioresour. Technol. Rep. 2021, 15, 100726. [Google Scholar] [CrossRef]
- Segal-Kischinevzky, C.; Romero-Aguilar, L.; Alcaraz, L.D.; López-Ortiz, G.; Martínez-Castillo, B.; Torres-Ramírez, N.; Sandoval, G.; González, J. Yeasts Inhabiting Extreme Environments and Their Biotechnological Applications. Microorganisms 2022, 10, 794. [Google Scholar] [CrossRef]
- Libkind, D.; Moliné, M.; Sampaio, J.P.; Van Broock, M. Yeasts from High-Altitude Lakes: Influence of UV Radiation. FEMS Microbiol. Ecol. 2009, 69, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Moliné, M.; Flores, M.R.; Libkind, D.; del Carmen Carmen Diéguez, M.; Farías, M.E.; van Broock, M. Photoprotection by Carotenoid Pigments in the Yeast Rhodotorula mucilaginosa: The Role of Torularhodin. Photochem. Photobiol. Sci. 2010, 9, 1145–1151. [Google Scholar] [CrossRef]
- Ueno, R.; Hamada-Sato, N.; Ishida, M.; Urano, N. Potential of Carotenoids in Aquatic Yeasts as a Phylogenetically Reliable Marker and Natural Colorant for Aquaculture. Biosci. Biotechnol. Biochem. 2011, 75, 1654–1661. [Google Scholar] [CrossRef]
- Mata-Gómez, L.C.; Mapelli-Brahm, P.; Meléndez-Martínez, A.J.; Méndez-Zavala, A.; Morales-Oyervides, L.; Montañez, J. Microbial Carotenoid Synthesis Optimization in Goat Cheese Whey Using the Robust Taguchi Method: A Sustainable Approach to Help Tackle Vitamin A Deficiency. Foods 2023, 12, 658. [Google Scholar] [CrossRef]
- Hernández-Almanza, A.; Montañez, J.; Martínez, G.; Aguilar-Jiménez, A.; Contreras-Esquivel, J.C.; Aguilar, C.N. Lycopene: Progress in Microbial Production. Trends Food Sci. Technol. 2016, 56, 142–148. [Google Scholar] [CrossRef]
- Cardoso, L.A.C.; Jäckel, S.; Karp, S.G.; Framboisier, X.; Chevalot, I.; Marc, I. Improvement of Sporobolomyces ruberrimus Carotenoids Production by the Use of Raw Glycerol. Bioresour. Technol. 2016, 200, 374–379. [Google Scholar] [CrossRef]
- Ambati, R.; Phang, S.-M.; Ravi, S.; Aswathanarayana, R. Astaxanthin: Sources, Extraction, Stability, Biological Activities and Its Commercial Applications—A Review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef] [PubMed]
- Kurtzman, C.P.; Mateo, R.Q.; Kolecka, A.; Theelen, B.; Robert, V.; Boekhout, T. Advances in Yeast Systematics and Phylogeny and Their Use as Predictors of Biotechnologically Important Metabolic Pathways. FEMS Yeast Res. 2015, 15, fov050. [Google Scholar] [CrossRef] [PubMed]
- Libkind, D.; Moliné, M.; Colabella, F. Isolation and Selection of New Astaxanthin-Producing Strains of Phaffia rhodozyma. In Methods in Molecular Biology; Springer: Berlin/Heidelberg, Germany, 2018; pp. 297–310. [Google Scholar]
- Buzzini, P.; Innocenti, M.; Turchetti, B.; Libkind, D.; van Broock, M.; Mulinacci, N. Carotenoid Profiles of Yeasts Belonging to the Genera Rhodotorula, Rhodosporidium, Sporobolomyces, and Sporidiobolus. Can. J. Microbiol. 2007, 53, 1024–1031. [Google Scholar] [CrossRef]
- Brandão, L.R.; Libkind, D.; Vaz, A.B.M.; Espírito Santo, L.C.; Moliné, M.; de García, V.; van Broock, M.; Rosa, C.A. Yeasts from an Oligotrophic Lake in Patagonia (Argentina): Diversity, Distribution and Synthesis of Photoprotective Compounds and Extracellular Enzymes. FEMS Microbiol. Ecol. 2011, 76, 1–13. [Google Scholar] [CrossRef]
- Pino-Maureira, N.L.; González-Saldía, R.R.; Capdeville, A.; Srain, B. Rhodotorula Strains Isolated from Seawater That Can Biotransform Raw Glycerol into Docosahexaenoic Acid (DHA) and Carotenoids for Animal Nutrition. Appl. Sci. 2021, 11, 2824. [Google Scholar] [CrossRef]
- Mannazzu, I.; Landolfo, S.; da Silva, T.L.; Buzzini, P. Red Yeasts and Carotenoid Production: Outlining a Future for Non-Conventional Yeasts of Biotechnological Interest. World J. Microbiol. Biotechnol. 2015, 31, 1665–1673. [Google Scholar] [CrossRef] [PubMed]
- Fotedar, R.; Chatting, M.; Kolecka, A.; Zeyara, A.; Al Malki, A.; Kaul, R.; Bukhari, S.J.; Moaiti, M.A.; Febbo, E.J.; Boekhout, T.; et al. Communities of Culturable Yeasts and Yeast-like Fungi in Oligotrophic Hypersaline Coastal Waters of the Arabian Gulf Surrounding Qatar. Antonie Van Leeuwenhoek 2022, 115, 609–633. [Google Scholar] [CrossRef] [PubMed]
- Moliné, M.; Libkind, D.; de Garcia, V.; Giraudo, M.R. Production of Pigments and Photo-Protective Compounds by Cold-Adapted Yeasts. In Cold-Adapted Yeasts; Springer: Berlin/Heidelberg, Germany, 2014; pp. 193–224. [Google Scholar]
- Herz, S.; Weber, R.W.S.; Anke, H.; Mucci, A.; Davoli, P. Intermediates in the Oxidative Pathway from Torulene to Torularhodin in the Red Yeasts Cystofilobasidium infirmominiatum and C. Capitatum (Heterobasidiomycetes, Fungi). Phytochemistry 2007, 68, 2503–2511. [Google Scholar] [CrossRef] [PubMed]
- Madhour, A.; Anke, H.; Mucci, A.; Davoli, P.; Weber, R.W.S. Biosynthesis of the Xanthophyll Plectaniaxanthin as a Stress Response in the Red Yeast Dioszegia (Tremellales, Heterobasidiomycetes, Fungi). Phytochemistry 2005, 66, 2617–2626. [Google Scholar] [CrossRef]
- Kot, A.M.; Błażejak, S.; Gientka, I.; Kieliszek, M.; Bryś, J. Torulene and Torularhodin: “New” Fungal Carotenoids for Industry? Microb. Cell Fact. 2018, 17, 49. [Google Scholar] [CrossRef]
- Moliné, M.; Libkind, D.; van Broock, M. Production of Torularhodin, Torulene, and β-Carotene by Rhodotorula Yeasts. In Methods Molecular Biology; Humana Press: Totowa, NJ, USA, 2012; pp. 275–283. [Google Scholar]
- Manimala, M.R.A.; Murugesan, R. In Vitro Antioxidant and Antimicrobial Activity of Carotenoid Pigment Extracted from Sporobolomyces sp. Isolated from Natural Source. J. Appl. Nat. Sci. 2014, 6, 649–653. [Google Scholar] [CrossRef]
- Ungureanu, C.; Ferdes, M. Evaluation of Antioxidant and Antimicrobial Activities of Torularhodin. Adv. Sci. Lett. 2012, 18, 50–53. [Google Scholar] [CrossRef]
- Ungureanu, C.; Popescu, S.; Purcel, G.; Tofan, V.; Popescu, M.; Sălăgeanu, A.; Pîrvu, C. Improved Antibacterial Behavior of Titanium Surface with Torularhodin–Polypyrrole Film. Mater. Sci. Eng. C 2014, 42, 726–733. [Google Scholar] [CrossRef]
- Ungureanu, C.; Dumitriu, C.; Popescu, S.; Enculescu, M.; Tofan, V.; Popescu, M.; Pirvu, C. Enhancing Antimicrobial Activity of TiO2/Ti by Torularhodin Bioinspired Surface Modification. Bioelectrochemistry 2016, 107, 14–24. [Google Scholar] [CrossRef]
- Du, C.; Li, Y.; Guo, Y.; Han, M.; Zhang, W.; Qian, H. The Suppression of Torulene and Torularhodin Treatment on the Growth of PC-3 Xenograft Prostate Tumors. Biochem. Biophys. Res. Commun. 2016, 469, 1146–1152. [Google Scholar] [CrossRef]
- Barahona, S.; Yuivar, Y.; Socias, G.; Alcaíno, J.; Cifuentes, V.; Baeza, M. Identification and Characterization of Yeasts Isolated from Sedimentary Rocks of Union Glacier at the Antarctica. Extremophiles 2016, 20, 479–491. [Google Scholar] [CrossRef] [PubMed]
- Leyton, A.; Flores, L.; Mäki-Arvela, P.; Lienqueo, M.E.; Shene, C. Macrocystis pyrifera Source of Nutrients for the Production of Carotenoids by a Marine Yeast Rhodotorula mucilaginosa. J. Appl. Microbiol. 2019, 127, 1069–1079. [Google Scholar] [CrossRef] [PubMed]
- Sperstad, S.; Lutnæs, B.F.; Stormo, S.K.; Liaaen-Jensen, S.; Landfald, B. Torularhodin and Torulene Are the Major Contributors to the Carotenoid Pool of Marine Rhodosporidium babjevae (Golubev). J. Ind. Microbiol. Biotechnol. 2006, 33, 269–273. [Google Scholar] [CrossRef]
- Libkind, D.; Van Broock, M. Biomass and Carotenoid Pigment Production by Patagonian Native Yeasts. World J. Microbiol. Biotechnol. 2006, 22, 687–692. [Google Scholar] [CrossRef]
- Allahkarami, S.; Sepahi, A.A.; Hosseini, H.; Razavi, M.R. Isolation and Identification of Carotenoid-Producing Rhodotorula sp. from Pinaceae Forest Ecosystems and Optimization of in Vitro Carotenoid Production. Biotechnol. Rep. 2021, 32, e00687. [Google Scholar] [CrossRef] [PubMed]
- Elfeky, N.; Elmahmoudy, M.; Bao, Y. Manipulation of Culture Conditions: Tool for Correlating/Improving Lipid and Carotenoid Production by Rhodotorula glutinis. Processes 2020, 8, 140. [Google Scholar] [CrossRef]
- Aksu, Z.; Eren, A.T. Production of Carotenoids by the Isolated Yeast of Rhodotorula glutinis. Biochem. Eng. J. 2007, 35, 107–113. [Google Scholar] [CrossRef]
- Cipolatti, E.P.; Remedi, R.D.; dos Santos Sá, C.; Rodrigues, A.B.; Gonçalves Ramos, J.M.; Veiga Burkert, C.A.; Furlong, E.B.; Fernandes de Medeiros Burkert, J. Use of Agroindustrial Byproducts as Substrate for Production of Carotenoids with Antioxidant Potential by Wild Yeasts. Biocatal. Agric. Biotechnol. 2019, 20, 101208. [Google Scholar] [CrossRef]
- de Carvalho, J.C.; Goyzueta-Mamani, L.D.; Molina-Aulestia, D.T.; Magalhães Júnior, A.I.; Iwamoto, H.; Ambati, R.; Ravishankar, G.A.; Soccol, C.R. Microbial Astaxanthin Production from Agro-Industrial Wastes-Raw Materials, Processes, and Quality. Fermentation 2022, 8, 484. [Google Scholar] [CrossRef]
- Igreja, W.S.; de Andrade Maia, F.; Lopes, A.S.; Chisté, R.C. Biotechnological Production of Carotenoids Using Low Cost-Substrates Is Influenced by Cultivation Parameters: A Review. Int. J. Mol. Sci. 2021, 22, 8819. [Google Scholar] [CrossRef] [PubMed]
- Braunwald, T.; Schwemmlein, L.; Graeff-Hönninger, S.; French, W.T.; Hernandez, R.; Holmes, W.E.; Claupein, W. Effect of Different C/N Ratios on Carotenoid and Lipid Production by Rhodotorula glutinis. Appl. Microbiol. Biotechnol. 2013, 97, 6581–6588. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, J.; Obledo, N.; Arellano, M.; Herrera, E. Astaxanthin Production by Phaffia Rhodozyma in a Fedbatch Culture Using a Low Cost Medium Feeding; Springer: Guadalajara, México, 2006; Volume 4. [Google Scholar]
- Tkáčová, J.; Čaplová, J.; Klempová, T.; Čertík, M. Correlation between Lipid and Carotenoid Synthesis in Torularhodin-Producing Rhodotorula glutinis. Ann. Microbiol. 2017, 67, 541–551. [Google Scholar] [CrossRef]
- Elfeky, N.; Elmahmoudy, M.; Zhang, Y.; Guo, J.; Bao, Y. Lipid and Carotenoid Production by Rhodotorula glutinis with a Combined Cultivation Mode of Nitrogen, Sulfur, and Aluminium Stress. Appl. Sci. 2019, 9, 2444. [Google Scholar] [CrossRef]
- Frengova, G.I.; Beshkova, D.M. Carotenoids from Rhodotorula and Phaffia: Yeasts of Biotechnological Importance. J. Ind. Microbiol. Biotechnol. 2009, 36, 163–180. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, X.; Tan, T. Lipid and Carotenoid Production by Rhodotorula glutinis under Irradiation/High-Temperature and Dark/Low-Temperature Cultivation. Bioresour. Technol. 2014, 157, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Pham, K.D.; Shida, Y.; Miyata, A.; Takamizawa, T.; Suzuki, Y.; Ara, S.; Yamazaki, H.; Masaki, K.; Mori, K.; Aburatani, S.; et al. Effect of Light on Carotenoid and Lipid Production in the Oleaginous Yeast Rhodosporidium toruloides. Biosci. Biotechnol. Biochem. 2020, 84, 1501–1512. [Google Scholar] [CrossRef]
- Stachowiak, B. Effect of Illumination Intensities on Astaxanthin Synthesis by Xanthophyllomyces dendrorhous and Its Mutants. Food Sci. Biotechnol. 2013, 22, 1033–1038. [Google Scholar] [CrossRef]
- Li, C.; Xu, Y.; Li, Z.; Cheng, P.; Yu, G. Transcriptomic and Metabolomic Analysis Reveals the Potential Mechanisms Underlying the Improvement of β-Carotene and Torulene Production in Rhodosporidiobolus colostri under Low Temperature Treatment. Food Res. Int. 2022, 156, 111158. [Google Scholar] [CrossRef]
- Cheng, Y.-T.; Yang, C.-F. Using Strain Rhodotorula mucilaginosa to Produce Carotenoids Using Food Wastes. J. Taiwan Inst. Chem. Eng. 2016, 61, 270–275. [Google Scholar] [CrossRef]
- Zhao, D.; Li, C. Multi-Omics Profiling Reveals Potential Mechanisms of Culture Temperature Modulating Biosynthesis of Carotenoids, Lipids, and Exopolysaccharides in Oleaginous Red Yeast Rhodotorula glutinis ZHK. LWT 2022, 171, 114103. [Google Scholar] [CrossRef]
- Davoli, P.; Mierau, V.; Weber, R.W.S. Carotenoids and Fatty Acids in Red Yeasts Sporobolomyces roseus and Rhodotorula glutinis. Appl. Biochem. Microbiol. 2004, 40, 392–397. [Google Scholar] [CrossRef]
- Akiba, Y.; Sato, K.; Takahashi, K.; Matsushita, K.; Komiyama, H.; Tsunekawa, H.; Nagao, H. Meat Color Modification in Broiler Chickens by Feeding Yeast Phaffia rhodozyma Containing High Concentrations of Astaxanthin. J. Appl. Poult. Res. 2001, 10, 154–161. [Google Scholar] [CrossRef]
- Zoz, L.; Carvalho, J.C.; Soccol, V.T.; Casagrande, T.C.; Cardoso, L. Torularhodin and Torulene: Bioproduction, Properties and Prospective Applications in Food and Cosmetics—A Review. Braz. Arch. Biol. Technol. 2015, 58, 278–288. [Google Scholar] [CrossRef]
- Varela, J.C.; Pereira, H.; Vila, M.; León, R. Production of Carotenoids by Microalgae: Achievements and Challenges. Photosynth. Res. 2015, 125, 423–436. [Google Scholar] [CrossRef]
- Chazan, A.; Das, I.; Fujiwara, T.; Murakoshi, S.; Rozenberg, A.; Molina-Márquez, A.; Sano, F.K.; Tanaka, T.; Gómez-Villegas, P.; Larom, S.; et al. Phototrophy by Antenna-Containing Rhodopsin Pumps in Aquatic Environments. Nature 2023, 615, 535–540. [Google Scholar] [CrossRef]
- Béjà, O.; Aravind, L.; Koonin, E.V.; Suzuki, M.T.; Hadd, A.; Nguyen, L.P.; Jovanovich, S.B.; Gates, C.M.; Feldman, R.A.; Spudich, J.L.; et al. Bacterial Rhodopsin: Evidence for a New Type of Phototrophy in the Sea. Science 2000, 289, 1902–1906. [Google Scholar] [CrossRef]
- Pinhassi, J.; DeLong, E.F.; Béjà, O.; González, J.M.; Pedrós-Alió, C. Marine Bacterial and Archaeal Ion-Pumping Rhodopsins: Genetic Diversity, Physiology, and Ecology. Microbiol. Mol. Biol. Rev. 2016, 80, 929–954. [Google Scholar] [CrossRef] [PubMed]
- Balashov, S.P.; Imasheva, E.S.; Boichenko, V.A.; Antón, J.; Wang, J.M.; Lanyi, J.K. Xanthorhodopsin: A Proton Pump with a Light-Harvesting Carotenoid Antenna. Science 2005, 309, 2061–2064. [Google Scholar] [CrossRef] [PubMed]
- Köcher, S.; Breitenbach, J.; Müller, V.; Sandmann, G. Structure, Function and Biosynthesis of Carotenoids in the Moderately Halophilic Bacterium Halobacillus halophilus. Arch. Microbiol. 2009, 191, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Kelman, D.; Ben-Amotz, A.; Berman-Frank, I. Carotenoids Provide the Major Antioxidant Defence in the Globally Significant N 2 -Fixing Marine Cyanobacterium trichodesmium. Environ. Microbiol. 2009, 11, 1897–1908. [Google Scholar] [CrossRef] [PubMed]
- Giani, M.; Garbayo, I.; Vílchez, C.; Martínez-Espinosa, R.M. Haloarchaeal Carotenoids: Healthy Novel Compounds from Extreme Environments. Mar. Drugs 2019, 17, 524. [Google Scholar] [CrossRef] [PubMed]
- Oren, A. Saltern Evaporation Ponds as Model Systems for the Study of Primary Production Processes under Hypersaline Conditions. Aquat. Microb. Ecol. 2009, 56, 193–204. [Google Scholar] [CrossRef]
- Sajjad, W.; Din, G.; Rafiq, M.; Iqbal, A.; Khan, S.; Zada, S.; Ali, B.; Kang, S. Pigment Production by Cold-Adapted Bacteria and Fungi: Colorful Tale of Cryosphere with Wide Range Applications. Extremophiles 2020, 24, 447–473. [Google Scholar] [CrossRef]
- Chattopadhyay, M.K.; Jagannadham, M.V.; Vairamani, M.; Shivaji, S. Carotenoid Pigments of an Antarctic Psychrotrophic Bacterium Micrococcus roseus: Temperature Dependent Biosynthesis, Structure, and Interaction with Synthetic Membranes. Biochem. Biophys. Res. Commun. 1997, 239, 85–90. [Google Scholar] [CrossRef]
- Jagannadham, M.V.; Chattopadhyay, M.K.; Subbalakshmi, C.; Vairamani, M.; Narayanan, K.; Mohan Rao, C.; Shivaji, S. Carotenoids of an Antarctic Psychrotolerant Bacterium, Sphingobacterium antarcticus, and a Mesophilic Bacterium, Sphingobacterium multivorum. Arch. Microbiol. 2000, 173, 418–424. [Google Scholar] [CrossRef]
- Flegler, A.; Lipski, A. The C50 Carotenoid Bacterioruberin Regulates Membrane Fluidity in Pink-Pigmented Arthrobacter Species. Arch. Microbiol. 2022, 204, 70. [Google Scholar] [CrossRef]
- Tian, B.; Hua, Y. Carotenoid Biosynthesis in Extremophilic Deinococcus-Thermus Bacteria. Trends Microbiol. 2010, 18, 512–520. [Google Scholar] [CrossRef]
- Seto, R.; Takaichi, S.; Kurihara, T.; Kishi, R.; Honda, M.; Takenaka, S.; Tsukatani, Y.; Madigan, M.T.; Wang-Otomo, Z.-Y.; Kimura, Y. Lycopene-Family Carotenoids Confer Thermostability on Photocomplexes from a New Thermophilic Purple Bacterium. Biochemistry 2020, 59, 2351–2358. [Google Scholar] [CrossRef]
- Maoka, T. Carotenoids in Marine Animals. Mar. Drugs 2011, 9, 278–293. [Google Scholar] [CrossRef]
- Eggersdorfer, M.; Wyss, A. Carotenoids in Human Nutrition and Health. Arch. Biochem. Biophys. 2018, 652, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.J. Role of Lutein and Zeaxanthin in Visual and Cognitive Function throughout the Lifespan. Nutr. Rev. 2014, 72, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Visioli, F.; Artaria, C. Astaxanthin in Cardiovascular Health and Disease: Mechanisms of Action, Therapeutic Merits, and Knowledge Gaps. Food Funct. 2017, 8, 39–63. [Google Scholar] [CrossRef] [PubMed]
- Meléndez-Martínez, A.J.; Böhm, V.; Borge, G.I.; Cano, M.P.; Fikselová, M.; Gruskiene, R.; Lavelli, V.; Loizzo, M.R.; Mandić, A.; Mapelli-Brahm, P.; et al. Carotenoids: Considerations for Their Use in Functional Foods, Nutraceuticals, Nutricosmetics, Supplements, Botanicals and Novel Foods in the Context of Sustainability, Circular Economy and Climate Change. Annu. Rev. Food Sci. Technol. 2021, 12, 433–460. [Google Scholar] [CrossRef]
- Checa, J.; Aran, J.M. Reactive Oxygen Species: Drivers of Physiological and Pathological Processes. J. Inflamm. Res. 2020, 13, 1057–1073. [Google Scholar] [CrossRef]
- Ranneh, Y.; Ali, F.; Akim, A.M.; Hamid, H.A.; Khazaai, H.; Fadel, A. Crosstalk between Reactive Oxygen Species and Pro-Inflammatory Markers in Developing Various Chronic Diseases: A Review. Appl. Biol. Chem. 2017, 60, 327–338. [Google Scholar] [CrossRef]
- Du, C.; Guo, Y.; Cheng, Y.; Han, M.; Zhang, W.; Qian, H. Anti-Cancer Effects of Torulene, Isolated from Sporidiobolus pararoseus, on Human Prostate Cancer LNCaP and PC-3 Cells via a Mitochondrial Signal Pathway and the down-Regulation of AR Expression. RSC Adv. 2017, 7, 2466–2474. [Google Scholar] [CrossRef]
- Nakao, R.; Nelson, O.L.; Park, J.S.; Mathison, B.D.; Thompson, P.A.; Chew, B.P. Effect of Dietary Astaxanthin at Different Stages of Mammary Tumor Initiation in BALB/c Mice. Anticancer Res. 2010, 30, 2171–2175. [Google Scholar]
- Prabhu, P.N.; Ashokkumar, P.; Sudhandiran, G. Antioxidative and Antiproliferative Effects of Astaxanthin during the Initiation Stages of 1,2-Dimethyl Hydrazine-Induced Experimental Colon Carcinogenesis. Fundam. Clin. Pharmacol. 2009, 23, 225–234. [Google Scholar] [CrossRef]
- Fassett, R.G.; Coombes, J.S. Astaxanthin: A Potential Therapeutic Agent in Cardiovascular Disease. Mar. Drugs 2011, 9, 447–465. [Google Scholar] [CrossRef]
- Sartaj, K.; Gupta, P.; Tripathi, S.; Poluri, K.M.; Prasad, R. Insights into the Extraction, Characterization and Antifungal Activity of Astaxanthin Derived from Yeast de-Oiled Biomass. Environ. Technol. Innov. 2022, 27, 102437. [Google Scholar] [CrossRef]
- Lakshminarayana, R.; Vijay, K.; Ambedkar, R.; Ranga Rao, A.; Ravishankar, G.A. Biological Activities and Health Benefits of Seaweed Carotenoids with Special Reference to Fucoxanthin. In Sustainable Global Resources of Seaweeds Volume 2: Food, Pharmaceutical and Health Applications; Ranga Rao, A., Ravishankar, G.A., Eds.; Springer International Publishing: Berlin, Germany, 2022; pp. 539–558. ISBN 978-3-030-92174-3. [Google Scholar]
- Sathasivam, R.; Ki, J.-S. A Review of the Biological Activities of Microalgal Carotenoids and Their Potential Use in Healthcare and Cosmetic Industries. Mar. Drugs 2018, 16, 26. [Google Scholar] [CrossRef]
- Afra, S.; Makhdoumi, A.; Matin, M.M.; Feizy, J. A Novel Red Pigment from Marine Arthrobacter sp. G20 with Specific Anticancer Activity. J. Appl. Microbiol. 2017, 123, 1228–1236. [Google Scholar] [CrossRef] [PubMed]
- Shindo, K.; Kikuta, K.; Suzuki, A.; Katsuta, A.; Kasai, H.; Yasumoto-Hirose, M.; Matsuo, Y.; Misawa, N.; Takaichi, S. Rare Carotenoids, (3R)-Saproxanthin and (3R,2′S)-Myxol, Isolated from Novel Marine Bacteria (Flavobacteriaceae) and Their Antioxidative Activities. Appl. Microbiol. Biotechnol. 2007, 74, 1350–1357. [Google Scholar] [CrossRef] [PubMed]
- Shindo, K.; Asagi, E.; Sano, A.; Hotta, E.; Minemura, N.; Mikami, K.; Tamesada, E.; Misawa, N.; Maoka, T. Diapolycopenedioic Acid Xylosyl Esters A, B, and C, Novel Antioxidative Glyco-C30-Carotenoic Acids Produced by a New Marine Bacterium Rubritalea squalenifaciens. J. Antibiot. 2008, 61, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Jinendiran, S.; Dahms, H.-U.; Dileep Kumar, B.S.; Kumar Ponnusamy, V.; Sivakumar, N. Diapolycopenedioic-Acid-Diglucosyl Ester and Keto-Myxocoxanthin Glucoside Ester: Novel Carotenoids Derived from Exiguobacterium acetylicum S01 and Evaluation of Their Anticancer and Anti-Inflammatory Activities. Bioorg. Chem. 2020, 103, 104149. [Google Scholar] [CrossRef]
- Osawa, A.; Ishii, Y.; Sasamura, N.; Morita, M.; Kasai, H.; Maoka, T.; Shindo, K. Characterization and Antioxidative Activities of Rare C50 Carotenoids-Sarcinaxanthin, Sarcinaxanthin Monoglucoside, and Sarcinaxanthin Diglucoside-Obtained from Micrococcus yunnanensis. J. Oleo Sci. 2010, 59, 653–659. [Google Scholar] [CrossRef]
- Metwally, R.A.; El-Sersy, N.A.; El Sikaily, A.; Sabry, S.A.; Ghozlan, H.A. Optimization and Multiple in Vitro Activity Potentials of Carotenoids from Marine Kocuria sp. RAM1. Sci. Rep. 2022, 12, 18203. [Google Scholar] [CrossRef]
- Osawa, A.; Ishii, Y.; Sasamura, N.; Morita, M.; Köcher, S.; Müller, V.; Sandmann, G.; Shindo, K. Hydroxy-3,4-Dehydro-Apo-8′-Lycopene and Methyl Hydroxy-3,4-Dehydro-Apo-8′-Lycopenoate, Novel C30 Carotenoids Produced by a Mutant of Marine Bacterium Halobacillus halophilus. J. Antibiot. 2010, 63, 291–295. [Google Scholar] [CrossRef]
- Shindo, K.; Endo, M.; Miyake, Y.; Wakasugi, K.; Morritt, D.; Bramley, P.M.; Fraser, P.D.; Kasai, H.; Misawa, N. Methyl Glucosyl-3,4-Dehydro-Apo-8′-Lycopenoate, a Novel Antioxidative Glyco-C30-Carotenoic Acid Produced by a Marine Bacterium Planococcus maritimus. J. Antibiot. 2008, 61, 729–735. [Google Scholar] [CrossRef]
- Styczynski, M.; Rogowska, A.; Gieczewska, K.; Garstka, M.; Szakiel, A.; Dziewit, L. Genome-Based Insights into the Production of Carotenoids by Antarctic Bacteria, Planococcus sp. ANT_H30 and Rhodococcus sp. ANT_H53B. Molecules 2020, 25, 4357. [Google Scholar] [CrossRef] [PubMed]
- Patias, L.D.; Fernandes, A.S.; Petry, F.C.; Mercadante, A.Z.; Jacob-Lopes, E.; Zepka, L.Q. Carotenoid Profile of Three Microalgae/Cyanobacteria Species with Peroxyl Radical Scavenger Capacity. Food Res. Int. 2017, 100, 260–266. [Google Scholar] [CrossRef]
- Lopes, G.; Clarinha, D.; Vasconcelos, V. Carotenoids from Cyanobacteria: A Biotechnological Approach for the Topical Treatment of Psoriasis. Microorganisms 2020, 8, 302. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Chuda, Y.; Suzuki, M.; Nagata, T. Fucoxanthin as the Major Antioxidant in Hijikia fusiformis, a Common Edible Seaweed. Biosci. Biotechnol. Biochem. 1999, 63, 605–607. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.-J.; Yoon, W.-J.; Kim, K.-N.; Ahn, G.-N.; Kang, S.-M.; Kang, D.-H.; Affan, A.; Oh, C.; Jung, W.-K.; Jeon, Y.-J. Evaluation of Anti-Inflammatory Effect of Fucoxanthin Isolated from Brown Algae in Lipopolysaccharide-Stimulated RAW 264.7 Macrophages. Food Chem. Toxicol. 2010, 48, 2045–2051. [Google Scholar] [CrossRef]
- Nazarudin, M.F.; Yasin, I.S.M.; Mazli, N.A.I.N.; Saadi, A.R.; Azizee, M.H.S.; Nooraini, M.A.; Saad, N.; Ferdous, U.T.; Fakhrulddin, I.M. Preliminary Screening of Antioxidant and Cytotoxic Potential of Green Seaweed, Halimeda opuntia (Linnaeus) Lamouroux. Saudi J. Biol. Sci. 2022, 29, 2698–2705. [Google Scholar] [CrossRef]
- Balasubramaniam, V.; June Chelyn, L.; Vimala, S.; Mohd Fairulnizal, M.N.; Brownlee, I.A.; Amin, I. Carotenoid Composition and Antioxidant Potential of Eucheuma denticulatum, Sargassum polycystum and Caulerpa lentillifera. Heliyon 2020, 6, e04654. [Google Scholar] [CrossRef]
- Chiu, H.-F.; Liao, J.; Lu, Y.-Y.; Han, Y.-C.; Shen, Y.-C.; Venkatakrishnan, K.; Golovinskaia, O.; Wang, C. Anti-Proliferative, Anti-in Fl Ammatory and pro-Apoptotic e Ff Ects of Dunaliella salina on Human KB Oral Carcinoma Cells. J. Food Biochem. 2017, 41, e12349. [Google Scholar] [CrossRef]
- Dokumacioglu, E.; Iskender, H.; Yenice, G.; Kapakin, K.; Sevim, C.; Hayirli, A.; Saral, S.; Comakli, A. Effects of Astaxanthin on Biochemical and Histopathological Parameters Related to Oxidative Stress on Testes of Rats on High Fructose Regime. Andrologia 2018, 50, e13042. [Google Scholar] [CrossRef]
- Lee, A.H.; Shin, H.Y.; Park, J.H.; Koo, S.Y.; Kim, S.M.; Yang, S.-H. Fucoxanthin from Microalgae Phaeodactylum tricornutum Inhibits pro– Inflammatory Cytokines by Regulating Both NF-ΚB and NLRP3 Inflammasome Activation. Sci. Rep. 2021, 11, 543. [Google Scholar] [CrossRef]
- Shiratori, K.; Ohgami, K.; Ilieva, I.; Jin, X.-H.; Koyama, Y.; Miyashita, K.; Yoshida, K.; Kase, S.; Ohno, S. Effects of Fucoxanthin on Lipopolysaccharide-Induced Inflammation in Vitro and in Vivo. Exp. Eye Res. 2005, 81, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-P.; Wu, Z.-H.; Jian, J.-C.; Zhang, X.-Z. Effect of Marine Red Yeast Rhodosporidium paludigenum on Growth and Antioxidant Competence of Litopenaeus vannamei. Aquaculture 2010, 309, 62–65. [Google Scholar] [CrossRef]
- Mussagy, C.U.; Khan, S.; Kot, A.M. Current Developments on the Application of Microbial Carotenoids as an Alternative to Synthetic Pigments. Crit. Rev. Food Sci. Nutr. 2022, 62, 6932–6946. [Google Scholar] [CrossRef]
- Mapelli-Brahm, P.; Barba, F.J.; Remize, F.; Garcia, C.; Fessard, A.; Mousavi Khaneghah, A.; Sant’Ana, A.S.; Lorenzo, J.M.; Montesano, D.; Meléndez-Martínez, A.J. The Impact of Fermentation Processes on the Production, Retention and Bioavailability of Carotenoids: An Overview. Trends Food Sci. Technol. 2020, 99, 389–401. [Google Scholar] [CrossRef]
- Amar, E.C.; Kiron, V.; Akutsu, T.; Satoh, S.; Watanabe, T. Resistance of Rainbow Trout Oncorhynchus mykiss to Infectious Hematopoietic Necrosis Virus (IHNV) Experimental Infection Following Ingestion of Natural and Synthetic Carotenoids. Aquaculture 2012, 330–333, 148–155. [Google Scholar] [CrossRef]
- Dufossé, L.; Galaup, P.; Yaron, A.; Arad, S.M.; Blanc, P.; Chidambara Murthy, K.N.; Ravishankar, G.A. Microorganisms and Microalgae as Sources of Pigments for Food Use: A Scientific Oddity or an Industrial Reality? Trends Food Sci. Technol. 2005, 16, 389–406. [Google Scholar] [CrossRef]
- Liu, X.; Xie, J.; Zhou, L.; Zhang, J.; Chen, Z.; Xiao, J.; Cao, Y.; Xiao, H. Recent Advances in Health Benefits and Bioavailability of Dietary Astaxanthin and Its Isomers. Food Chem. 2023, 404, 134605. [Google Scholar] [CrossRef]
- Miyashita, K.; Beppu, F.; Hosokawa, M.; Liu, X.; Wang, S. Nutraceutical Characteristics of the Brown Seaweed Carotenoid Fucoxanthin. Arch. Biochem. Biophys. 2020, 686, 108364. [Google Scholar] [CrossRef] [PubMed]
- Guedes, A.C.; Amaro, H.M.; Malcata, F.X. Microalgae as Sources of Carotenoids. Mar. Drugs 2011, 9, 625–644. [Google Scholar] [CrossRef]
- Saleh, R.; Bearth, A.; Siegrist, M. “Chemophobia” Today: Consumers’ Knowledge and Perceptions of Chemicals. Risk Anal. 2019, 39, 2668–2682. [Google Scholar] [CrossRef] [PubMed]

| Species | Carotenoids | Collection Sites | Experimental Conditions | Quantification/Identification Methodologies | Contents | References |
|---|---|---|---|---|---|---|
| Halobacterium halobium | Total carotenoids (mainly bacterioruberin) | Saltern brine, Sfax, Tunisia | Liquid culture (10 g/L yeast extract, 7.5 g/L casamino acids, 250 g/L NaCl, 20 g/L MgSO4.7H2O, 2 g/L KCl, and 3 g/L trisodium citrate), 37 °C, 240 rpm, 7 days | Spectrophotometry | 5.66–7.63 mg/L, FW | [48] |
| Haloarcula japonica | Total carotenoids (mainly bacterioruberin) | Saltern soil | Liquid culture, 37 °C, 10 days | HPLC-MS | 335 μg/g, DW. Bacterioruberin was up to 68.1% of the total carotenoids (mol%) | [49] |
| Halorubrum sp. | Total carotenoids (mainly bacterioruberin) | Saltern brine | Liquid culture, 37 °C | Spectrophotometry | 25 mg/L, FW | [50] |
| Haloterrigena turkmenica | Total carotenoids (mainly bacterioruberin) | Salt soil crust | Liquid culture, 37 °C, 180 rpm | Spectrophotometry | 74.5 μg/g, DW | [51] |
| Halorubrum sp. | Total carotenoids (mainly bacterioruberin) | Seven distinct saline habitats, Algeria | Liquid culture, 37 °C | Spectrophotometry | 3.68 mg/L, FW | [52] |
| Haloarcula sp. | Total carotenoids (mainly bacterioruberin) | Tebenquiche Lake of the Atacama Saltern, Chile | Liquid culture (5 g/L proteose-peptone, 10 g/L yeast extract, 1 g/L glucose, with 25% (w/v) total salts), 40 °C, 120 rpm, 10 days | HPLC-MS | 488.88–871.53 mg/g, DW | [53] |
| Haloferax mediterranei strain R-4 (ATCC33500) | Total carotenoids (mainly bacterioruberin) | Salt pond | Liquid culture, 36.5 °C | Spectrophotometry | 92.2 µg/mL, FW | [54] |
| Organisms | Species | Carotenoids | Biological Activities | Methodologies | References |
|---|---|---|---|---|---|
| Archaea | Halobacterium halobium | Bacterioruberin 1 | Antioxidant | Exposing hepatoma cell lines with carotenoid extract to arachidonic acid or H2O2. The protective effect against oxidative stress was also assessed by the MTT assay | [48] |
| Haloarcula japonica | Bacterioruberin 1 | Antioxidant | DPPH assay | [49] | |
| Haloterrigena turkmenica | Bacterioruberin 1 | Antioxidant | DPPH and FRP assays | [51] | |
| Haloferax volcanii, Halogranum rubrum, Haloplanus inordinatus, Halogeometricum limi, and Haloplanus vescus | Bacterioruberin 1 | Antioxidant | DPPH assay and the evaluation of the inhibition of H2O2-induced hemolysis of mouse erythrocytes | [57] | |
| Haloarcula hispanica and Halobacterium salinarum | Bacterioruberin 1 | Antioxidant/ anti-inflammatory | Antioxidant: DPPH, ABTS, NO, FRAP, CCA, and ICA assays. Anti-inflammatory: against COX-2 | [59] | |
| Halorubrum sp. | Bacterioruberin 1 | Antioxidant | DPPH and ABTS assays | [52] | |
| Haloarcula sp. and Halorubrum tebenquichense | Bacterioruberin 1 | Antioxidant | DPPH, ABTS, and FRAP assays | [53] | |
| Haloferax mediterranei | Bacterioruberin 1 | Antioxidant | DPPH, ABTS, and FRAP assays | [54] | |
| Arthrobacter sp. G20 | Crocin 1 | Antioxidant | DPPH assay | [300] | |
| strain 04OKA-13-27 | (3R)-saproxanthin 2 | Antioxidant | Against free-radical-induced lipid peroxidation in a rat brain homogenate | [301] | |
| Bacteria | strain YM6-073 | (3R,2′S)-myxo, (3R,3′R)-zeaxanthin 2 | Antioxidant | Against free-radical-induced lipid peroxidation in a rat brain homogenate | [301] |
| strain 04OKA-17-12 | (3R,3′R)-zeaxanthin 2 | Antioxidant | Against free-radical-induced lipid peroxidation in a rat brain homogenate | [301] | |
| Rubritalea squalenifaciens | Diapolycopenedioic acid 2 xylosyl esters | Antioxidant | 1O2 suppression model | [302] | |
| Exiguobacterium acetylicum S01 | Diapolycopenedioic-acid-diglucosyl ester 2 | Antioxidant/ anti-inflammatory | Antioxidant: DPPH assay. Anti-inflammatory: inhibition of NO production and TNF-α protein levels in LPS-induced oxidative stress in PBMC | [303] | |
| Exiguobacterium acetylicum S01 | Keto-myxocoxanthinglucoside ester 2 | Antioxidant/ anti-inflammatory | Antioxidant: DPPH assay. Anti-inflammatory: inhibition of NO production and TNF-α protein levels in LPS-induced oxidative stress in PBMC | [303] | |
| Micrococcus yunnanensis | Sarcinaxanthin 2 | Antioxidant | 1O2 suppression model | [304] | |
| Micrococcus yunnanensis | Sarcinaxanthin monoglucoside 2 | Antioxidant | 1O2 suppression model | [304] | |
| Micrococcus yunnanensis | Sarcinaxanthin diglucoside 2 | Antioxidant | 1O2 suppression model | [304] | |
| Kocuria sp. RAM1 | Bisanhydrobacterioruberin derivative, trisanhydrobacterioruberin, and 3,4,3′,4′-tetrahydrospirilloxanthin 1 | Antioxidant/ anti-inflammatory | Antioxidant: DPPH assay. Anti-inflammatory: hypotonic solution-induced hemolysis | [305] | |
| Halobacillus halophilus (mutant) | Hydroxy-3,4-dehydro-apo-8′-lycopene 2 | Antioxidant | 1O2 suppression model | [306] | |
| Halobacillus halophilus (mutant) | Methyl hydroxy-3,4-dehydro-apo-8′-lycopenoate 2 | Antioxidant | 1O2 suppression model | [306] | |
| Planococcus maritimus | Methyl glucosyl-3,4-dehydro-apo-8′-lycopenoate 2 | Antioxidant | 1O2 suppression model | [307] | |
| Planococcus sp. ANT_H30 | Unidentified 1 | Antioxidant | DPPH assay | [308] | |
| Rhodococcus sp. ANT_H53B | Dihydroxyneurosporene, hydroxyechinenone, and 4 unidentified 1 | Antioxidant | DPPH assay | [308] | |
| Planococcus sp. Eg-Natrun | Astaxanthin and β-carotene 1 | Antioxidant | Fenton reaction | [87] | |
| Erythrobacter citreus LAMA 915 | Zeaxanthin, caloxanthin, nostoxanthin, adonixanthin, canthaxanthin, and erythroxanthin sulfate 1 | Antioxidant | DPPH assay | [85] | |
| Cyanobacteria | Trichodesmiumemi IMS101 | Zeaxanthin, all-trans- and 9-cis-β-carotene 1 | Antioxidant | FRAP method | [277] |
| Aphanothece microscopica Nageli | 13-cis-Antheroxanthin, 15-cis- and all-trans-lutein, all-trans-zeaxanthin, all-trans-cantaxanthin, all-trans-myxoxanthophyll, β-carotene-5,6-epoxide, all-trans-β-cryptoxanthin, all-trans-crocoxanthin, all-trans- and 9-cis-echineone, and all-trans- 9-cis-, and 13-cis-β-carotene 1 | Antioxidant | Fluorescence decay resulting from the ROO· induced oxidation of the C11-BODIPY581/591 probe | [309] | |
| Alkalinema aff. pantanalense | Zeaxanthin, lutein derivatives, echinenone derivative, and unknown carotenoids 1 | Antioxidant | 1O2 suppression model | [310] | |
| Cuspidothrix issatschenkoi | Canthaxanthin, all-trans- and 13-cis-β-carotene, α-carotene derivative, and unknown carotenoids 1 | Antioxidant | 1O2 suppression model | [310] | |
| Leptolyngbya-like sp. | β-carotene oxygenated derivatives, lutein derivative, lutein, zeaxanthin, echinenone, all-trans and 13-cis-β-carotene, α-carotene derivative, and unknown carotenoids 1 | Anti-inflammatory | Inhibition of NO production in macrophage cells | [310] | |
| Macroalga (brown) | Hijikia fusiformis | Fucoxanthin 1 | Antioxidant | DPPH assay | [311] |
| Myagropsis myagroide | Fucoxanthin 1 | Anti-inflammatory | Inhibition of NO in LPS-induced macrophage cells | [312] | |
| Sargassum muticum | - | Antioxidant | Evaluation of the total antioxidant capacity after supplementation in humans | [174] | |
| Sargassum hemiphyllum | Fucoxanthin and fucoidan (polysaccharide) 1 | Anti-inflammatory | Evaluation of hepatic inflammation through modulation of leptin/adiponectin axis after supplementation in humans with NAFLD | [175] | |
| Macroalga (green) | Halimeda opuntia | Unknown carotenoids 1 | Antioxidant | DPPH assay | [313] |
| Macroalga (red) | Eucheuma denticulatum | Lutein and zeaxanthin 1 | Antioxidant | ORAC assay | [314] |
| Microalga | Dunaliella salina | Lutein, zeaxanthin, α-carotene, all-trans- and 9-cis-β-carotene, and one unknown compound 1 | Antioxidant/anti-inflammatory | Antioxidant: reducing capacity, chelating activity, DPPH and 1O2 suppression model. Anti-inflammatory: against COX-2 on human oral squamous carcinoma cells | [315] |
| Haematococcus pluvialis | Astaxanthin 3 | Antioxidant | Evaluation of oxidative damage in rats caused by high fructose consumption after supplementation of astaxanthin | [316] | |
| Phaeodactylum tricornutum | Fucoxanthin 1 | Anti-inflammatory | Inhibition of NF-κB and NLRP3 inflammasome activation induced by the combination of LPS and ATP in bone marrow-derived immune cells and astrocytes | [317] | |
| Haematococcus pluvialis | 3S,3′S-astaxanthin and 3S,3′S-astaxanthin esters 1 | Antioxidant | 1O2 suppression model | [209] | |
| Brown microalga | Fucoxanthin 1 | Anti-inflammatory | Inhibition of COX-2 and iNOS expression in in macrophage cells incubated with LPS | [318] | |
| Yeast | Rhodosporidium paludigenum | Carotenoids | Antioxidant | Evaluation of MDA levels in the muscle, and the activities of serum T-AOC, CAT, SOD and GPx, and hepatopancreases SOD and GPx after supplementation of shrimps with live yeast | [319] |
| Rhodotorula sp. | Torularhodin | Antioxidant | Chemiluminescence and photochemiluminescence (Trolox) methods | [243] 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mapelli-Brahm, P.; Gómez-Villegas, P.; Gonda, M.L.; León-Vaz, A.; León, R.; Mildenberger, J.; Rebours, C.; Saravia, V.; Vero, S.; Vila, E.; et al. Microalgae, Seaweeds and Aquatic Bacteria, Archaea, and Yeasts: Sources of Carotenoids with Potential Antioxidant and Anti-Inflammatory Health-Promoting Actions in the Sustainability Era. Mar. Drugs 2023, 21, 340. https://doi.org/10.3390/md21060340
Mapelli-Brahm P, Gómez-Villegas P, Gonda ML, León-Vaz A, León R, Mildenberger J, Rebours C, Saravia V, Vero S, Vila E, et al. Microalgae, Seaweeds and Aquatic Bacteria, Archaea, and Yeasts: Sources of Carotenoids with Potential Antioxidant and Anti-Inflammatory Health-Promoting Actions in the Sustainability Era. Marine Drugs. 2023; 21(6):340. https://doi.org/10.3390/md21060340
Chicago/Turabian StyleMapelli-Brahm, Paula, Patricia Gómez-Villegas, Mariana Lourdes Gonda, Antonio León-Vaz, Rosa León, Jennifer Mildenberger, Céline Rebours, Verónica Saravia, Silvana Vero, Eugenia Vila, and et al. 2023. "Microalgae, Seaweeds and Aquatic Bacteria, Archaea, and Yeasts: Sources of Carotenoids with Potential Antioxidant and Anti-Inflammatory Health-Promoting Actions in the Sustainability Era" Marine Drugs 21, no. 6: 340. https://doi.org/10.3390/md21060340
APA StyleMapelli-Brahm, P., Gómez-Villegas, P., Gonda, M. L., León-Vaz, A., León, R., Mildenberger, J., Rebours, C., Saravia, V., Vero, S., Vila, E., & Meléndez-Martínez, A. J. (2023). Microalgae, Seaweeds and Aquatic Bacteria, Archaea, and Yeasts: Sources of Carotenoids with Potential Antioxidant and Anti-Inflammatory Health-Promoting Actions in the Sustainability Era. Marine Drugs, 21(6), 340. https://doi.org/10.3390/md21060340