Effect of Ishophloroglucin A Isolated from Ishige okamurae on In Vitro Osteoclastogenesis and Osteoblastogenesis
Abstract
:1. Introduction
2. Results
2.1. Effect of IPA on Cell Viability and TRAP Activity in RANKL-Induced RAW 264.7 Cells
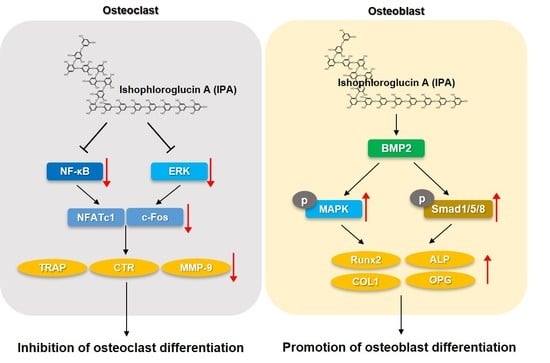
2.2. IPA Inhibits RANKL-Induced Expression of Osteoclast Differentiation-Related Protein in RAW 264.7 Cells
2.3. IPA Inhibits RANKL-Induced Expression of Osteoclast-Differentiation-Related Transcriptional Factors in RAW 264.7 Cells
2.4. IPA Inhibits RANKL-Induced Activation of NF-κB and ERK in RAW 264.7 Cells
2.5. IPA Promotes Osteoblasts Differentiation in MG-63 Cells
3. Discussion
4. Materials and Methods
4.1. Sample Preparation
4.2. Cell Culture
4.3. MTT Assay
4.4. Tartrate-Resistant Acid Phosphatase (TRAP) Staining
4.5. Alkaline Phosphatase (ALP) Activity
4.6. Western Blot Analysis
4.7. Confocal Laser Scanning Microscopy (CLSM)
4.8. Statistical Analysis
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, X.; Wang, Z.; Duan, N.; Zhu, G.; Schwarz, E.M.; Xia, C. Osteoblast-osteoclast interactions. Connect. Tissue Res. 2018, 59, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, K.; Park, K.A.; Ito, M.; Ikeda, K.; Takeshita, S. Osteoclast-derived complement component 3a stimulates osteoblast differentiation. J. Bone Miner. Res. 2014, 29, 1522–1530. [Google Scholar] [CrossRef] [PubMed]
- Torregrosa Suas, O. Anti-resorptives in the management of osteoporosis. Rev. Osteoporos. Metab. Miner. 2021, 13 (Suppl. 1), S18–S22. [Google Scholar]
- Park, S.H.; Jeon, M.J.; Jang, M.K.; Lee, S.J.; Kim, B.K.; Jeon, M.J.; Kim, S.Y.; Kim, M.; Lee, D.G.; Lee, T.H.; et al. Verification of estrogen like activities of herbal medicines using an in vitro detection system. J. Physiol. Pathol. Korean Med. 2013, 27, 752–758. [Google Scholar]
- Blick, S.K.A.; Dhillon, S.; Keam, S.J. Teriparatide. Drugs 2008, 68, 2709–2737. [Google Scholar] [CrossRef] [PubMed]
- Yoon, B.H.; Kim, K.C. Does Teriparatide improve fracture union?: A systematic review. J. Bone Metab. 2020, 27, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Kobza, A.O.; Papaioannou, A.; Lau, A.N.; Adachi, J.D. Romosozumab in the treatment of osteoporosis. Immunotherapy 2020, 12, 13. [Google Scholar] [CrossRef] [PubMed]
- McDonald, M.M.; Khoo, W.H.; Ng, P.Y.; Xiao, Y.; Zamerli, J.; Thatcher, P.; Kyaw, W.; Pathmanandavel, K.; Grootveld, A.K.; Moran, I.; et al. Osteoclasts recycles via osteomorphs during RANKL-stimulated bone resorption. Cell 2021, 184, 1330–1347. [Google Scholar] [CrossRef]
- Kang, J.H.; Lim, H.; Jeong, J.E.; Yim, M. Attenuation of RANKL-induced osteoclast formation via p38-mediated NFATc1 signaling pathways by extract of Euphorbia lathyris L. J. Bone Metab. 2016, 23, 207–214. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.Y.; Kim, J.H.; Kim, E.Y.; Yeom, M.; Jung, H.S.; Sohn, Y. Water extract of Cnidii Rhizoma suppresses RANKL-induced osteoclastogenesis in RAW 264.7 cell by inhibiting NFATc1/c-Fos signaling and prevents ovariectomized bone loss in SD-rat. BMC Complement. Med. Ther. 2019, 19, 207. [Google Scholar] [CrossRef]
- Han, Y.; Kim, Y.M.; Kim, H.S.; Lee, K.Y. Melatonin promotes osteoblasts differentiation by regulating Osterix protein stability and expression. Sci. Rep. 2017, 7, 5716. [Google Scholar] [CrossRef] [Green Version]
- Yun, H.M.; Park, K.R.; Quang, T.H.; Oh, H.; Hong, J.T.; Kim, Y.C.; Kim, E.C. 2,4,5-Trimethoxylalbergiquinol promotes osteoblastic differentiation and mineralization via the BMP and Wnt/β-catenin pathway. Cell Death Dis. 2015, 6, e1819. [Google Scholar] [CrossRef] [Green Version]
- Cho, Y.E.; Kwun, I.S. Cellular zinc deficiency inhibits the mineralized nodule formation and downregulates bone-specific gene expression in osteoblastic MC3T3-E1 cells. J. Nutr. Health 2018, 51, 379–385. [Google Scholar] [CrossRef] [Green Version]
- Menshova, R.V.; Shevchenko, N.M.; Imbs, T.I.; Zvyaintseva, T.N.; Malyarenko, O.S.; Zaporoshets, T.S.; Besednova, N.N.; Ermakova, S.P. Fucoidans from brown alga Fucus evanescens: Structure and biological activity. Front. Mar. Sci. 2016, 3, 129. [Google Scholar] [CrossRef] [Green Version]
- Catarino, M.D.; Silva, A.; Cruz, M.T.; Mateus, N.; Silva, A.M.S.; Cardoso, S.M. Phlorotannins from Fucus vesiculosus: Modulation of inflammatory response by blocking NF-κB signaling pathway. Int. J. Mol. Sci. 2020, 21, 6897. [Google Scholar] [CrossRef]
- Liu, X.; Yuan, W.; Sharma-Shivappa, R.; van Zanten, J. Antioxidant activity of phlorotannins from brown algae. Int. J. Agric. Biol. 2017, 10, 2017. [Google Scholar] [CrossRef]
- Gheda, S.; Naby, M.A.; Mohamed, T.; Pereira, L.; Khamis, A. Antidiabetic and antioxidant activity of phlorotannins extracted from the brown seaweed Cystoseria compressa in streptozotocin-induced diabetic rats. Environ. Sci. Pollut. Res. 2021, 28, 22886–22901. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.H.; Kwon, T.H.; Jeong, H.; Kim, J.S.; Kim, S.R.; Jeong, M.S.; Park, S.; Choi, M.; Woo, J.H.; Ahn, J.; et al. Dieckol isolated from Eisenia bicylis extract suppresses RANKL-induced osteoclastogenesis in murine RAW 264.7 cells. Asian Pac. J. Trop. Biomed. 2022, 12, 262–269. [Google Scholar]
- Karadeniz, F.; Ahn, B.N.; Kim, J.A.; Seo, Y.; Jang, M.S.; Nam, K.H.; Kim, M.; Lee, S.H.; Kong, C.S. Phlorotannins suppress adipogenesis in pre-adipocytes while enhancing osteoblastogenesis in pre-osteoblasts. Arch. Pharm. Res. 2015, 38, 2172–2182. [Google Scholar] [CrossRef] [PubMed]
- Ihn, H.J.; Kim, J.A.; Cho, H.S.; Shin, H.I.; Kim, G.Y.; Choi, Y.H.; Joen, Y.J.; Park, E.K. Diphlorethohydroxycarmalol from Ishige okamurae suppresses osteoclast differentiation by downregulating the NF-κB signaling pathway. Int. J. Mol. Sci. 2017, 18, 2635. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Yang, H.W.; Jiang, Y.; Oh, J.Y.; Jeon, Y.J.; Ryu, B. Ishophlroglucin A isolated from Ishige okamurae suppresses melanogenesis induced by α-MSH: In vitro and in vivo. Mar. Drugs 2020, 18, 470. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.W.; Son, M.; Choi, J.; Oh, S.; Jeon, Y.J.; Byun, K.; Ryu, B. Effect of Ishophloroglucin A, a component of Ishige okamurae, on glucose homeostasis in the pancreas and muscle of high fat diet-fed mice. Mar. Drugs 2019, 17, 608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, I.S.; Kim, C. Taurine chloramine inhibits osteoclastic differentiation and osteoclast marker expression in RAW 264.7 cells. Adv. Exp. Med. Biol. 2019, 1155, 61–70. [Google Scholar] [PubMed]
- Ihn, H.J.; Lee, T.; Lee, D.; Bae, J.S.; Kim, S.H.; Jang, I.H.; Bae, Y.C.; Shin, H.I.; Park, E.K. Inhibitory effect of KP-A038 on osteoclastogenesis and inflammatory bone loss is associated with downregulation of blimp1. Front. Pharmacol. 2019, 10, 367. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Wang, W.; Geng, L.; Qin, Y.; Dong, W.; Zhang, X.; Qin, A.; Zhang, M. Inhibition of RANKL-induced osteoclastogenesis through the suppression of the ERK signaling pathway by astragaloside Ⅳ and attenuation of titanium-particle-induced osteolysis. Int. J. Mol. Med. 2015, 36, 1335–1344. [Google Scholar] [CrossRef] [Green Version]
- Ikeda, R.; Nishimurae, R.; Matsubara, T.; Tanaka, S.; Inoue, J.; Reddy, S.V.; Hata, K.; Tamashita, K.; Hiraga, T.; Watanabe, T.; et al. Critical roles of c-Jun singalong in regulation of NFAT family and RANKL-regulated osteoclast differentiation. J. Clin. Investig. 2004, 114, 475–484. [Google Scholar] [CrossRef] [Green Version]
- Giridharan, S.; Srinivasan, M. Mechanisms of NF-κB p65 and strategies for therapeutic manipulation. J. Inflamm. Res. 2018, 11, 407–419. [Google Scholar] [CrossRef] [Green Version]
- Su, P.; Tian, Y.; Yang, C.; Ma, X.; Wang, X.; Pei, J.; Qian, A. Mesenchymal stem cell migration during bone formation and bone diseases therapy. Int. J. Mol. Sci. 2018, 19, 2343. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.M.; Kim, M.G.; Byun, J.H.; Kim, G.C.; Ro, J.H.; Hwang, D.S.; Choi, B.B.; Park, G.C.; Kim, U.K. The effect of biomechanical stimulation on osteoblast differentiation of human jaw periosteum-derived stem cells. Maxillofac. Plast. Reconstr. Surg. 2017, 39, 7. [Google Scholar] [CrossRef] [Green Version]
- Huang, R.L.; Yuan, Y.; Tu, J.; Zou, G.M.; Li, Q. Opposing TNF-α/IL-1β- and BMP-2-activated MAPK signaling pathways converge on Runx2 to regulate BMP-2-induced osteoblastic differentiation. Cell Death Dis. 2014, 5, e1187. [Google Scholar] [CrossRef] [Green Version]
- James, A.W. Review of signaling pathways governing MSC osteogenic and adipogenic differentiation. Scientifica 2013, 2013, 684736. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Feng, T.; Guo, D.; Zhang, M.; Chen, L.; Zhou, Y. Sanggenon C stimulates osteoblastic proliferation and differentiation, inhibits osteoclastic resorption, and ameliorates prednisolone-induced osteoporosis in zebrafish model. Molecules 2018, 23, 2343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shang, N.; Bhullar, K.S.; Hubbard, B.P.; Wu, J. Tripeptide IRW initiates differentiation in osteoblasts via the RUNX2 pathway. Biochim. Biophys. Acta—Gen. Subj. 2019, 1863, 1138–1146. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Wang, L.; Jayawardena, T.U.; Kim, E.A.; Heo, S.J.; Fernano, I.P.S.; Lee, J.H.; Jeon, Y.J. High-performance centrifugal partition chromatography (HPCPC) for efficient isolation of diphlorethohydroxycarmalol (DPHC) and screening of its antioxidant activity in a zebrafish model. Process Biochem. 2020, 88, 189–196. [Google Scholar] [CrossRef]
- Cho, S.H.; Park, S.; Jeong, H.; Kim, S.R.; Jeong, M.S.; Choi, M.; Kim, S.H.; Kim, K.N. Anti-Inflammatory Activity of 4-((1R,2R)-3-Hydroxy-1-(4-Hydroxyphenyl)-1-Methoxypropan-2-yl)-2-Methoxyphenol Isolated from Juglans mandshurica Maxim. in LPS-Stimulated RAW 264.7 Macrophages and Zebrafish Larvae Model. Pharmaceuticals 2021, 14, 771. [Google Scholar] [CrossRef]
- Baek, M.; Seo, M.; Lee, J.H.; Kim, I.W.; Kim, M.A.; Hwang, J.S. Osteoblastogenic activity of Locusta migratoria ethanol extracts on pre-osteoblastic MG-63 cells. J. Life Sci. 2018, 28, 1448–1454. [Google Scholar]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, S.-H.; Kim, H.-S.; Jung, H.-Y.; Park, J.-I.; Jang, Y.-J.; Ahn, J.; Kim, K.-N. Effect of Ishophloroglucin A Isolated from Ishige okamurae on In Vitro Osteoclastogenesis and Osteoblastogenesis. Mar. Drugs 2023, 21, 377. https://doi.org/10.3390/md21070377
Cho S-H, Kim H-S, Jung H-Y, Park J-I, Jang Y-J, Ahn J, Kim K-N. Effect of Ishophloroglucin A Isolated from Ishige okamurae on In Vitro Osteoclastogenesis and Osteoblastogenesis. Marine Drugs. 2023; 21(7):377. https://doi.org/10.3390/md21070377
Chicago/Turabian StyleCho, Su-Hyeon, Hyun-Soo Kim, Hye-Yeon Jung, Jae-Il Park, You-Jee Jang, Juhee Ahn, and Kil-Nam Kim. 2023. "Effect of Ishophloroglucin A Isolated from Ishige okamurae on In Vitro Osteoclastogenesis and Osteoblastogenesis" Marine Drugs 21, no. 7: 377. https://doi.org/10.3390/md21070377
APA StyleCho, S. -H., Kim, H. -S., Jung, H. -Y., Park, J. -I., Jang, Y. -J., Ahn, J., & Kim, K. -N. (2023). Effect of Ishophloroglucin A Isolated from Ishige okamurae on In Vitro Osteoclastogenesis and Osteoblastogenesis. Marine Drugs, 21(7), 377. https://doi.org/10.3390/md21070377