Valorisation of the Invasive Macroalgae Undaria pinnatifida (Harvey) Suringar for the Green Synthesis of Gold and Silver Nanoparticles with Antimicrobial and Antioxidant Potential
Abstract
:1. Introduction
2. Results and Discussion
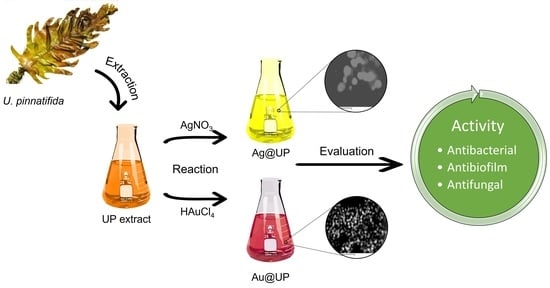
2.1. Synthesis and Characterization of Gold and Silver Nanoparticles
2.2. Carbohydrate Analysis
2.3. Antioxidant Activity
2.4. Antibacterial Activity of UP Extract, Ag@UP and Au@UP
2.5. Antifungal Activity Assessment of UP Extract, Ag@UP and Au@UP
3. Materials and Methods
3.1. Seaweed Collection and Extract Preparation
3.2. Synthesis of Gold and Silver Nanoparticles
3.3. Characterization Techniques
3.4. Carbohydrate Analysis
3.5. In Vitro Antioxidant Activity
3.5.1. Reducing Power
3.5.2. Total Phenolic Content
3.5.3. DPPH Scavenging Activity
3.5.4. Statistical Analysis
3.6. Antibacterial Activity
3.7. Inhibition of Biofilm Formation
3.8. Antifungal Assays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organitation. Antimicrobial Resistance; WHO: Geneva, Switzerland, 2021. [Google Scholar]
- EClinicalMedicine. Antimicrobial Resistance: A Top Ten Global Public Health Threat. EClinicalMedicine 2021, 41, 101221. [Google Scholar] [CrossRef] [PubMed]
- Vestby, L.K.; Grønseth, T.; Simm, R.; Nesse, L.L. Bacterial Biofilm and its Role in the Pathogenesis of Disease. Antibiotics 2020, 9, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. WHO Fungal Priority Pathogens List to Guide Research, Development and Public Health Action; WHO: Geneva, Switzerland, 2022. [Google Scholar]
- De Oliveira, D.M.P.; Forde, B.M.; Kidd, T.J.; Harris, P.N.A.; Schembri, M.A.; Beatson, S.A.; Paterson, D.L.; Walker, M.J. Antimicrobial Resistance in ESKAPE Pathogens. Clin. Microbiol. Rev. 2020, 33, e00181-19. [Google Scholar] [CrossRef] [PubMed]
- Pappas, P.C.; Lionakis, M.S.; Arenup, M.C.; Ostrosky-Zeichner, L.; Kullberg, B.J. Invasive Candidiasis. Nature reviews. Dis. Primers 2018, 4, 18026. [Google Scholar] [CrossRef]
- Nami, S.; Aghebati-Maleki, A.; Morovati, H.; Aghebati-Maleki, L. Current Antifungal Drugs and Immunotherapeutic Approaches as Promising Strategies to Treatment of Fungal Diseases. Biomed. Pharmacother. 2019, 110, 857–868. [Google Scholar] [CrossRef] [PubMed]
- Mussin, J.; Giusiano, G. Biogenic Silver Nanoparticles as Antifungal Agents. Front. Chem. 2022, 10, 1023542. [Google Scholar] [CrossRef]
- Pereira, A.M.; Costa, A.D.; Dias, S.C.; Casal, M.; Machado, R. Production and Purification of Two Bioactive Antimicrobial Peptides using a Two-Step Approach Involving an Elastin-Like Fusion Tag. Pharmaceuticals 2021, 14, 956. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, C.; Li, Y.; Li, J.; Wan, Q.; Chen, J.; Tay, F.R.; Niu, L. Considerations and Caveats in Combating ESKAPE Pathogens Against Nosocomial Infections. Adv. Sci. 2019, 7, 1901872. [Google Scholar] [CrossRef] [Green Version]
- Lv, X.; Wang, L.; Mei, A.; Xu, Y.; Ruan, X.; Wang, W.; Shao, J.; Yang, D.; Dong, X. Recent Nanotechnologies to Overcome the Bacterial Biofilm Matrix Barriers. Small 2022, 19, 2206220. [Google Scholar] [CrossRef]
- Ren, R.; Lim, C.; Li, S.; Wang, Y.; Song, J.; Lin, T.; Muir, B.W.; Hsu, H.; Shen, H. Recent Advances in the Development of Lipid-, Metal-, Carbon-, and Polymer-Based Nanomaterials for Antibacterial Applications. Nanomaterials 2022, 12, 3855. [Google Scholar] [CrossRef]
- Luzala, M.M.; Muanga, C.K.; Kyana, J.; Safari, J.B.; Zola, E.N.; Mbusa, G.V.; Nuapia, Y.B.; Liesse, J.I.; Nkanga, C.I.; Krause, R.W.M.; et al. A Critical Review of the Antimicrobial and Antibiofilm Activities of Green-Synthesized Plant-Based Metallic Nanoparticles. Nanomaterials 2022, 12, 1841. [Google Scholar] [CrossRef] [PubMed]
- Jin, R. The Impacts of Nanotechnology on Catalysis by Precious Metal Nanoparticles. Nanotechnol. Rev. 2012, 1, 31–56. [Google Scholar] [CrossRef]
- Ye, J.; Chen, X. Current Promising Strategies Against Antibiotic-Resistant Bacterial Infections. Antibiotics 2022, 12, 67. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Thakur, V.; Kumar, V.; Raj, M.; Gupta, S.; Devi, N.; Upadhyay, S.K.; Macho, M.; Banerjee, A.; Ewe, D.; et al. Silver Nanoparticles and its Mechanistic Insight for Chronic Wound Healing: Review on Recent Progress. Molecules 2022, 27, 5587. [Google Scholar] [CrossRef]
- Tripathi, N.; Goshisht, M.K. Recent Advances and Mechanistic Insights into Antibacterial Activity, Antibiofilm Activity, and Cytotoxicity of Silver Nanoparticles. ACS Appl. Bio Mater. 2022, 5, 1391. [Google Scholar]
- Aljarba, N.H.; Imtiaz, S.; Anwar, N.; Alanazi, I.S.; Alkahtani, S. Anticancer and Microbial Activities of Gold Nanoparticles: A Mechanistic Review. J. King Saud Univ. Sci. 2022, 34, 101907. [Google Scholar] [CrossRef]
- Zhan, X.; Yan, J.; Tang, H.; Xia, D.; Lin, H. Antibacterial Properties of Gold Nanoparticles in the Modification of Medical Implants: A Systematic Review. Pharmaceutics 2022, 14, 2654. [Google Scholar] [CrossRef]
- Suringar, W.F.R. Illustrationes Des Algues Du Japon. Musée Bot. Leide 1873, 1, 77–82. [Google Scholar]
- Epstein, G.; Smale, D.A. Undaria pinnatifida: A Case Study to Highlight Challenges in Marine Invasion Ecology and Management. Ecol. Evol. 2017, 7, 8624–8642. [Google Scholar] [CrossRef]
- Blanco, A.; Larrinaga, A.R.; Neto, J.M.; Troncoso, J.; Méndez, G.; Domínguez-Lapido, P.; Ovejero, A.; Pereira, L.; Mouga, T.M.; Gaspar, R.; et al. Spotting Intruders: Species Distribution Models for Managing Invasive Intertidal Macroalgae. J. Environ. Manag. 2021, 281, 111861. [Google Scholar] [CrossRef]
- Gallardo, B. Europe’s Top 10 Invasive Species: Relative Importance of Climatic, Habitat and Socio-Economic Factors. Ethol. Ecol. Evol. 2014, 26, 130–151. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Pang, Z.; Han, C. Undaria pinnatifida (Wakame): A Seaweed with Pharmacological Properties. Sci. Int. 2014, 2, 32–36. [Google Scholar] [CrossRef] [Green Version]
- Zeng, J.; Luan, F.; Hu, J.; Liu, Y.; Zhang, X.; Qin, T.; Zhang, X.; Liu, R.; Zeng, N. Recent Research Advances in Polysaccharides from Undaria pinnatifida: Isolation, Structures, Bioactivities, and Applications. Int. J. Biol. Macromol. 2022, 206, 325–354. [Google Scholar] [CrossRef] [PubMed]
- Kolb, N.; Vallorani, L.; Milanovi, N.; Stocchi, V. Evaluation of Marine Algae Wakame (Undaria pinnatifida) and Kombu (Laminaria digitata japonica) as Food Supplements. Food Technol. Biotechnol. 2004, 42, 57–61. [Google Scholar]
- Balboa, E.M.; Gallego-Fabrega, C.; Moure, A.; Dominguez, H. Study of the Seasonal Variation on Proximate Composition of Oven-Dried Sargassum muticum Biomass Collected in Vigo Ria, Spain. J. Appl. Phycol. 2016, 28, 1943–1953. [Google Scholar] [CrossRef]
- Rodrigues, D.; Freitas, A.C.; Pereira, L.; Rocha-Santos, T.A.P.; Vasconcelos, M.W.; Roriz, M.; Rodríguez-Alcalá, L.M.; Gomes, A.M.P.; Duarte, A.C. Chemical Composition of Red, Brown and Green Macroalgae from Buarcos Bay in Central West Coast of Portugal. Food Chem. 2015, 183, 197–207. [Google Scholar] [CrossRef]
- Pereira, L.; Gheda, S.F.; Ribeiro-Claro, P.J.A. Analysis by Vibrational Spectroscopy of Seaweed Polysaccharides with Potential use in Food, Pharmaceutical, and Cosmetic Industries. Int. J. Carbohydr. Chem. 2013, 2013, 1–7. [Google Scholar]
- Koh, H.S.A.; Lu, J.; Zhou, W. Structure Characterization and Antioxidant Activity of Fucoidan Isolated from Undaria pinnatifida Grown in New Zealand. Carbohydr. Polym. 2019, 212, 178–185. [Google Scholar] [CrossRef]
- Synytsya, A.; Kim, W.J.; Kim, S.M.; Pohl, R.; Synytsya, A.; Kvasnicka, F.; Copíková, J.; Park, Y.I. Structure and Antitumour Activity of Fucoidan Isolated from Sporophyll of Korean Brown Seaweed Undaria pinnatifida. Carbohydr. Polym. 2010, 81, 41–48. [Google Scholar] [CrossRef]
- Ferreira, C.A.M.; Félix, R.; Félix, C.; Januário, A.P.; Alves, N.; Novais, S.C.; Dias, J.R.; Lemos, M.F.L. A Biorefinery Approach to the Biomass of the Seaweed Undaria pinnatifida (Harvey Suringar, 1873): Obtaining Phlorotannins-Enriched Extracts for Wound Healing. Biomolecules 2021, 11, 461. [Google Scholar] [CrossRef]
- Balquinta, M.L.; Dellatorre, F.G.; Andrés, S.C.; Lorenzo, G. Effect of pH and Seaweed (Undaria pinnatifida) Meal Level on Rheological and Antioxidant Properties of Model Aqueous Systems. Algal Res. 2022, 62, 102629. [Google Scholar] [CrossRef]
- Plaza-Cazón, J.; Landea, M.P.S.; Donati, E.R. Bioreduction and Biosorption of Chromium by Undaria pinntifida. Algal Res. 2022, 65, 102729. [Google Scholar] [CrossRef]
- Jiménez-Escrig, A.; Sánchez-Muniz, F.J. Dietary Fibre from Edible Seaweeds: Chemical Structure, Physicochemical Properties and Effects on Cholesterol Metabolism. Nutr. Res. 2000, 20, 585. [Google Scholar] [CrossRef]
- González-Ballesteros, N.; Rodríguez-Argüelles, M.C.; Prado-López, S.; Lastra, M.; Grimaldi, M.; Cavazza, A.; Nasi, L.; Salviati, G.; Bigi, F. Macroalgae to Nanoparticles: Study of Ulva lactuca Role in Biosynthesis of Gold and Silver Nanoparticles and of their Cytotoxicity on Colon Cancer Cell Lines. Mat. Scie. Eng. C 2019, 97, 498–509. [Google Scholar] [CrossRef] [PubMed]
- González-Ballesteros, N.; Diego-González, L.; Lastra-Valdor, M.; Rodríguez-Argüelles, M.C.; Grimaldi, M.; Cavazza, A.; Bigi, F.; Simón-Vázquez, R. Immunostimulant and Biocompatible Gold and Silver Nanoparticles Synthesized by the Ulva intestinalis L. Aqueous Extract. J. Mater. Chem. B 2019, 7, 4677–4691. [Google Scholar] [CrossRef]
- Eda, M.; Kuda, T.; Kataoka, M.; Takahashi, H.; Kimura, B. Anti-Glycation Properties of the Aqueous Extract Solutions of Dried Algae Products Harvested and made in the Miura Peninsula, Japan, and Effect of Lactic Acid Fermentation on the Properties. J. Appl. Phycol. 2016, 28, 3617–3624. [Google Scholar] [CrossRef]
- Patra, J.K.; Lee, S.W.; Park, J.G.; Baek, K.H. Antioxidant and Antibacterial Properties of Essential Oil Extracted from an Edible Seaweed Undaria pinnatifida. J. Food Biochem. 2017, 41, e12278. [Google Scholar] [CrossRef]
- González-Ballesteros, N.; Prado-López, S.; Rodríguez-González, J.B.; Lastra, M.; Rodríguez-Argüelles, M.C. Green Synthesis of Gold Nanoparticles using Brown Algae Cystoseira baccata: Its Activity in Colon Cancer Cells. Colloids Surf. B Biointerfaces 2017, 153, 190–198. [Google Scholar] [CrossRef]
- González-Ballesteros, N.; Diego-González, L.; Lastra-Valdor, M.; Grimaldi, M.; Cavazza, A.; Bigi, F.; Rodríguez-Argüelles, M.C.; Simón-Vázquez, R. Saccorhiza polyschides used to Synthesize Gold and Silver Nanoparticles with Enhanced Antiproliferative and Immunostimulant Activity. Mater. Sci. Eng. C 2021, 123, 111960. [Google Scholar] [CrossRef]
- González-Ballesteros, N.; Rodríguez-Argüelles, M.C.; Lastra-Valdor, M.; González-Mediero, G.; Rey-Cao, S.; Grimaldi, M.; Cavazza, A.; Bigi, F. Synthesis of Silver and Gold Nanoparticles by Sargassum muticum Biomolecules and Evaluation of their Antioxidant Activity and Antibacterial Properties. J. Nanostructure Chem. 2020, 10, 317–330. [Google Scholar] [CrossRef]
- González-Ballesteros, N.; Rodríguez-Argüelles, M.C.; Lastra-Valdor, M. Evaluation of the Antioxidant Capacities of Antarctic Macroalgae and their use for Nanoparticles Production. Molecules 2021, 26, 1182. [Google Scholar] [CrossRef] [PubMed]
- González-Ballesteros, N.; Rodríguez-González, J.B.; Lastra-Valdor, M.; Rodríguez-Argüelles, M.C. New Application of Two Antarctic Macroalgae Palmaria decipiens and Desmarestia menziesii in the Synthesis of Gold and Silver Nanoparticles. Polar Sci. 2018, 15, 49–54. [Google Scholar] [CrossRef]
- Phull, A.R.; Majid, M.; Haq, I.; Khan, M.R.; Kim, S.J. In Vitro and in Vivo Evaluation of Anti-Arthritic, Antioxidant Efficacy of Fucoidan from Undaria pinnatifida (Harvey) Suringar. Int. J. Biol. Macromol. 2017, 97, 468–480. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.T.; Sun, X.Y.; Yu, K.; Gui, B.S.; Gui, Q.; Ouyang, J.M. Effect of Content of Sulfate Groups in Seaweed Polysaccharides on Antioxidant Activity and Repair Effect of Subcellular Organelles in Injured HK-2 Cells. Oxid. Med. Cell. Longev. 2017, 2017, 1–13. [Google Scholar] [CrossRef] [Green Version]
- López-Hortas, L.; Domínguez, H.; Torres, M.D. Valorisation of Edible Brown Seaweeds by the Recovery of Bioactive Compounds from Aqueous Phase using MHG to Develop Innovative Hydrogels. Process. Biochem. 2019, 78, 100–107. [Google Scholar] [CrossRef]
- Fernandes, M.; González-Ballesteros, N.; Da Costa, A.; Machado, R.; Gomes, A.C.; Rodríguez-Argüelles, M.C. Antimicrobial and Anti-Biofilm Activity of Silver Nanoparticles Biosynthesized with Cystoseira Algae Extracts. J. Biol. Inorg. Chem. 2023, 28, 439–450. [Google Scholar] [CrossRef]
- Thirumalairaj, V.K.; Vijayan, M.P.; Durairaj, G.; Shanmu-gaasokan, L.; Yesudas, R.; Gunasekaran, S. Potential Antibacterial Activity of Crude Extracts and Silver Nanoparticles Synthesized from Sargassum wightii. Int. Curr. Pharm. J. 2014, 3, 322–325. [Google Scholar] [CrossRef] [Green Version]
- Chitrikha Suresh, T.; Poonguzhali, T.V.; Anuradha, V.; Ramesh, B.; Suresh, G. Aqueous Extract of Turbinaria conoides (J. Agardh) Kützing Mediated Fabrication of Silver Nanoparticles used Against Bacteria Associated with Diabetic Foot Ulcer. Mater. Today Proc. 2021, 43, 3038–3043. [Google Scholar] [CrossRef]
- Manuel Xavier, H.F.; Nadar, V.M.; Patel, P.; Umapathy, D.; Velanganni Joseph, A.; Manivannan, S.; Santhiyagu, P.; Pandi, B.; Muthusamy, G.; Rathinam, Y.; et al. Selective Antibacterial and Apoptosis-Inducing Effects of Hybrid Gold Nanoparticles—A Green Approach. J. Drug Deliv. Sci. Technol. 2020, 59, 101890. [Google Scholar] [CrossRef]
- Dharul Salam, F.; Nadar Vinita, M.; Puja, P.; Prakash, S.; Yuvakkumar, R.; Kumar, P. Anti-Bacterial and Anti-Biofilm Efficacies of Bioinspired Gold Nanoparticles. Mater. Lett. 2020, 261, 126998. [Google Scholar] [CrossRef]
- Sampaio, P.; Santos, M.; Correia, A.; Amaral, F.E.; Chavéz-Galarza, J.; Costa-de-Oliveira, S.; Castro, A.G.; Pedrosa, J.; Pais, C. Virulence Attenuation of Candida albicans Genetic Variants Isolated from a Patient with a Recurrent Bloodstream Infection. PLoS ONE 2010, 5, e10155. [Google Scholar] [CrossRef] [Green Version]
- Costa-de-Oliveira, S.; Sousa, I.; Correia, A.; Sampaio, P.; Pais, C.; Rodrigues, A.G.; Pina-Vaz, C. Genetic Relatedness and Antifungal Susceptibility Profile of Candida albicans Isolates from Fungaemia Patients. Med. Mycol. (Oxford) 2011, 49, 248–252. [Google Scholar] [CrossRef] [Green Version]
- Bravo-Chaucanés, C.P.; Vargas-Casanova, Y.; Chitiva-Chitiva, L.C.; Ceballos-Garzon, A.; Modesti-Costa, G.; Parra-Giraldo, C.M. Evaluation of Anti-Candida Potential of Piper nigrum Extract in Inhibiting Growth, Yeast-Hyphal Transition, Virulent Enzymes, and Biofilm Formation. J. Fungi 2022, 8, 784. [Google Scholar] [CrossRef]
- Kayalvizhi, K.; Asmathunisha, N.; Subramanian, V.; Kathiresan, K.; Kathiresan, K.; Kayalvizhi, K.; Asmathunisha, N.; Subramanian, V.; Kathiresan, K. Purification of Silver and Gold Nanoparticles from Two Species of Brown Seaweeds (Padina tetrastromatica and Turbinaria ornata). J. Med. Plants Stud. 2014, 2, 32–37. [Google Scholar]
- Sathyaseelan, T.; Subbiah, M.; Sivamugugan, V. Green Synthesis of Silver Nano Particles using Marine Brown Alga Lobophora variegata and its Efficacy in Antifungal Activity. World J. Pharm. Res. 2015, 4, 2137–2145. [Google Scholar]
- Sivagnanam, S.P.; Tilahun Getachew, A.; Choi, J.H.; Park, Y.B.; Woo, H.C.; Chun, B.S. Green Synthesis of Silver Nanoparticles from Deoiled Brown Algal Extract Via Box-Behnken Based Design and their Antimicrobial and Sensing Properties. Green Process. Synth. 2016, 6, 147–160. [Google Scholar] [CrossRef]
- Rajeshkumar, S.; Malarkodi, C.; Paulkumar, K.; Vanaja, M.; Gnanajobitha, G.; Annadurai, G. Algae Mediated Green Fabrication of Silver Nanoparticles and Examination of its Antifungal Activity Against Clinical Pathogens. Int. J. Met. 2014, 2014, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Thiurunavukkarau, R.; Shanmugam, S.; Subramanian, K.; Pandi, P.; Muralitharan, G.; Arokiarajan, M.; Kasinathan, K.; Sivaraj, A.; Kalyanasundaram, R.; AlOmar, S.Y.; et al. Silver Nanoparticles Synthesized from the Seaweed Sargassum polycystum and Screening for their Biological Potential. Sci. Rep. 2022, 12, 14757. [Google Scholar] [CrossRef] [PubMed]
- Dananjaya, S.H.S.; Thu Thao, N.T.; Wijerathna, H.M.S.M.; Lee, J.; Edussuriya, M.; Choi, D.; Saravana Kumar, R. In Vitro and in Vivo Anticandidal Efficacy of Green Synthesized Gold Nanoparticles using Spirulina maxima Polysaccharide. Process. Biochem. 2020, 92, 138–148. [Google Scholar] [CrossRef]
- González-Ballesteros, N.; Diego-González, L.; Lastra-Valdor, M.; Grimaldi, M.; Cavazza, A.; Bigi, F.; Rodríguez-Argüelles, M.C.; Simón-Vázquez, R. Immunomodulatory and Antitumoral Activity of Gold Nanoparticles Synthesized by Red Algae Aqueous Extracts. Mar. Drugs 2022, 20, 182. [Google Scholar] [CrossRef] [PubMed]
- Cendra, M.D.M.; Blanco-Cabra, N.; Pedraz, L.; Torrents, E. Optimal Environmental and Culture Conditions Allow the in Vitro Coexistence of Pseudomonas aeruginosa and Staphylococcus aureus in Stable Biofilms. Sci. Rep. 2019, 9, 16284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and Broth Dilution Methods to Determine the Minimal Inhibitory Concentration (MIC) of Antimicrobial Substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef] [PubMed]








| E. coli | P. aeruginosa | S. aureus | ||||
|---|---|---|---|---|---|---|
| MIC (µg/mL) | MBC (µg/mL) | MIC (µg/mL) | MBC (µg/mL) | MIC (µg/mL) | MBC (µg/mL) | |
| UP extract | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. |
| Ag@UP | 1.96 | 3.94 | 1.96 | 3.94 | 1.96 | 3.94 |
| Au@UP | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. |
| Ampicillin | 40 | 60 | 60 | 60 | 60 | 60 |
| Kanamycin | 60 | 60 | 60 | 60 | 60 | 60 |
| Silver nitrate | 10.19 | 10.19 | 10.19 | 10.19 | 6.79 | 10.19 |
| P. aeruginosa | S. aureus | |
|---|---|---|
| MIC (µg/mL) | MIC (µg/mL) | |
| UP extract | n.a. | n.a. |
| Ag@UP | 7.88 | 7.88 |
| Au@UP | 11.81 | 11.81 |
| Ampicillin | 20 | 20 |
| Kanamycin | 20 | 20 |
| Silver nitrate | 4.31 | 2.16 |
| Candida albicans 124a | Candida albicans SC5314 | Candida auris 117 | Candida auris 120 | |
|---|---|---|---|---|
| MFC (µg/mL) | MFC (µg/mL) | MFC (µg/mL) | MFC (µg/mL) | |
| UP extract | n.a. | n.a. | n.a. | n.a. |
| Ag@UP | 0.54 | 1.97 | 0.54 | 0.54 |
| Au@UP | 0.54 | n.a. | 0.54 | 0.54 |
| Silver nitrate | n.a. | 6.79 | 4.31 | n.a. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Ballesteros, N.; Fernandes, M.; Machado, R.; Sampaio, P.; Gomes, A.C.; Cavazza, A.; Bigi, F.; Rodríguez-Argüelles, M.C. Valorisation of the Invasive Macroalgae Undaria pinnatifida (Harvey) Suringar for the Green Synthesis of Gold and Silver Nanoparticles with Antimicrobial and Antioxidant Potential. Mar. Drugs 2023, 21, 397. https://doi.org/10.3390/md21070397
González-Ballesteros N, Fernandes M, Machado R, Sampaio P, Gomes AC, Cavazza A, Bigi F, Rodríguez-Argüelles MC. Valorisation of the Invasive Macroalgae Undaria pinnatifida (Harvey) Suringar for the Green Synthesis of Gold and Silver Nanoparticles with Antimicrobial and Antioxidant Potential. Marine Drugs. 2023; 21(7):397. https://doi.org/10.3390/md21070397
Chicago/Turabian StyleGonzález-Ballesteros, Noelia, Mário Fernandes, Raúl Machado, Paula Sampaio, Andreia C. Gomes, Antonella Cavazza, Franca Bigi, and Maria Carmen Rodríguez-Argüelles. 2023. "Valorisation of the Invasive Macroalgae Undaria pinnatifida (Harvey) Suringar for the Green Synthesis of Gold and Silver Nanoparticles with Antimicrobial and Antioxidant Potential" Marine Drugs 21, no. 7: 397. https://doi.org/10.3390/md21070397
APA StyleGonzález-Ballesteros, N., Fernandes, M., Machado, R., Sampaio, P., Gomes, A. C., Cavazza, A., Bigi, F., & Rodríguez-Argüelles, M. C. (2023). Valorisation of the Invasive Macroalgae Undaria pinnatifida (Harvey) Suringar for the Green Synthesis of Gold and Silver Nanoparticles with Antimicrobial and Antioxidant Potential. Marine Drugs, 21(7), 397. https://doi.org/10.3390/md21070397