Marine Microbial Polysaccharides: An Untapped Resource for Biotechnological Applications
Abstract
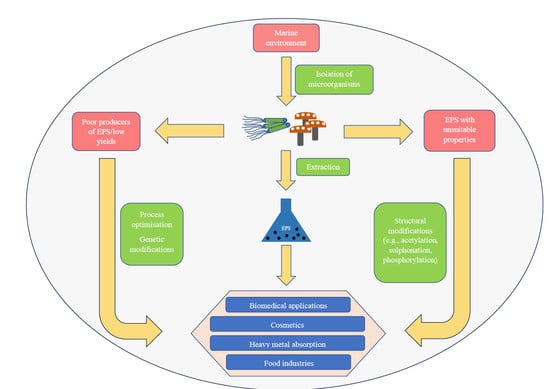
:1. Introduction
2. The Case of Marine Polysaccharides
3. Current Research on Marine Microbial Polysaccharides
3.1. Biomedical Applications
3.1.1. Anticancer Activity
3.1.2. Antimicrobial Activity
3.1.3. Anti-Oxidant Activity
3.1.4. Drug Delivery
3.2. Bioremediation
4. Challenges for the Commercialization of Marine Microbial Polysaccharides
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baranwal, J.; Barse, B.; Fais, A.; Delogu, G.L.; Kumar, A. Biopolymer: A Sustainable Material for Food and Medical Applications. Polymers 2022, 14, 983. [Google Scholar] [CrossRef] [PubMed]
- Osemwegie, O.O.; Adetunji, C.O.; Ayeni, E.A.; Adejobi, O.I.; Arise, R.O.; Nwonuma, C.O.; Oghenekaro, A.O. Exopolysaccharides from bacteria and fungi: Current status and perspectives in Africa. Heliyon 2020, 6, e04205. [Google Scholar] [CrossRef] [PubMed]
- Zaghloul, E.H.; Ibrahim, M.I.A. Production and Characterization of Exopolysaccharide from Newly Isolated Marine Probiotic Lactiplantibacillus plantarum EI6 with in vitro Wound Healing Activity. Front. Microbiol. 2022, 13, 903363. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.; Shah, M.D.; Shah, L.; Lee, P.-C.; Khan, I. Bacterial polysaccharides—A big source for prebiotics and therapeutics. Front. Nutr. 2022, 9, 1031935. [Google Scholar] [CrossRef]
- Ibrahim, H.A.H.; Elhassayeb, H.E.A.; El-Sayed, W.M.M. Potential functions and applications of diverse microbial exopolysaccharides in marine environments. J. Genet. Eng. Biotechnol. 2022, 20, 151. [Google Scholar] [CrossRef]
- Liu, Z.-X.; Huang, S.-L.; Hou, J.; Guo, X.-P.; Wang, F.-S.; Sheng, J.-Z. Cell-based high-throughput screening of polysaccharide biosynthesis hosts. Microb. Cell Factories 2021, 20, 62. [Google Scholar] [CrossRef]
- Mahmoud, Y.A.-G.; El-Naggar, M.E.; Abdel-Megeed, A.; El-Newehy, M. Recent Advancements in Microbial Polysaccharides: Synthesis and Applications. Polymers 2021, 13, 4136. [Google Scholar] [CrossRef]
- Zeidan, A.A.; Poulsen, V.K.; Janzen, T.; Buldo, P.; Derkx, P.M.F.; Øregaard, G.; Neves, A.R. Polysaccharide production by lactic acid bacteria: From genes to industrial applications. FEMS Microbiol. Rev. 2017, 41 (Suppl. 1), S168–S200. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, N.H.; Mustafa, S.; Man, Y.B.C. Microbial Polysaccharides and Their Modification Approaches: A Review. Int. J. Food Prop. 2014, 18, 332–347. [Google Scholar] [CrossRef] [Green Version]
- Mohammed, A.S.A.; Naveed, M.; Jost, N. Polysaccharides; Classification, Chemical Properties, and Future Perspective Applications in Fields of Pharmacology and Biological Medicine (A Review of Current Applications and Upcoming Potentialities). J. Polym. Environ. 2021, 29, 2359–2371. [Google Scholar] [CrossRef]
- Zeitz, M.A.; Tanveer, Z.; Openshaw, A.T.; Schmidt, M. Genetic Regulators and Physiological Significance of Glycogen Storage in Candida albicans. J. Fungi 2019, 5, 102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Rubio, R.; De Oliveira, H.C.; Rivera, J.; Trevijano-Contador, N. The Fungal Cell Wall: Candida, Cryptococcus, and Aspergillus Species. Front. Microbiol. 2020, 10, 2993. [Google Scholar] [CrossRef] [PubMed]
- Gow, N.A.R.; Latge, J.-P.; Munro, C.A. The Fungal Cell Wall: Structure, Biosynthesis, and Function. Microbiol. Spectr. 2017, 5, 28513415. [Google Scholar] [CrossRef] [Green Version]
- Agustinho, D.P.; Miller, L.C.; Li, L.X.; Doering, T.L. Peeling the onion: The outer layers of Cryptococcus neoformans. Memórias Inst. Oswaldo Cruz 2018, 113, e180040. [Google Scholar] [CrossRef] [Green Version]
- Mahapatra, S.; Banerjee, D. Fungal Exopolysaccharide: Production, Composition and Applications. Microbiol. Insights 2013, 6, MBI-S10957. [Google Scholar] [CrossRef] [Green Version]
- Pereira, S.B.; Sousa, A.; Santos, M.; Araújo, M.; Serôdio, F.; Granja, P.; Tamagnini, P. Strategies to Obtain Designer Polymers Based on Cyanobacterial Extracellular Polymeric Substances (EPS). Int. J. Mol. Sci. 2019, 20, 5693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruocco, N.; Costantini, S.; Guariniello, S.; Costantini, M. Polysaccharides from the Marine Environment with Pharmacological, Cosmeceutical and Nutraceutical Potential. Molecules 2016, 21, 551. [Google Scholar] [CrossRef] [PubMed]
- Qi, M.; Zheng, C.; Wu, W.; Yu, G.; Wang, P. Exopolysaccharides from Marine Microbes: Source, Structure and Application. Mar. Drugs 2022, 20, 512. [Google Scholar] [CrossRef]
- Casillo, A.; Lanzetta, R.; Parrilli, M.; Corsaro, M.M. Exopolysaccharides from Marine and Marine Extremophilic Bacteria: Structures, Properties, Ecological Roles and Applications. Mar. Drugs 2018, 16, 69. [Google Scholar] [CrossRef] [Green Version]
- Mac Rygaard, A.; Thøgersen, M.S.; Nielsen, K.F.; Gram, L.; Bentzon-Tilia, M. Effects of Gelling Agent and Extracellular Signaling Molecules on the Culturability of Marine Bacteria. Appl. Environ. Microbiol. 2017, 83, e00243-17. [Google Scholar] [CrossRef] [Green Version]
- Sen, K.; Sen, B.; Wang, G. Diversity, Abundance, and Ecological Roles of Planktonic Fungi in Marine Environments. J. Fungi 2022, 8, 491. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, M.F.M.; Esteves, A.C.; Alves, A. Marine Fungi: Opportunities and Challenges. Encyclopedia 2022, 2, 559–577. [Google Scholar] [CrossRef]
- Srivastava, N.; Kumari, S.; Kurmi, S.; Pinnaka, A.K.; Choudhury, A.R. Isolation, purification, and characterization of a novel exopolysaccharide isolated from marine bacteria Brevibacillus borstelensis M42. Arch. Microbiol. 2022, 204, 399. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Huang, L.; Zhang, Y.; Yan, Y. Production, Characterization and Immunomodulatory Activity of an Extracellular Polysaccharide from Rhodotorula mucilaginosa YL-1 Isolated from Sea Salt Field. Mar. Drugs 2020, 18, 595. [Google Scholar] [CrossRef] [PubMed]
- Shyam, K.P.; Rajkumar, P.; Ramya, V.; Sivabalan, S.; Kings, A.J.; Miriam, L.M. Exopolysaccharide production by optimized medium using novel marine Enterobacter cloacae MBB8 isolate and its antioxidant potential. Carbohydr. Polym. Technol. Appl. 2021, 2, 100070. [Google Scholar] [CrossRef]
- Wang, C.; Mao, W.; Chen, Z.; Zhu, W.; Chen, Y.; Zhao, C.; Li, N.; Yan, M.; Liu, X.; Guo, T. Purification, structural characterization and antioxidant property of an extracellular polysaccharide from Aspergillus terreus. Process. Biochem. 2013, 48, 1395–1401. [Google Scholar] [CrossRef]
- Chen, Y.; Mao, W.-J.; Yan, M.-X.; Liu, X.; Wang, S.-Y.; Xia, Z.; Xiao, B.; Cao, S.-J.; Yang, B.-Q.; Li, J. Purification, Chemical Characterization, and Bioactivity of an Extracellular Polysaccharide Produced by the Marine Sponge Endogenous Fungus Alternaria sp. SP-32. Mar. Biotechnol. 2016, 18, 301–313. [Google Scholar] [CrossRef]
- Alshawwa, S.Z.; Alshallash, K.S.; Ghareeb, A.; Elazzazy, A.M.; Sharaf, M.; Alharthi, A.; Abdelgawad, F.E.; El-Hossary, D.; Jaremko, M.; Emwas, A.-H.; et al. Assessment of Pharmacological Potential of Novel Exopolysaccharide Isolated from Marine Kocuria sp. Strain AG5: Broad-Spectrum Biological Investigations. Life 2022, 12, 1387. [Google Scholar] [CrossRef]
- Asker, M.S.; El Sayed, O.H.; Mahmoud, M.G.; Yahya, S.M.; Mohamed, S.S.; Selim, M.S.; El Awady, M.S.; Abdelnasser, S.M.; Elsoud, M.M.A. Production of exopolysaccharides from novel marine bacteria and anticancer activity against hepatocellular carcinoma cells (HepG2). Bull. Natl. Res. Cent. 2018, 42, 30. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, G.; Liu, R.; Wei, M.; Zhang, J.; Sun, C. EPS364, a Novel Deep-Sea Bacterial Exopolysaccharide, Inhibits Liver Cancer Cell Growth and Adhesion. Mar. Drugs 2021, 19, 171. [Google Scholar] [CrossRef]
- Liu, G.; Liu, R.; Shan, Y.; Sun, C. Marine bacterial exopolysaccharide EPS11 inhibits migration and invasion of liver cancer cells by directly targeting collagen I. J. Biol. Chem. 2021, 297, 101133. [Google Scholar] [CrossRef]
- Cao, R.; Jin, W.; Shan, Y.; Wang, J.; Liu, G.; Kuang, S.; Sun, C. Marine Bacterial Polysaccharide EPS11 Inhibits Cancer Cell Growth via Blocking Cell Adhesion and Stimulating Anoikis. Mar. Drugs 2018, 16, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Liu, G.; Ma, W.; Lu, Z.; Sun, C. Marine Bacterial Polysaccharide EPS11 Inhibits Cancer Cell Growth and Metastasis via Blocking Cell Adhesion and Attenuating Filiform Structure Formation. Mar. Drugs 2019, 17, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sran, K.S.; Bisht, B.; Mayilraj, S.; Choudhury, A.R. Structural characterization and antioxidant potential of a novel anionic exopolysaccharide produced by marine Microbacterium aurantiacum FSW-25. Int. J. Biol. Macromol. 2019, 131, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Sran, K.S.; Sundharam, S.S.; Krishnamurthi, S.; Choudhury, A.R. Production, characterization and bio-emulsifying activity of a novel thermostable exopolysaccharide produced by a marine strain of Rhodobacter johrii CDR-SL 7Cii. Int. J. Biol. Macromol. 2019, 127, 240–249. [Google Scholar] [CrossRef]
- Liu, S.-B.; Chen, X.-L.; He, H.-L.; Zhang, X.-Y.; Xie, B.-B.; Yu, Y.; Chen, B.; Zhou, B.-C.; Zhang, Y.-Z. Structure and Ecological Roles of a Novel Exopolysaccharide from the Arctic Sea Ice Bacterium Pseudoalteromonas sp. Strain SM20310. Appl. Environ. Microbiol. 2013, 79, 224–230. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Wahab, B.A.; El-Kareem, H.F.A.; Alzamami, A.; Fahmy, C.A.; Elesawy, B.H.; Mahmoud, M.M.; Ghareeb, A.; El Askary, A.; Nahas, H.H.A.; Attallah, N.G.M.; et al. Novel Exopolysaccharide from Marine Bacillus subtilis with Broad Potential Biological Activities: Insights into Antioxidant, Anti-Inflammatory, Cytotoxicity, and Anti-Alzheimer Activity. Metabolites 2022, 12, 715. [Google Scholar] [CrossRef]
- Zhang, Z.; Cai, R.; Zhang, W.; Fu, Y.; Jiao, N. A Novel Exopolysaccharide with Metal Adsorption Capacity Produced by a Marine Bacterium Alteromonas sp. JL2810. Mar. Drugs 2017, 15, 175. [Google Scholar] [CrossRef] [Green Version]
- Roychowdhury, R.; Srivastava, N.; Kumari, S.; Pinnaka, A.K.; Choudhury, A.R. Isolation of an exopolysaccharide from a novel marine bacterium Neorhizobium urealyticum sp. nov. and its utilization in nanoemulsion formation for encapsulation and stabilization of astaxanthin. LWT 2021, 151, 112105. [Google Scholar] [CrossRef]
- Spanò, A.; Gugliandolo, C.; Lentini, V.; Maugeri, T.L.; Anzelmo, G.; Poli, A.; Nicolaus, B. A Novel EPS-Producing Strain of Bacillus licheniformis Isolated from a Shallow Vent Off Panarea Island (Italy). Curr. Microbiol. 2013, 67, 21–29. [Google Scholar] [CrossRef]
- Gugliandolo, C.; Spanò, A.; Lentini, V.; Arena, A.; Maugeri, T.L. Antiviral and immunomodulatory effects of a novel bacterial exopolysaccharide of shallow marine vent origin. J. Appl. Microbiol. 2014, 116, 1028–1034. [Google Scholar] [CrossRef] [PubMed]
- Sahana, T.; Rekha, P. A novel exopolysaccharide from marine bacterium Pantoea sp. YU16-S3 accelerates cutaneous wound healing through Wnt/β-catenin pathway. Carbohydr. Polym. 2020, 238, 116191. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Geng, L.; Wang, Q.; Yue, Y.; Wang, J.; Wu, N.; Wang, X.; Sun, C.; Zhang, Q. Purification, characterization and immunostimulatory activity of a novel exopolysaccharide from Bacillus sp. H5. Int. J. Biol. Macromol. 2021, 189, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Sran, K.S.; Pinnaka, A.K.; Choudhury, A.R. Purification, characterization and functional properties of exopolysaccharide from a novel halophilic Natronotalea sambharensis sp. nov. Int. J. Biol. Macromol. 2019, 136, 547–558. [Google Scholar] [CrossRef]
- Guo, S.; Mao, W.; Yan, M.; Zhao, C.; Li, N.; Shan, J.; Lin, C.; Liu, X.; Guo, T.; Guo, T.; et al. Galactomannan with novel structure produced by the coral endophytic fungus Aspergillus ochraceus. Carbohydr. Polym. 2014, 105, 325–333. [Google Scholar] [CrossRef]
- Wu, K.; Li, Y.; Lin, Y.; Xu, B.; Yang, J.; Mo, L.; Huang, R.; Zhang, X. Structural characterization and immunomodulatory activity of an exopolysaccharide from marine-derived Aspergillus versicolor SCAU141. Int. J. Biol. Macromol. 2023, 227, 329–339. [Google Scholar] [CrossRef]
- Shao, Z.; Tian, Y.; Liu, S.; Chu, X.; Mao, W. Anti-Diabetic Activity of a Novel Exopolysaccharide Produced by the Mangrove Endophytic Fungus Penicillium janthinellum N29. Mar. Drugs 2023, 21, 270. [Google Scholar] [CrossRef]
- Roca, C.; Lehmann, M.; Torres, C.A.; Baptista, S.; Gaudêncio, S.P.; Freitas, F.; Reis, M.A. Exopolysaccharide production by a marine Pseudoalteromonas sp. strain isolated from Madeira Archipelago ocean sediments. New Biotechnol. 2016, 33, 460–466. [Google Scholar] [CrossRef]
- Poli, A.; Finore, I.; Romano, I.; Gioiello, A.; Lama, L.; Nicolaus, B. Microbial Diversity in Extreme Marine Habitats and Their Biomolecules. Microorganisms 2017, 5, 25. [Google Scholar] [CrossRef] [Green Version]
- Laurienzo, P. Marine Polysaccharides in Pharmaceutical Applications: An Overview. Mar. Drugs 2010, 8, 2435–2465. [Google Scholar] [CrossRef] [Green Version]
- López-Ortega, M.A.; Chavarría-Hernández, N.; del Rocio Lopez-Cuellar, M.; Rodríguez-Hernández, A.I. A review of extracellular polysaccharides from extreme niches: An emerging natural source for the biotechnology. From the adverse to diverse! Int. J. Biol. Macromol. 2021, 177, 559–577. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.-L.; Zhao, F.; Shi, M.; Zhang, X.-Y.; Zhou, B.-C.; Zhang, Y.-Z.; Chen, X.-L. Characterization and Biotechnological Potential Analysis of a New Exopolysaccharide from the Arctic Marine Bacterium Polaribacter sp. SM1127. Sci. Rep. 2015, 5, 18435. [Google Scholar] [CrossRef] [Green Version]
- Courtois, A.; Berthou, C.; Guézennec, J.; Boisset, C.; Bordron, A. Exopolysaccharides Isolated from Hydrothermal Vent Bacteria Can Modulate the Complement System. PLoS ONE 2014, 9, e94965. [Google Scholar] [CrossRef]
- Carrión, O.; Delgado, L.; Mercade, E. New emulsifying and cryoprotective exopolysaccharide from Antarctic Pseudomonas sp. ID1. Carbohydr. Polym. 2015, 117, 1028–1034. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.-L.; Liu, S.-B.; Qiao, L.-P.; Chen, X.-L.; Pang, X.; Shi, M.; Zhang, X.-Y.; Qin, Q.-L.; Zhou, B.-C.; Zhang, Y.-Z.; et al. A novel exopolysaccharide from deep-sea bacterium Zunongwangia profunda SM-A87: Low-cost fermentation, moisture retention, and antioxidant activities. Appl. Microbiol. Biotechnol. 2014, 98, 7437–7445. [Google Scholar] [CrossRef] [PubMed]
- Chikkanna, A.; Ghosh, D.; Kishore, A. Expression and characterization of a potential exopolysaccharide from a newly isolated halophilic thermotolerant bacteria Halomonas nitroreducensstrain WB1. PeerJ 2018, 6, e4684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nadzir, M.M.; Nurhayati, R.W.; Idris, F.N.; Nguyen, M.H. Biomedical Applications of Bacterial Exopolysaccharides: A Review. Polymers 2021, 13, 530. [Google Scholar] [CrossRef]
- Li, N.; Wang, C.; Georgiev, M.I.; Bajpai, V.K.; Tundis, R.; Simal-Gandara, J.; Lu, X.; Xiao, J.; Tang, X.; Qiao, X. Advances in dietary polysaccharides as anticancer agents: Structure-activity relationship. Trends Food Sci. Technol. 2021, 111, 360–377. [Google Scholar] [CrossRef]
- Selim, M.S.; Amer, S.K.; Mohamed, S.S.; Mounier, M.M.; Rifaat, H.M. Production and characterisation of exopolysaccharide from Streptomyces carpaticus isolated from marine sediments in Egypt and its effect on breast and colon cell lines. J. Genet. Eng. Biotechnol. 2017, 16, 23–28. [Google Scholar] [CrossRef]
- Martos-Benítez, F.D.; Gutiérrez-Noyola, A.; Echevarría-Víctores, A. Postoperative complications and clinical outcomes among patients undergoing thoracic and gastrointestinal cancer surgery: A prospective cohort study. Rev. Bras. Ter. Intensiv. 2016, 28, 40–48. [Google Scholar] [CrossRef]
- Boogaard, W.M.C.V.D.; Komninos, D.S.J.; Vermeij, W.P. Chemotherapy Side-Effects: Not All DNA Damage Is Equal. Cancers 2022, 14, 627. [Google Scholar] [CrossRef] [PubMed]
- Do, T.T.H.; Lai, T.N.B.; Stephenson, S.L.; Tran, H.T.M. Cytotoxicity activities and chemical characteristics of exopolysaccharides and intracellular polysaccharides of Physarum polycephalum microplasmodia. BMC Biotechnol. 2021, 21, 28. [Google Scholar] [CrossRef] [PubMed]
- Abdelnasser, S.M.; Yahya, S.M.M.; Mohamed, W.F.; Asker, M.M.S.; Abu Shady, H.M.; Mahmoud, M.G.; Gadallah, M.A. Antitumor Exopolysaccharides Derived from Novel Marine Bacillus: Isolation, Characterization Aspect and Biological Activity. Asian Pac. J. Cancer Prev. 2017, 18, 1847–1854. [Google Scholar] [CrossRef]
- Amer, M.S.; Zaghloul, E.H.; Ibrahim, M.I. Characterization of exopolysaccharide produced from marine-derived Aspergillus terreus SEI with prominent biological activities. Egypt. J. Aquat. Res. 2020, 46, 363–369. [Google Scholar] [CrossRef]
- Mahgoub, A.M.; Mahmoud, M.G.; Selim, M.S.; EL Awady, M.E. Exopolysaccharide from Marine Bacillus velezensis MHM3 Induces Apoptosis of Human Breast Cancer MCF-7 Cells through a Mitochondrial Pathway. Asian Pac. J. Cancer Prev. 2018, 19, 1957–1963. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.Y.; Youness, E.R.; Mahmoud, M.G.; Asker, M.S.; El-Newary, S.A. Acidic Exopolysaccharide Produced from Marine Bacillus amyloliquefaciens 3MS 2017 for the Protection and Treatment of Breast Cancer. Breast Cancer Basic Clin. Res. 2020, 14, 1178223420902075. [Google Scholar] [CrossRef] [Green Version]
- Tukenmez, U.; Aktas, B.; Aslim, B.; Yavuz, S. The relationship between the structural characteristics of lactobacilli-EPS and its ability to induce apoptosis in colon cancer cells in vitro. Sci. Rep. 2019, 9, 8268. [Google Scholar] [CrossRef] [Green Version]
- Nussinov, R.; Tsai, C.-J.; Jang, H. Anticancer drug resistance: An update and perspective. Drug Resist. Updat. 2021, 59, 100796. [Google Scholar] [CrossRef]
- Terreni, M.; Taccani, M.; Pregnolato, M. New Antibiotics for Multidrug-Resistant Bacterial Strains: Latest Research Developments and Future Perspectives. Molecules 2021, 26, 2671. [Google Scholar] [CrossRef]
- Miethke, M.; Pieroni, M.; Weber, T.; Brönstrup, M.; Hammann, P.; Halby, L.; Arimondo, P.B.; Glaser, P.; Aigle, B.; Bode, H.B.; et al. Towards the sustainable discovery and development of new antibiotics. Nat. Rev. Chem. 2021, 5, 726–749. [Google Scholar] [CrossRef]
- Aullybux, A.A.; Puchooa, D.; Bahorun, T.; Jeewon, R. Phylogenetics and antibacterial properties of exopolysaccharides from marine bacteria isolated from Mauritius seawater. Ann. Microbiol. 2019, 69, 957–972. [Google Scholar] [CrossRef]
- Arayes, M.A.; Mabrouk, M.E.M.; Sabry, S.A.; Abdella, B. Exopolysaccharide production from Alkalibacillus sp. w3: Statistical optimization and biological activity. Biologia 2022, 78, 229–240. [Google Scholar] [CrossRef]
- Zhou, Y.; Cui, Y.; Qu, X. Exopolysaccharides of lactic acid bacteria: Structure, bioactivity and associations: A review. Carbohydr. Polym. 2018, 207, 317–332. [Google Scholar] [CrossRef] [PubMed]
- Rajoka, M.S.R.; Mehwish, H.M.; Hayat, H.F.; Hussain, N.; Sarwar, S.; Aslam, H.; Nadeem, A.; Shi, J. Characterization, the Antioxidant and Antimicrobial Activity of Exopolysaccharide Isolated from Poultry Origin Lactobacilli. Probiotics Antimicrob. Proteins 2018, 11, 1132–1142. [Google Scholar] [CrossRef]
- Abdalla, A.K.; Ayyash, M.M.; Olaimat, A.N.; Osaili, T.M.; Al-Nabulsi, A.A.; Shah, N.P.; Holley, R. Exopolysaccharides as Antimicrobial Agents: Mechanism and Spectrum of Activity. Front. Microbiol. 2021, 12, 664395. [Google Scholar] [CrossRef]
- Andrew, M.; Jayaraman, G. Structural features of microbial exopolysaccharides in relation to their antioxidant activity. Carbohydr. Res. 2019, 487, 107881. [Google Scholar] [CrossRef] [PubMed]
- Selim, M.S.; Mohamed, S.S.; Mahmoud, M.G.; Ibrahim, A.Y.; A Ghazy, E. Production, characterization, and antioxidant activities of bacterial exopolysaccharides extracted from petroleum oil water. Egypt. Pharm. J. 2019, 18, 42. [Google Scholar] [CrossRef]
- Martemucci, G.; Costagliola, C.; Mariano, M.; D’andrea, L.; Napolitano, P.; D’alessandro, A.G. Free Radical Properties, Source and Targets, Antioxidant Consumption and Health. Oxygen 2022, 2, 48–78. [Google Scholar] [CrossRef]
- Adwas, A.A.; Elsayed, A.; Azab, A.E.; Quwaydir, F.A. Oxidative stress and antioxidant mechanisms in human body. J. Appl. Biotechnol. Bioeng. 2019, 6, 43–47. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [Green Version]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef] [PubMed]
- Sarangarajan, R.; Meera, S.; Rukkumani, R.; Sankar, P.; Anuradha, G. Antioxidants: Friend or foe? Asian Pac. J. Trop. Med. 2017, 10, 1111–1116. [Google Scholar] [CrossRef]
- Yan, M.-X.; Mao, W.-J.; Liu, X.; Wang, S.-Y.; Xia, Z.; Cao, S.-J.; Li, J.; Qin, L.; Xian, H.-L. Extracellular polysaccharide with novel structure and antioxidant property produced by the deep-sea fungus Aspergillus versicolor N2bc. Carbohydr. Polym. 2016, 147, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Prathyusha, A.; Sheela, G.M.; Bramhachari, P. Chemical characterization and antioxidant properties of exopolysaccharides from mangrove filamentous fungi Fusarium equiseti ANP2. Biotechnol. Rep. 2018, 19, e00277. [Google Scholar] [CrossRef] [PubMed]
- Meulmeester, F.L.; Luo, J.; Martens, L.G.; Mills, K.; van Heemst, D.; Noordam, R. Antioxidant Supplementation in Oxidative Stress-Related Diseases: What Have We Learned from Studies on Alpha-Tocopherol? Antioxidants 2022, 11, 2322. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, Y.; Jiang, Y.; Chu, W. Prebiotic, Antioxidant, and Immunomodulatory Properties of Acidic Exopolysaccharide from Marine Rhodotorula RY1801. Front. Nutr. 2021, 8, 710668. [Google Scholar] [CrossRef] [PubMed]
- Ayyash, M.; Abu-Jdayil, B.; Olaimat, A.; Esposito, G.; Itsaranuwat, P.; Osaili, T.; Obaid, R.S.; Kizhakkayil, J.; Liu, S.-Q. Physicochemical, bioactive and rheological properties of an exopolysaccharide produced by a probiotic Pediococcus pentosaceus M41. Carbohydr. Polym. 2020, 229, 115462. [Google Scholar] [CrossRef]
- Li, C.; Wang, J.; Wang, Y.; Gao, H.; Wei, G.; Huang, Y.; Yu, H.; Gan, Y.; Wang, Y.; Mei, L. Recent progress in drug delivery. Acta Pharm. Sin. B 2019, 9, 1145–1162. [Google Scholar] [CrossRef]
- Adepu, S.; Ramakrishna, S. Controlled Drug Delivery Systems: Current Status and Future Directions. Molecules 2021, 26, 5905. [Google Scholar] [CrossRef]
- Tabernero, A.; Cardea, S. Microbial Exopolysaccharides as Drug Carriers. Polymers 2020, 12, 2142. [Google Scholar] [CrossRef]
- Laubach, J.; Joseph, M.; Brenza, T.; Gadhamshetty, V.; Sani, R.K. Exopolysaccharide and biopolymer-derived films as tools for transdermal drug delivery. J. Control. Release 2021, 329, 971–987. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Montes, E. Dextran: Sources, Structures, and Properties. Polysaccharides 2021, 2, 554–565. [Google Scholar] [CrossRef]
- Huang, G.; Huang, H. Application of dextran as nanoscale drug carriers. Nanomedicine 2018, 13, 3149–3158. [Google Scholar] [CrossRef]
- Wang, H.; Dai, T.; Zhou, S.; Huang, X.; Li, S.; Sun, K.; Zhou, G.; Dou, H. Self-Assembly Assisted Fabrication of Dextran-Based Nanohydrogels with Reduction-Cleavable Junctions for Applications as Efficient Drug Delivery Systems. Sci. Rep. 2017, 7, 40011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, Y.; Wang, H.; Dou, H.-J.; Fan, X.; Fei, X.-C.; Wang, L.; Cheng, S.; Janin, A.; Zhao, W.L. Doxorubicin-loaded dextran-based nano-carriers for highly efficient inhibition of lymphoma cell growth and synchronous reduction of cardiac toxicity. Int. J. Nanomed. 2018, 13, 5673–5683. [Google Scholar] [CrossRef] [Green Version]
- de Siqueira, E.C.; Rebouças, J.d.S.; Pinheiro, I.O.; Formiga, F.R. Levan-based nanostructured systems: An overview. Int. J. Pharm. 2020, 580, 119242. [Google Scholar] [CrossRef]
- Garg, U.; Chauhan, S.; Nagaich, U.; Jain, N. Current Advances in Chitosan Nanoparticles Based Drug Delivery and Targeting. Adv. Pharm. Bull. 2019, 9, 195–204. [Google Scholar] [CrossRef] [Green Version]
- Sultan, M.H.; Moni, S.S.; Madkhali, O.A.; Bakkari, M.A.; Alshahrani, S.; Alqahtani, S.S.; Alhakamy, N.A.; Mohan, S.; Ghazwani, M.; Bukhary, H.A.; et al. Characterization of cisplatin-loaded chitosan nanoparticles and rituximab-linked surfaces as target-specific injectable nano-formulations for combating cancer. Sci. Rep. 2022, 12, 468. [Google Scholar] [CrossRef]
- Cinan, E.; Cesur, S.; Haskoylu, M.E.; Gunduz, O.; Oner, E.T. Resveratrol-Loaded Levan Nanoparticles Produced by Electrohydrodynamic Atomization Technique. Nanomaterials 2021, 11, 2582. [Google Scholar] [CrossRef]
- Sun, L.; Chen, Y.; Zhou, Y.; Guo, D.; Fan, Y.; Guo, F.; Zheng, Y.; Chen, W. Preparation of 5-fluorouracil-loaded chitosan nanoparticles and study of the sustained release in vitro and in vivo. Asian J. Pharm. Sci. 2017, 12, 418–423. [Google Scholar] [CrossRef]
- Amer, M.S.; Ibrahim, H.A.H. Chitosan from marine-derived Penicillum spinulosum MH2 cell wall with special emphasis on its antimicrobial and antifouling properties. Egypt. J. Aquat. Res. 2019, 45, 359–365. [Google Scholar] [CrossRef]
- Poli, A.; Kazak, H.; Gürleyendağ, B.; Tommonaro, G.; Pieretti, G.; Öner, E.T.; Nicolaus, B. High level synthesis of levan by a novel Halomonas species growing on defined media. Carbohydr. Polym. 2009, 78, 651–657. [Google Scholar] [CrossRef]
- Zykwinska, A.; Marquis, M.; Sinquin, C.; Cuenot, S.; Colliec-Jouault, S. Assembly of HE800 exopolysaccharide produced by a deep-sea hydrothermal bacterium into microgels for protein delivery applications. Carbohydr. Polym. 2016, 142, 213–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zykwinska, A.; Marquis, M.; Godin, M.; Marchand, L.; Sinquin, C.; Garnier, C.; Jonchère, C.; Chédeville, C.; Le Visage, C.; Guicheux, J.; et al. Microcarriers Based on Glycosaminoglycan-Like Marine Exopolysaccharide for TGF-β1 Long-Term Protection. Mar. Drugs 2019, 17, 65. [Google Scholar] [CrossRef] [Green Version]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef] [PubMed]
- Mathivanan, K.; Chandirika, J.U.; Mathimani, T.; Rajaram, R.; Annadurai, G.; Yin, H. Production and functionality of exopolysaccharides in bacteria exposed to a toxic metal environment. Ecotoxicol. Environ. Saf. 2020, 208, 111567. [Google Scholar] [CrossRef] [PubMed]
- Velkova, Z.; Kirova, G.; Stoytcheva, M.; Kostadinova, S.; Todorova, K.; Gochev, V. Immobilized microbial biosorbents for heavy metals removal. Eng. Life Sci. 2018, 18, 871–881. [Google Scholar] [CrossRef] [Green Version]
- Mohapatra, R.K.; Parhi, P.K.; Patra, J.K.; Panda, C.R.; Thatoi, H.N. Biodetoxification of Toxic Heavy Metals by Marine Metal Resistant Bacteria—A Novel Approach for Bioremediation of the Polluted Saline Environment. In Microbial Biotechnology; Patra, J., Vishnuprasad, C., Das, G., Eds.; Springer: Singapore, 2017; pp. 343–376. [Google Scholar] [CrossRef]
- Nanda, M.; Kumar, V.; Sharma, D. Multimetal tolerance mechanisms in bacteria: The resistance strategies acquired by bacteria that can be exploited to ‘clean-up’ heavy metal contaminants from water. Aquat. Toxicol. 2019, 212, 1–10. [Google Scholar] [CrossRef]
- Gupta, P.; Diwan, B. Bacterial Exopolysaccharide mediated heavy metal removal: A Review on biosynthesis, mechanism and remediation strategies. Biotechnol. Rep. 2016, 13, 58–71. [Google Scholar] [CrossRef]
- Concórdio-Reis, P.; Reis, M.A.M.; Freitas, F. Biosorption of Heavy Metals by the Bacterial Exopolysaccharide FucoPol. Appl. Sci. 2020, 10, 6708. [Google Scholar] [CrossRef]
- Ntozonke, N.; Okaiyeto, K.; Okoli, A.S.; Olaniran, A.O.; Nwodo, U.U.; Okoh, A.I. A Marine Bacterium, Bacillus sp. Isolated from the Sediment Samples of Algoa Bay in South Africa Produces a Polysaccharide-Bioflocculant. Int. J. Environ. Res. Public Health 2017, 14, 1149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Li, Z.; Liu, P.; Liu, Y.; Wang, Y.; Li, Q.; Zhen, C. Characterization of a novel bioflocculant from a marine bacterium and its application in dye wastewater treatment. BMC Biotechnol. 2017, 17, 84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaisuwan, W.; Jantanasakulwong, K.; Wangtueai, S.; Phimolsiripol, Y.; Chaiyaso, T.; Techapun, C.; Phongthai, S.; You, S.; Regenstein, J.M.; Seesuriyachan, P. Microbial exopolysaccharides for immune enhancement: Fermentation, modifications and bioactivities. Food Biosci. 2020, 35, 100564. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Wu, Y.-J.; Hu, C.-Y. Monosaccharide composition influence and immunomodulatory effects of probiotic exopolysaccharides. Int. J. Biol. Macromol. 2019, 133, 575–582. [Google Scholar] [CrossRef]
- Rajoka, M.S.R.; Wu, Y.; Mehwish, H.M.; Bansal, M.; Zhao, L. Lactobacillus exopolysaccharides: New perspectives on engineering strategies, physiochemical functions, and immunomodulatory effects on host health. Trends Food Sci. Technol. 2020, 103, 36–48. [Google Scholar] [CrossRef]
- Verkhnyatskaya, S.; Ferrari, M.; de Vos, P.; Walvoort, M.T.C. Shaping the Infant Microbiome with Non-digestible Carbohydrates. Front. Microbiol. 2019, 10, 343. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Li, W.; Rui, X.; Li, T.; Chen, X.; Jiang, M.; Dong, M. Chemical modification, characterization and bioactivity of a released exopolysaccharide (r-EPS1) from Lactobacillus plantarum. Glycoconj. J. 2014, 32, 17–27. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, Z.; Tao, X.; Wei, H. Characterization and sulfated modification of an exopolysaccharide from Lactobacillus plantarum ZDY2013 and its biological activities. Carbohydr. Polym. 2016, 153, 25–33. [Google Scholar] [CrossRef]
- Senni, K.; Gueniche, F.; Changotade, S.; Septier, D.; Sinquin, C.; Ratiskol, J.; Lutomski, D.; Godeau, G.; Guezennec, J.; Colliec-Jouault, S. Unusual Glycosaminoglycans from a Deep Sea Hydrothermal Bacterium Improve Fibrillar Collagen Structuring and Fibroblast Activities in Engineered Connective Tissues. Mar. Drugs 2013, 11, 1351–1369. [Google Scholar] [CrossRef] [Green Version]
- Di Donato, P.; Poli, A.; Taurisano, V.; Abbamondi, G.R.; Nicolaus, B.; Tommonaro, G. Recent Advances in the Study of Marine Microbial Biofilm: From the Involvement of Quorum Sensing in Its Production up to Biotechnological Application of the Polysaccharide Fractions. J. Mar. Sci. Eng. 2016, 4, 34. [Google Scholar] [CrossRef] [Green Version]
- García, A.; Fernández-Sandoval, M.T.; Morales-Guzmán, D.; Martínez-Morales, F.; Trejo-Hernández, M.R. Advances in exopolysaccharide production from marine bacteria. J. Chem. Technol. Biotechnol. 2022, 97, 2694–2705. [Google Scholar] [CrossRef]
- Hereher, F.; ElFallal, A.; Abou-Dobara, M.; Toson, E.; Abdelaziz, M.M. Cultural optimization of a new exopolysaccharide producer Micrococcus roseus. Beni-Suef Univ. J. Basic Appl. Sci. 2018, 7, 632–639. [Google Scholar] [CrossRef]
- Biswas, J.; Paul, A.K. Optimization of factors influencing exopolysaccharide production by Halomonas xianhensis SUR308 under batch culture. AIMS Microbiol. 2017, 3, 564–579. [Google Scholar] [CrossRef]
- Nguyen, P.-T.; Nguyen, T.-T.; Bui, D.-C.; Hong, P.-T.; Hoang, Q.-K.; Nguyen, H.-T. Exopolysaccharide production by lactic acid bacteria: The manipulation of environmental stresses for industrial applications. AIMS Microbiol. 2020, 6, 451–469. [Google Scholar] [CrossRef] [PubMed]
- Gudiña, E.J.; Couto, M.R.; Silva, S.P.; Coelho, E.; Coimbra, M.A.; Teixeira, J.A.; Rodrigues, L.R. Sustainable Exopolysaccharide Production by Rhizobium viscosum CECT908 Using Corn Steep Liquor and Sugarcane Molasses as Sole Substrates. Polymers 2022, 15, 20. [Google Scholar] [CrossRef]
- Delbarre-Ladrat, C.; Sinquin, C.; Lebellenger, L.; Zykwinska, A.; Colliec-Jouault, S. Exopolysaccharides produced by marine bacteria and their applications as glycosaminoglycan-like molecules. Front. Chem. 2014, 2, 85. [Google Scholar] [CrossRef] [Green Version]
- Ma, Z.-C.; Liu, N.-N.; Chi, Z.; Liu, G.-L.; Chi, Z.-M. Genetic Modification of the Marine-Isolated Yeast Aureobasidium melanogenum P16 for Efficient Pullulan Production from Inulin. Mar. Biotechnol. 2015, 17, 511–522. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, J. Bacterial exopolysaccharides: Chemical structures, gene clusters and genetic engineering. Int. J. Biol. Macromol. 2021, 173, 481–490. [Google Scholar] [CrossRef]
- Li, S.; Xiong, Q.; Lai, X.; Li, X.; Wan, M.; Zhang, J.; Yan, Y.; Cao, M.; Lu, L.; Guan, J.; et al. Molecular Modification of Polysaccharides and Resulting Bioactivities. Compr. Rev. Food Sci. Food Saf. 2015, 15, 237–250. [Google Scholar] [CrossRef]
- Wang, J.; Salem, D.R.; Sani, R.K. Extremophilic exopolysaccharides: A review and new perspectives on engineering strategies and applications. Carbohydr. Polym. 2018, 205, 8–26. [Google Scholar] [CrossRef]
- Chopin, N.; Sinquin, C.; Ratiskol, J.; Zykwinska, A.; Weiss, P.; Cérantola, S.; Le Bideau, J.; Colliec-Jouault, S. A Direct Sulfation Process of a Marine Polysaccharide in Ionic Liquid. BioMed Res. Int. 2015, 2015, 508656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, S.; Zhai, Y.; Wang, X.; Fan, Q.; Dong, X.; Chen, M.; Han, T. Phosphorylation of polysaccharides: A review on the synthesis and bioactivities. Int. J. Biol. Macromol. 2021, 184, 946–954. [Google Scholar] [CrossRef] [PubMed]
- Vitorino, L.C.; Bessa, L.A. Microbial Diversity: The Gap between the Estimated and the Known. Diversity 2018, 10, 46. [Google Scholar] [CrossRef] [Green Version]
| Organism Name | Composition | Molecular Weight (kDa) | Possible Structure(s) | Biological Activity (If Any) | Level of Characterization | Reference |
|---|---|---|---|---|---|---|
| Vibrio alginolyticus | Mannose, glucosamine, gluconic acid, galactosamine and arabinose (5:9:3.4:0.5:0.8) | 14.8 | N/A | Antitumor activity | Molecular weight, monosaccharide composition, functional groups, surface morphology, and element composition | [30] |
| Bacillus sp. | Mannose, glucosamine, galacturonic acid, glucose and xylose (1:2.58:0.68:0.13:3.09:1.41) | 22.3 | N/A | Anticancer through different mechanisms | Molecular weight and monosaccharide composition | [31,32,33] |
| Microbacterium aurantiacum | Glucose, mannose, fucose and glucuronic acid | 7000 |  | Anti-oxidant activity and viscosifying property | Molecular weight, total sugar, total protein and uronic acid content, functional groups, element analysis, monosaccharide composition, and partial structure | [34] |
| Rhodobacter johrii | Glucose, galactose, rhamnose and glucuronic acid (3:1.5:0.25:0.25) | 2000 |  | Bioemulsification property | Molecular weight, total sugar, total protein and uronic acid content, functional groups, element analysis, monosaccharide composition, and partial structure | [35] |
| Pseudoalteromonas sp. | Mannose, glucose, galactose, rhamnose, xylose, N-acetylgalactosamine and N-acetylglucosamine | >2000 |  | - | Molecular weight, total sugar content, monosaccharide composition, linkage analysis, and structure | [36] |
| Bacillus subtilis | Glucose, rhamnose and arabinose | 14.8 |  | Anti-oxidant, anti-inflammatory, cytotoxicity, and anti-Alzheimer activities | Molecular weight, functional groups, uronic acid and sulfate content, monosaccharide composition, surface morphology, and crystal structure | [37] |
| Alteromonas sp. | Rhamnose, mannose and galacturonic acid | 167 |  | Biosorption of heavy metals | Molecular weight, total sugar content, functional groups, linkage analysis and structure | [38] |
| Neorhizobium urealyticum K1T sp. nov. | Glucose and galacturonic acid | 207 | N/A | Emulsification | Molecular weight, total sugar and total protein content, functional groups, element analysis, monosaccharide composition, and partial structure | [39] |
| B. licheniformis | Fructose, fucose, glucose, galactosamine and mannose (1.0:0.75:0.28:tr:tr) * | 1000 |  | Cytotoxic, antiviral and immunomodulatory properties | Molecular weight, monosaccharide composition, polar lipids and fatty acids content, and structure | [40,41] |
| Pantoea sp. | Glucose, galactose, N-acetyl galactosamine and glucosamine (1.9:1:0.4:0.02) | 175 | N/A | Cutaneous wound healing | Molecular weight, monosaccharide composition, total sugar and total protein contents, and functional groups | [42] |
| Bacillus sp. | Mannose, glucosamine, glucose, and galactose (1.00:0.02:0.07:0.02) | 89 |  | Immunomodulation | Molecular weight, monosaccharide composition, linkage analysis, functional groups, and structure | [43] |
| Natronotalea sambharensis sp. nov. | Mannose, glucose and glucuronic acid | 4.6 × 106 |  | Anti-oxidant | Molecular weight, total sugar, total protein, nucleic acid and uronic acid content, element analysis, functional groups, surface morphology, monosaccharide composition, and partial structure | [44] |
| Aspergillus ochraceus | Mannose and galactose (2.16:1.00) | 29 |  | - | Molecular weight, total sugar, protein and uronic acid content, monosaccharide composition, linkage analysis, sugar configuration, and structure | [45] |
| Aspergillus versicolor | Glucose | 5.1 |  | Immunomodulation | Molecular weight, functional groups, monosaccharide composition, and linkage | [46] |
| Penicillium janthinellum | Mannose and galactose | 10.24 |  | Anti-diabetic activity | Molecular weight, total sugar and total protein content, functional groups, monosaccharide composition, linkage analysis, sugar configurations, and structure | [47] |
| Species Name | Type of Environment | EPS Name | EPS Composition | Distinguishing Features | Reference |
|---|---|---|---|---|---|
| Polaribacter sp. | Polar region (Arctic) | EPS | N-acetyl glucosamine, mannose, glucuronic acid, moderate amounts of galactose and fucose, and minor amounts of glucose and rhamnose | Tolerance to high salinity and a wide pH range | [52] |
| Alteromonas infernus | Deep-sea hydrothermal vent | GY785 | Glucose, galactose, galacturonic acid and glucuronic acid | - | [53] |
| Vibrio diabolicus | Deep-sea hydrothermal vent | HE800 | N-acetyl glucosamine, N-acetyl galactosamine and glucuronic acid | - | [53] |
| Pseudomonas sp. | Polar region (Antarctica) | EPS | Glucose, galactose, fucose, and uronic acid | Cryoprotection and emulsification | [54] |
| Zunongwangia profunda | Deep-sea (1245 m) | EPS | - | High moisture retention and anti-oxidant potential | [55] |
| Halomonas nitroreducens | Hydrothermal vent | EPS | Three different EPSs made up of glucose, mannose, galactose, and small quantities of rhamnose, arabinose, and galacturonic acid in variable amounts | Pseudoplastic nature with high emulsifying, anti-oxidant, and heavy metal-binding activities | [56] |
| Patent Number | Species | Source | Characteristic of Polysaccharide | Patented Application |
|---|---|---|---|---|
| AU2016330332B2 | Alteromonas sp. | Deep-sea hydrothermal environment | 15 kDa over-sulfated exopolysaccharide (GYS15) | Anti-metastatic and/or related uses for various cancers |
| CN116120477A | - | Antarctic sea | 4350–4360 kDa extracellular polysaccharide with low temperature resistance and moisturizing functions | Preparation method and application |
| ES2585398B1 | Pseudomonas sp. | Marine sediment | 2000 kDa exopolysaccharide with cryoprotective, emulsifying, thickening, stabilizing, or texturing properties | Cosmetic application |
| CN105087450A | Alteromonas marina | - | 167 kDa exopolysaccharide | Culture of organism and preparation of polysaccharide |
| US10993434B2 | Pseudoalteromonas sp. | Polar region | 100–430 kDa exopolysaccharide | Cryoprotection of cells |
| CN107523515A | Pseudoalteromonas sp. | - | - | Absorption of heavy metals from drinking water |
| LU501700B1 | Aerococcus urinaeequi | - | - | Growth of microorganism and polysaccharide production |
| CN109457001A | - | - | Polysaccharide with mannose, glucosamine, ribose, rhamnose, glucuronic acid, galacturonic acid, glucose, galactolipin, xylose, and arabinose | Preparation and application as decoloring agent |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeewon, R.; Aullybux, A.A.; Puchooa, D.; Nazurally, N.; Alrefaei, A.F.; Zhang, Y. Marine Microbial Polysaccharides: An Untapped Resource for Biotechnological Applications. Mar. Drugs 2023, 21, 420. https://doi.org/10.3390/md21070420
Jeewon R, Aullybux AA, Puchooa D, Nazurally N, Alrefaei AF, Zhang Y. Marine Microbial Polysaccharides: An Untapped Resource for Biotechnological Applications. Marine Drugs. 2023; 21(7):420. https://doi.org/10.3390/md21070420
Chicago/Turabian StyleJeewon, Rajesh, Aadil Ahmad Aullybux, Daneshwar Puchooa, Nadeem Nazurally, Abdulwahed Fahad Alrefaei, and Ying Zhang. 2023. "Marine Microbial Polysaccharides: An Untapped Resource for Biotechnological Applications" Marine Drugs 21, no. 7: 420. https://doi.org/10.3390/md21070420
APA StyleJeewon, R., Aullybux, A. A., Puchooa, D., Nazurally, N., Alrefaei, A. F., & Zhang, Y. (2023). Marine Microbial Polysaccharides: An Untapped Resource for Biotechnological Applications. Marine Drugs, 21(7), 420. https://doi.org/10.3390/md21070420