Microalgae: A Promising Source of Bioactive Phycobiliproteins
Abstract
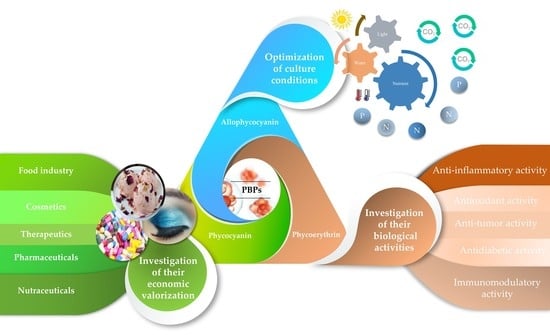
:1. Introduction
2. Impact of Culture Conditions on PBP Production by Microalgae
3. Phycobiliproteins from Microalgae
4. Pharmaceutical Potentials of Phycobiliproteins
4.1. Antioxidant Activity
4.2. Antitumor Activity
4.3. Anti-Inflammatory Activity
4.4. Antidiabetic Activity
4.5. Other Biological Activities
5. Economic Valorization of PBPs from Microalgae
5.1. Phycobiliproteins from Microalgae in Food Field
5.2. Phycobiliproteins from Microalgae in Biotechnology and Therapeutic Field
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johnson, T.J.; Katuwal, S.; Anderson, G.A.; Gu, L.; Zhou, R.; Gibbons, W.R. Photobioreactor Cultivation Strategies for Microalgae and Cyanobacteria. Biotechnol. Prog. 2018, 34, 811–827. [Google Scholar] [CrossRef]
- Sarnaik, A.; Sawant, K.; Khadilkar, J.; Pillai, G.; Pandit, R.; Lali, A. Cyanobacterial Cell Factories for Improved Carotenoid Biosynthesis through a Synthetic Biology Approach. In ACS Symposium Series; Rathinam, N.K., Sani, R.K., Eds.; American Chemical Society: Washington, DC, USA, 2019; Volume 1329, pp. 23–39. ISBN 978-0-8412-3500-7. [Google Scholar]
- Nobre, B.P.; Villalobos, F.; Barragán, B.E.; Oliveira, A.C.; Batista, A.P.; Marques, P.A.S.S.; Mendes, R.L.; Sovová, H.; Palavra, A.F.; Gouveia, L. A Biorefinery from Nannochloropsis sp. Microalga—Extraction of Oils and Pigments. Production of Biohydrogen from the Leftover Biomass. Bioresour. Technol. 2013, 135, 128–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, P.; Sharma, N. Industrial and Biotechnological Applications of Algae: A Review. J. Asia-Pac. Biodivers. 2017, 1, 1–25. [Google Scholar] [CrossRef]
- Stengel, D.B.; Connan, S.; Popper, Z.A. Algal Chemodiversity and Bioactivity: Sources of Natural Variability and Implications for Commercial Application. Biotechnol. Adv. 2011, 29, 483–501. [Google Scholar] [CrossRef] [PubMed]
- Schubert, N.; García-Mendoza, E.; Pacheco-Ruiz, I. Carotenoid Composition of Marine Red Algae. J. Phycol. 2006, 42, 1208–1216. [Google Scholar] [CrossRef]
- Mendes, A.; Reis, A.; Vasconcelos, R.; Guerra, P.; Lopes da Silva, T. Crypthecodinium Cohnii with Emphasis on DHA Production: A Review. J. Appl. Phycol. 2009, 21, 199–214. [Google Scholar] [CrossRef]
- Niu, J.-F.; Wang, G.-C.; Tseng, C.-K. Method for Large-Scale Isolation and Purification of R-Phycoerythrin from Red Alga Polysiphonia Urceolata Grev. Protein Expr. Purif. 2006, 49, 23–31. [Google Scholar] [CrossRef]
- Qiang, X.; Wang, L.; Niu, J.; Gong, X.; Wang, G. Phycobiliprotein as Fluorescent Probe and Photosensitizer: A Systematic Review. Int. J. Biol. Macromol. 2021, 193, 1910–1917. [Google Scholar] [CrossRef]
- Waggoner, A. Fluorescent Labels for Proteomics and Genomics. Curr. Opin. Chem. Biol. 2006, 10, 62–66. [Google Scholar] [CrossRef]
- Gargouch, N.; Karkouch, I.; Elleuch, J.; Elkahoui, S.; Michaud, P.; Abdelkafi, S.; Laroche, C.; Fendri, I. Enhanced B-Phycoerythrin Production by the Red Microalga Porphyridium marinum: A Powerful Agent in Industrial Applications. Int. J. Biol. Macromol. 2018, 120, 2106–2114. [Google Scholar] [CrossRef]
- Kannaujiya, V.K.; Kumar, D.; Singh, V.; Sinha, R.P. Advances in Phycobiliproteins Research: Innovations and Commercialization. In Natural Bioactive Compounds; Elsevier: Amsterdam, The Netherlands, 2021; pp. 57–81. [Google Scholar]
- de Morais, M.G.; da Fontoura Prates, D.; Moreira, J.B.; Duarte, J.H.; Costa, J.A.V. Phycocyanin from Microalgae: Properties, Extraction and Purification, with Some Recent Applications. Ind. Biotechnol. 2018, 14, 30–37. [Google Scholar] [CrossRef]
- Borowitzka, M.A. High-Value Products from Microalgae—Their Development and Commercialisation. J. Appl. Phycol. 2013, 25, 743–756. [Google Scholar] [CrossRef]
- Phycocyanin Market: Food & Beverage Application to Hold Close to 85% Value Share Throughout the Forecast Period: Global Industry Analysis (2013–2017) & Opportunity Assessment (2018–2028). Available online: https://www.marketresearch.com/Future-Market-Insights-v4066/Phycocyanin-Food-Beverage-Application-Hold-11865287/ (accessed on 29 October 2022).
- Pagels, F.; Guedes, A.C.; Amaro, H.M.; Kijjoa, A.; Vasconcelos, V. Phycobiliproteins from Cyanobacteria: Chemistry and Biotechnological Applications. Biotechnol. Adv. 2019, 37, 422–443. [Google Scholar] [CrossRef] [PubMed]
- Schulze, P.S.C.; Barreira, L.A.; Pereira, H.G.C.; Perales, J.A.; Varela, J.C.S. Light Emitting Diodes (LEDs) Applied to Microalgal Production. Trends Biotechnol. 2014, 32, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Keithellakpam, O.S.; Nath, T.O.; Oinam, A.S.; Thingujam, I.; Oinam, G.; Dutt, S.G. Effect of External PH on Cyanobacterial Phycobiliproteins Production and Ammonium Excretion. J. Appl. Biol. Biotechnol. 2015, 3, 38–42. [Google Scholar] [CrossRef] [Green Version]
- Mishra, S.K.; Shrivastav, A.; Maurya, R.R.; Patidar, S.K.; Haldar, S.; Mishra, S. Effect of Light Quality on the C-Phycoerythrin Production in Marine Cyanobacteria Pseudanabaena sp. Isolated from Gujarat Coast, India. Protein Expr. Purif. 2012, 81, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Hemlata; Fatma, T. Screening of Cyanobacteria for Phycobiliproteins and Effect of Different Environmental Stress on Its Yield. Bull. Environ. Contam. Toxicol. 2009, 83, 509–515. [Google Scholar] [CrossRef]
- Khattar, J.I.S.; Kaur, S.; Kaushal, S.; Singh, Y.; Singh, D.P.; Rana, S.; Gulati, A. Hyperproduction of Phycobiliproteins by the Cyanobacterium Anabaena fertilissima PUPCCC 410.5 under Optimized Culture Conditions. Algal Res. 2015, 12, 463–469. [Google Scholar] [CrossRef]
- Kim, N.N.; Shin, H.S.; Park, H.G.; Lee, J.; Kil, G.-S.; Choi, C.Y. Profiles of Photosynthetic Pigment Accumulation and Expression of Photosynthesis-Related Genes in the Marine Cyanobacteria Synechococcus sp.: Effects of LED Wavelengths. Biotechnol. Bioprocess. Eng. 2014, 19, 250–256. [Google Scholar] [CrossRef]
- Ma, R.; Lu, F.; Bi, Y.; Hu, Z. Effects of Light Intensity and Quality on Phycobiliprotein Accumulation in the Cyanobacterium Nostoc sphaeroides Kützing. Biotechnol. Lett. 2015, 37, 1663–1669. [Google Scholar] [CrossRef] [Green Version]
- Baer, S.; Heining, M.; Schwerna, P.; Buchholz, R.; Hübner, H. Optimization of Spectral Light Quality for Growth and Product Formation in Different Microalgae Using a Continuous Photobioreactor. Algal Res. 2016, 14, 109–115. [Google Scholar] [CrossRef]
- Coward, T.; Fuentes-Grünewald, C.; Silkina, A.; Oatley-Radcliffe, D.L.; Llewellyn, G.; Lovitt, R.W. Utilising Light-Emitting Diodes of Specific Narrow Wavelengths for the Optimization and Co-Production of Multiple High-Value Compounds in Porphyridium purpureum. Bioresour. Technol. 2016, 221, 607–615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castro, G.F.; Rizzo, R.F.; Passos, T.S.; Santos, B.N.; Dias, D.D.; Domingues, J.R.; Araújo, K.G. Biomass Production by Arthrospira platensis under Different Culture Conditions. Food Sci. Technol. 2015, 35, 18–24. [Google Scholar] [CrossRef] [Green Version]
- Gris, B.; Sforza, E.; Morosinotto, T.; Bertucco, A.; La Rocca, N. Influence of Light and Temperature on Growth and High-Value Molecules Productivity from Cyanobacterium aponinum. J. Appl. Phycol. 2017, 29, 1781–1790. [Google Scholar] [CrossRef]
- Mihova, S.G.; Georgiev, D.I.; Minkova, K.M.; Tchernov, A.A. Phycobiliproteins in Rhodella reticulata and Photoregulatory Effects on Their Content. J. Biotechnol. 1996, 48, 251–257. [Google Scholar] [CrossRef]
- Jahn, W.; Steinbiss, J.; Zetsche, K. Light Intensity Adaptation of the Phycobiliprotein Content of the Red Alga Porphyridium. Planta 1984, 161, 536–539. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, Y.; Guo, S. Growth and Phycocyanin Formation of Spirulina platensis in Photoheterotrophic Culture. Biotechnol. Lett. 1996, 18, 603–608. [Google Scholar] [CrossRef]
- Soletto, D.; Binaghi, L.; Ferrari, L.; Lodi, A.; Carvalho, J.C.M.; Zilli, M.; Converti, A. Effects of Carbon Dioxide Feeding Rate and Light Intensity on the Fed-Batch Pulse-Feeding Cultivation of Spirulina platensis in Helical Photobioreactor. Biochem. Eng. J. 2008, 39, 369–375. [Google Scholar] [CrossRef]
- Chaneva, G.; Furnadzhieva, S.; Minkova, K.; Lukavsky, J. Effect of Light and Temperature on the Cyanobacterium Arthronema africanum—A Prospective Phycobiliprotein-Producing Strain. J. Appl. Phycol. 2007, 19, 537–544. [Google Scholar] [CrossRef]
- Derbel, H.; Elleuch, J.; Tounsi, L.; Nicolo, M.S.; Rizzo, M.G.; Michaud, P.; Fendri, I.; Abdelkafi, S. Improvement of Biomass and Phycoerythrin Production by a Strain of Rhodomonas Sp. Isolated from the Tunisian Coast of Sidi Mansour. Biomolecules 2022, 12, 885. [Google Scholar] [CrossRef]
- Kumar, M.; Kulshreshtha, J.; Singh, G.P. Growth and Biopigment Accumulation of Cyanobacterium Spirulina platensis at Different Light Intensities and Temperature. Braz. J. Microbiol. 2011, 42, 1128–1135. [Google Scholar] [CrossRef] [Green Version]
- Maurya, S.S.; Maurya, J.N.; Pandey, V.D. Factors regulating phycobiliprotein production in cyanobacteria. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 764–771. [Google Scholar]
- Gaignard, C.; Gargouch, N.; Dubessay, P.; Delattre, C.; Pierre, G.; Laroche, C.; Fendri, I.; Abdelkafi, S.; Michaud, P. New Horizons in Culture and Valorization of Red Microalgae. Biotechnol. Adv. 2019, 37, 193–222. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.M.; Kumar, K.; Das, D. Physicochemical Parameters Optimization, and Purification of Phycobiliproteins from the Isolated Nostoc sp. Bioresour. Technol. 2014, 166, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, T.; Bryant, D.A. Growth at Low Temperature Causes Nitrogen Limitation in the Cyanobacterium Synechococcus sp. PCC 7002. Arch. Microbiol. 1997, 169, 10–19. [Google Scholar] [CrossRef]
- Gerloff-Elias, A.; Spijkerman, E.; Proschold, T. Effect of External PH on the Growth, Photosynthesis and Photosynthetic Electron Transport of Chlamydomonas acidophila Negoro, Isolated from an Extremely Acidic Lake (PH 2.6). Plant Cell Environ. 2005, 28, 1218–1229. [Google Scholar] [CrossRef]
- Warren, M.J.; Smith, A.G. Tetrapyrroles: Birth, Life, and Death; Molecular biology intelligence unit; Landes Bioscience: Austin, TX, USA; Springer Science & Business Media: New York, NY, USA, 2009; ISBN 978-0-387-78518-9. [Google Scholar]
- Battersby, A.R. Tetrapyrroles: The Pigments of Life. Nat. Prod. Rep. 2000, 17, 507–526. [Google Scholar] [CrossRef]
- Kaushal, S.; Singh, Y.; Khattar, J.I.S.; Singh, D.P. Phycobiliprotein Production by a Novel Cold Desert Cyanobacterium Nodularia sphaerocarpa PUPCCC 420.1. J. Appl. Phycol. 2017, 29, 1819–1827. [Google Scholar] [CrossRef]
- Hifney, A.F.; Adam, M.S.; Ghareib, G.; Issa, A.A. Allelopathic effects of some weeds on rhizosphere algae at El-Kharga Oasis (New Valley), Egypt. J. Biol. Earth Sci. 2013, 3, B42–B53. [Google Scholar]
- Bahavar, N.; Shokravi, S. Acclimation Response and Ability of Growth and Photosynthesis of Terrestrial Cyanobacterium Cylindrospermum sp. Strain FS 64 under Combined Environmental Factors. Arch. Microbiol. 2022, 204, 165. [Google Scholar] [CrossRef]
- Ismaiel, M.M.S.; El-Ayouty, Y.M.; Piercey-Normore, M. Role of PH on Antioxidants Production by Spirulina (Arthrospira) platensis. Braz. J. Microbiol. 2016, 47, 298–304. [Google Scholar] [CrossRef] [Green Version]
- Mohite, Y.S.; Wakte, P.S. Assessment of Factors Influencing Growth and C-Phycocyanin Production of Arthrospira platensis from Meteoritic Crater Lake. J. Algal Biomass Util. 2011, 2, 53–68. [Google Scholar]
- Deshmukh, D.V.; Puranik, P.R. Statistical Evaluation of Nutritional Components Impacting Phycocyanin Production in Synechocystis sp. Braz. J. Microbiol. 2012, 43, 348–355. [Google Scholar] [CrossRef] [Green Version]
- Velea, S.; Ilie, L.; Filipescu, L. Optimization of Porphyridium purpureum culture growth using two variables experimental design: Light and sodium bicarbonate. Sci. Bull. 2011, 73, 81–94. [Google Scholar]
- Simeunović, J.B.; Marković, S.B.; Kovač, D.J.; Mišan, A.Č.; Mandić, A.I.; Svirčev, Z.B. Filamentous cyanobacteria from Vojvodina region as source of phycobiliprotein pigments as potential natural colorants. Food Feed. Res. 2012, 39, 23–32. [Google Scholar]
- Sarada, R.; Pillai, M.G.; Ravishankar, G.A. Phycocyanin from Spirulina sp: Influence of Processing of Biomass on Phycocyanin Yield, Analysis of Efficacy of Extraction Methods and Stability Studies on Phycocyanin. Process Biochem. 1999, 34, 795–801. [Google Scholar] [CrossRef]
- Borsari, R.R.J.; Morioka, L.R.I.; Ribeiro, M.L.L.; Buzato, J.B.; Pinotti, M.H.P. Mixotrophic Growth of Nostoc sp. on Glucose, Sucrose and Sugarcane Molasses for Phycobiliprotein Production. Acta Sci.–Biol. Sci. 2007, 29, 9–13. [Google Scholar]
- Kovač, D.; Babić, O.; Milovanović, I.; Mišan, A.; Simeunović, J. The Production of Biomass and Phycobiliprotein Pigments in Filamentous Cyanobacteria: The Impact of Light and Carbon Sources. Appl. Biochem. Microbiol. 2017, 53, 539–545. [Google Scholar] [CrossRef]
- Venugopal, V.; Prasanna, R.; Sood, A.; Jaiswal, P.; Kaushik, B.D. Stimulation of Pigment Accumulation In Anabaena azollae Strains: Effect of Light Intensity and Sugars. Folia Microbiol. 2006, 51, 50–56. [Google Scholar] [CrossRef]
- Zeng, X.; Danquah, M.K.; Zhang, S.; Zhang, X.; Wu, M.; Chen, X.D.; Ng, I.-S.; Jing, K.; Lu, Y. Autotrophic Cultivation of Spirulina platensis for CO2 Fixation and Phycocyanin Production. Chem. Eng. J. 2012, 183, 192–197. [Google Scholar] [CrossRef]
- Sharma, G. Effect of Carbon Content, Salinity and PH on Spirulina platensis for Phycocyanin, Allophycocyanin and Phycoerythrin Accumulation. J. Microb. Biochem. Technol. 2014, 6, 202–206. [Google Scholar] [CrossRef] [Green Version]
- Khazi, M.I.; Demirel, Z.; Dalay, M.C. Evaluation of Growth and Phycobiliprotein Composition of Cyanobacteria Isolates Cultivated in Different Nitrogen Sources. J. Appl. Phycol. 2018, 30, 1513–1523. [Google Scholar] [CrossRef]
- Simeunović, J.; Bešlin, K.; Svirčev, Z.; Kovač, D.; Babić, O. Impact of Nitrogen and Drought on Phycobiliprotein Content in Terrestrial Cyanobacterial Strains. J. Appl. Phycol. 2013, 25, 597–607. [Google Scholar] [CrossRef]
- Chentir, I.; Doumandji, A.; Ammar, J.; Zili, F.; Jridi, M.; Markou, G.; Ben Ouada, H. Induced Change in Arthrospira sp. (Spirulina) Intracellular and Extracellular Metabolites Using Multifactor Stress Combination Approach. J. Appl. Phycol. 2018, 30, 1563–1574. [Google Scholar] [CrossRef]
- Singh, N.K.; Parmar, A.; Madamwar, D. Optimization of Medium Components for Increased Production of C-Phycocyanin from Phormidium Ceylanicum and Its Purification by Single Step Process. Bioresour. Technol. 2009, 100, 1663–1669. [Google Scholar] [CrossRef]
- Lemus, N.; Guevara, M.; Lodeiros, C.; Vásquez, A.; Freites, L.; Licet, B. Crecimiento y composición bioquímica de Limnothrix sp. a diferentes salinidades y concentraciones de nitrato. Rev. Colomb. Biotecnol. 2013, 15, 159–166. [Google Scholar]
- Fuenmayor, G.; Jonte, L.; Rosales-Loaiza, N.; Morales, E. Growth of the Marine Cyanobacteria Oscillatoria sp MOF-06 in Relation to PH in Discontinuous Cultures. Rev. Soc. Venez. Microbiol. 2009, 29, 21–25. [Google Scholar]
- Chaloub, R.M.; Motta, N.M.S.; de Araujo, S.P.; de Aguiar, P.F.; da Silva, A.F. Combined Effects of Irradiance, Temperature and Nitrate Concentration on Phycoerythrin Content in the Microalga Rhodomonas sp. (Cryptophyceae). Algal Res. 2015, 8, 89–94. [Google Scholar] [CrossRef]
- Mogany, T.; Swalaha, F.M.; Kumari, S.; Bux, F. Elucidating the Role of Nutrients in C-Phycocyanin Production by the Halophilic Cyanobacterium Euhalothece sp. J. Appl. Phycol. 2018, 30, 2259–2271. [Google Scholar] [CrossRef]
- Rafiqul, I.M.; Hassan, A.; Sulebele, G.; Orosco, C.A.; Roustaian, P.; Jalal, K.C.A. Salt Stress Culture of Blue-Green Algae Spirulina fusiformis. Pak. J. Biol. Sci. 2003, 6, 648–650. [Google Scholar] [CrossRef] [Green Version]
- Jonte, L.; Rosales-Loaiza, N.; Bermúdez-González, J.L.; Morales Avendaño, E.D. Cultivos Discontinuos Alimentados con Urea de la Cianobacteria Phormidium sp. en Función de la Salinidad y Edad del Cultivo. Rev. Colomb. Biotecnol. 2013, 15, 38. [Google Scholar] [CrossRef]
- Lee, H.; Noh, Y.; Hong, S.-J.; Lee, H.; Kim, D.-M.; Cho, B.-K.; Lee, C.-G.; Choi, H.-K. Photosynthetic Pigment Production and Metabolic and Lipidomic Alterations in the Marine Cyanobacteria Synechocystis sp. PCC 7338 under Various Salinity Conditions. J. Appl. Phycol. 2021, 33, 197–209. [Google Scholar] [CrossRef]
- Sonani, R.R.; Rastogi, R.P.; Patel, S.N.; Chaubey, M.G.; Singh, N.K.; Gupta, G.D.; Kumar, V.; Madamwar, D. Phylogenetic and Crystallographic Analysis of Nostoc Phycocyanin Having Blue-Shifted Spectral Properties. Sci. Rep. 2019, 9, 9863. [Google Scholar] [CrossRef] [Green Version]
- Patel, S.N.; Sonani, R.R.; Jakharia, K.; Bhastana, B.; Patel, H.M.; Chaubey, M.G.; Singh, N.K.; Madamwar, D. Antioxidant Activity and Associated Structural Attributes of Halomicronema Phycoerythrin. Int. J. Biol. Macromol. 2018, 111, 359–369. [Google Scholar] [CrossRef]
- Wang, L.; Qu, Y.; Fu, X.; Zhao, M.; Wang, S.; Sun, L. Isolation, Purification and Properties of an R-Phycocyanin from the Phycobilisomes of a Marine Red Macroalga Polysiphonia urceolata. PLoS ONE 2014, 9, e87833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pott, R.W.M. The Release of the Blue Biological Pigment C-phycocyanin through Calcium-aided Cytolysis of Live Spirulina sp. Color. Technol. 2019, 135, 17–21. [Google Scholar] [CrossRef] [Green Version]
- Kannaujiya, V.K.; Sundaram, S.; Sinha, R.P. Structural and Functional Significance of Phycobiliproteins. In Phycobiliproteins: Recent Developments and Future Applications; Springer: Singapore, 2017; pp. 21–44. ISBN 978-981-10-6459-3. [Google Scholar]
- Kathiresan, S.; Sarada, R.; Bhattacharya, S.; Ravishankar, G.A. Culture Media Optimization for Growth and Phycoerythrin Production From Porphyridium purpureum. Biotechnol. Bioeng. 2007, 96, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Su, H.-N.; Pu, Y.; Chen, J.; Liu, L.-N.; Liu, Q.; Qin, S. Phycobiliproteins: Molecular Structure, Production, Applications, and Prospects. Biotechnol. Adv. 2019, 37, 340–353. [Google Scholar] [CrossRef] [PubMed]
- Onishi, A.; Aikawa, S.; Kondo, A.; Akimoto, S. Energy Transfer in Anabaena variabilis Filaments under Nitrogen Depletion, Studied by Time-Resolved Fluorescence. Photosynth. Res. 2015, 125, 191–199. [Google Scholar] [CrossRef]
- Zhang, Z.; Lambrev, P.H.; Wells, K.L.; Garab, G.; Tan, H.-S. Direct Observation of Multistep Energy Transfer in LHCII with Fifth-Order 3D Electronic Spectroscopy. Nat. Commun. 2015, 6, 7914. [Google Scholar] [CrossRef] [Green Version]
- Chang, L.; Liu, X.; Li, Y.; Liu, C.-C.; Yang, F.; Zhao, J.; Sui, S.-F. Structural Organization of an Intact Phycobilisome and Its Association with Photosystem II. Cell Res. 2015, 25, 726–737. [Google Scholar] [CrossRef] [Green Version]
- Domínguez-Martín, M.A.; Sauer, P.V.; Kirst, H.; Sutter, M.; Bína, D.; Greber, B.J.; Nogales, E.; Polívka, T.; Kerfeld, C.A. Structures of a Phycobilisome in Light-Harvesting and Photoprotected States. Nature 2022, 609, 835–845. [Google Scholar] [CrossRef]
- Udayan, A.; Arumugam, M.; Pandey, A. Nutraceuticals From Algae and Cyanobacteria. In Algal Green Chemistry; Elsevier: Amsterdam, The Netherlands, 2017; pp. 65–89. ISBN 978-0-444-63784-0. [Google Scholar]
- Durvasula, R.V.; Rao, D.V.S. Extremophiles: From Biology to Biotechnology; CRC Press: Boca Raton, FL, USA, 2018; ISBN 978-1-4987-7493-2. [Google Scholar]
- Kent, M.; Welladsen, H.M.; Mangott, A.; Li, Y. Nutritional Evaluation of Australian Microalgae as Potential Human Health Supplements. PLoS ONE 2015, 10, e0118985. [Google Scholar] [CrossRef]
- Chen, H.; Qi, H.; Xiong, P. Phycobiliproteins—A Family of Algae-Derived Biliproteins: Productions, Characterization and Pharmaceutical Potentials. Mar. Drugs 2022, 20, 450. [Google Scholar] [CrossRef]
- Chakdar, H.; Pabbi, S. Algal Pigments for Human Health and Cosmeceuticals. In Algal Green Chemistry; Elsevier: Amsterdam, The Netherlands, 2017; pp. 171–188. ISBN 978-0-444-63784-0. [Google Scholar]
- Manirafasha, E.; Ndikubwimana, T.; Zeng, X.; Lu, Y.; Jing, K. Phycobiliprotein: Potential Microalgae Derived Pharmaceutical and Biological Reagent. Biochem. Eng. J. 2016, 109, 282–296. [Google Scholar] [CrossRef]
- Cornejo, J.; Beale, S.I. Phycobilin biosynthetic reactions in extracts of cyanobacteria. Photosynth. Res. 1997, 51, 223–230. [Google Scholar] [CrossRef]
- Henriques, M.; Silva, A.; Rocha, J. Extraction and Quantification of Pigments from a Marine Microalga: A Simple and Reproducible Method. Commun. Curr. Res. Educ. Top. Trends Appl. Microbiol. 2007, 2, 586–593. [Google Scholar]
- Tran, T.; Denimal, E.; Lafarge, C.; Journaux, L.; Lee, J.A.; Winckler, P.; Perrier-Cornet, J.-M.; Pradelles, R.; Loupiac, C.; Cayot, N. Effect of High Hydrostatic Pressure on Extraction of B-Phycoerythrin from Porphyridium cruentum: Use of Confocal Microscopy and Image Processing. Algal Res. 2019, 38, 101394. [Google Scholar] [CrossRef]
- Sekar, S.; Chandramohan, M. Phycobiliproteins as a Commodity: Trends in Applied Research, Patents and Commercialization. J. Appl. Phycol. 2008, 20, 113–136. [Google Scholar] [CrossRef]
- Yu, P.; Wu, Y.; Wang, G.; Jia, T.; Zhang, Y. Purification and Bioactivities of Phycocyanin. Crit. Rev. Food Sci. Nutr. 2017, 57, 3840–3849. [Google Scholar] [CrossRef]
- Pan-utai, W.; Iamtham, S. Physical Extraction and Extrusion Entrapment of C-Phycocyanin from Arthrospira platensis. J. King Saud. Univ.–Sci. 2019, 31, 1535–1542. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, X.B.; Wang, K.B.; Li, Y.X.; Bai, K.Z.; Kuang, T.Y.; Ji, H.B. A Simple Method for Extracting C-Phycocyanin from Spirulina Platensis Using Klebsiella pneumoniae. Appl. Microbiol. Biotechnol. 2007, 74, 244–248. [Google Scholar] [CrossRef]
- Bermejo, R.; Acién, F.G.; Ibáñez, M.J.; Fernández, J.M.; Molina, E.; Alvarez-Pez, J.M. Preparative Purification of B-Phycoerythrin from the Microalga Porphyridium cruentum by Expanded-Bed Adsorption Chromatography. Anal. Technol. Biomed. Life Sci. 2003, 790, 317–325. [Google Scholar] [CrossRef]
- Moraes, C.C.; Sala, L.; Cerveira, G.P.; Kalil, S.J. C-Phycocyanin Extraction from Spirulina Platensis Wet Biomass. Braz. J. Chem. Eng. 2011, 28, 45–49. [Google Scholar] [CrossRef] [Green Version]
- Günerken, E.; D’Hondt, E.; Eppink, M.H.M.; Garcia-Gonzalez, L.; Elst, K.; Wijffels, R.H. Cell Disruption for Microalgae Biorefineries. Biotechnol. Adv. 2015, 33, 243–260. [Google Scholar] [CrossRef]
- Golberg, A.; Sack, M.; Teissie, J.; Pataro, G.; Pliquett, U.; Saulis, G.; Stefan, T.; Miklavcic, D.; Vorobiev, E.; Frey, W. Energy-Efficient Biomass Processing with Pulsed Electric Fields for Bioeconomy and Sustainable Development. Biotechnol. Biofuels Bioprod. 2016, 9, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez, J.M.; Luengo, E.; Saldaña, G.; Álvarez, I.; Raso, J. C-Phycocyanin Extraction Assisted by Pulsed Electric Field from Artrosphira platensis. Food Res. Int. 2017, 99, 1042–1047. [Google Scholar] [CrossRef]
- Carullo, D.; Pataro, G.; Donsì, F.; Ferrari, G. Pulsed Electric Fields-Assisted Extraction of Valuable Compounds From Arthrospira platensis: Effect of Pulse Polarity and Mild Heating. Front. Bioeng. Biotechnol. 2020, 8, 551272. [Google Scholar] [CrossRef]
- Molina Grima, E.; Belarbi, E.-H.; Acién Fernández, F.G.; Robles Medina, A.; Chisti, Y. Recovery of Microalgal Biomass and Metabolites: Process Options and Economics. Biotechnol. Adv. 2003, 20, 491–515. [Google Scholar] [CrossRef] [PubMed]
- Juin, C.; Chérouvrier, J.-R.; Thiéry, V.; Gagez, A.-L.; Bérard, J.-B.; Joguet, N.; Kaas, R.; Cadoret, J.-P.; Picot, L. Microwave-Assisted Extraction of Phycobiliproteins from Porphyridium purpureum. Biotechnol. Appl. Biochem. 2015, 175, 1–15. [Google Scholar] [CrossRef]
- Ibáñez-González, M.J.; Mazzuca-Sobczuk, T.; Redondo-Miranda, R.M.; Molina-Grima, E.; Cooney, C.L. A Novel Vortex Flow Reactor for the Purification of B-Phycoerythrin from Porphyridium cruentum. Chem. Eng. Res. Des. 2016, 111, 24–33. [Google Scholar] [CrossRef]
- Lauceri, R.; Chini Zittelli, G.; Torzillo, G. A Simple Method for Rapid Purification of Phycobiliproteins from Arthrospira Platensis and Porphyridium cruentum Biomass. Algal Res. 2019, 44, 101685. [Google Scholar] [CrossRef]
- Khan, Z.; Wan Maznah, W.O.; Faradina Merican, M.S.M.; Convey, P.; Najimudin, N.; Alias, S.A. A Comparative Study of Phycobilliprotein Production in Two Strains of Pseudanabaena Isolated from Arctic and Tropical Regions in Relation to Different Light Wavelengths and Photoperiods. Polar Sci. 2019, 20, 3–8. [Google Scholar] [CrossRef]
- Choi, W.; Lee, H. Effect of Ultrasonic Extraction on Production and Structural Changes of C-Phycocyanin from Marine Spirulina maxima. Int. J. Mol. Sci. 2018, 19, 220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viskari, P.J.; Colyer, C.L. Rapid Extraction of Phycobiliproteins from Cultured Cyanobacteria Samples. Anal. Biochem. 2003, 319, 263–271. [Google Scholar] [CrossRef]
- Gantar, M.; Simović, D.; Djilas, S.; Gonzalez, W.W.; Miksovska, J. Isolation, Characterization and Antioxidative Activity of C-Phycocyanin from Limnothrix sp. Strain 37-2-1. J. Biotechnol. 2012, 159, 21–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bermejo Román, R.; Alvárez-Pez, J.M.; Acién Fernández, F.G.; Molina Grima, E. Recovery of Pure B-Phycoerythrin from the Microalga Porphyridium cruentum. J. Biotechnol. 2002, 93, 73–85. [Google Scholar] [CrossRef]
- Parmar, A.; Singh, N.K.; Kaushal, A.; Madamwar, D. Characterization of an Intact Phycoerythrin and Its Cleaved 14kDa Functional Subunit from Marine Cyanobacterium Phormidium sp. A27DM. Process Biochem. 2011, 46, 1793–1799. [Google Scholar] [CrossRef]
- Hrishikesh, A.T.; Prasad, V.; Raghavarao, K.S.M.S. Synergistic Method for Extraction of High Purity Allophycocyanin from Dry Biomass of Arthrospira platensis and Utilization of Spent Biomass for Recovery of Carotenoids. Sep. Purif. Technol. 2019, 225, 97–111. [Google Scholar]
- Chittapun, S.; Jonjaroen, V.; Khumrangsee, K.; Charoenrat, T. C-Phycocyanin Extraction from Two Freshwater Cyanobacteria by Freeze Thaw and Pulsed Electric Field Techniques to Improve Extraction Efficiency and Purity. Algal Res. 2020, 46, 101789. [Google Scholar] [CrossRef]
- Kumar, J.I.N.; Kumar, R.N.; Bora, A.; Amb, M.K.; Chakraborty, S. An Evaluation of the Pigment Composition of Eighteen Marine Macroalgae Collected from Okha Coast, Gulf of Kutch, India. Our Nat. 2010, 7, 48–55. [Google Scholar] [CrossRef] [Green Version]
- Barufi, J.B.; Figueroa, F.L.; Plastino, E.M. Effects of Light Quality on Reproduction, Growth and Pigment Content of Gracilaria birdiae (Rhodophyta: Gracilariales). Sci. Mar. 2015, 79, 15–24. [Google Scholar] [CrossRef] [Green Version]
- Munier, M.; Morançais, M.; Dumay, J.; Jaouen, P.; Fleurence, J. One-Step Purification of R-Phycoerythrin from the Red Edible Seaweed Grateloupia turuturu. J. Chromatogr. B 2015, 992, 23–29. [Google Scholar] [CrossRef]
- Baghel, R.S.; Kumari, P.; Reddy, C.R.K.; Jha, B. Growth, Pigments and Biochemical Composition of Marine Red Alga Gracilaria crassa. J. Appl. Phycol. 2014, 26, 2143–2150. [Google Scholar] [CrossRef]
- Punampalam, R.; Khoo, K.S.; Sit, N.W. Evaluation of Antioxidant Properties of Phycobiliproteins and Phenolic Compounds Extracted from Bangia atropurpurea. Malays. J. Fundam. Appl. Sci. 2018, 14, 289–297. [Google Scholar] [CrossRef]
- Reuter, W.; Nickel, C.; Wehrmeyer, W. Isolation of Allophycocyanin B from Rhodella violacea Results in a Model of the Core from Hemidiscoidal Phycobilisomes of Rhodophyceae. FEBS Lett. 1990, 273, 155–158. [Google Scholar] [CrossRef] [Green Version]
- Abalde, J.; Betancourt, L.; Torres, E.; Cid, A.; Barwell, C. Purification and Characterization of Phycocyanin from the Marine Cyanobacterium Synechococcus sp. IO9201. Plant Sci. 1998, 136, 109–120. [Google Scholar] [CrossRef] [Green Version]
- Sørensen, L.; Hantke, A.; Eriksen, N.T. Purification of the Photosynthetic Pigment C-Phycocyanin from Heterotrophic Galdieria sulphuraria. J. Sci. Food Agric. 2013, 93, 2933–2938. [Google Scholar] [CrossRef] [PubMed]
- Patil, G.; Chethana, S.; Sridevi, A.S.; Raghavarao, K.S.M.S. Method to Obtain C-Phycocyanin of High Purity. J. Chromatogr. A 2006, 1127, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.-F.; Wang, G.-C.; Lin, X.; Zhou, B.-C. Large-Scale Recovery of C-Phycocyanin from Spirulina platensis Using Expanded Bed Adsorption Chromatography. J. Chromatogr. B 2007, 850, 267–276. [Google Scholar] [CrossRef]
- Patil, G.; Raghavarao, K.S.M.S. Aqueous Two Phase Extraction for Purification of C-Phycocyanin. Biochem. Eng. J. 2007, 34, 156–164. [Google Scholar] [CrossRef]
- Mishra, S.K.; Shrivastav, A.; Mishra, S. Preparation of Highly Purified C-Phycoerythrin from Marine Cyanobacterium Pseudanabaena sp. Protein Expr. Purif. 2011, 80, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Sonani, R.R.; Singh, N.K.; Kumar, J.; Thakar, D.; Madamwar, D. Concurrent Purification and Antioxidant Activity of Phycobiliproteins from Lyngbya sp. A09DM: An Antioxidant and Anti-Aging Potential of Phycoerythrin in Caenorhabditis Elegans. Process Biochem. 2014, 49, 1757–1766. [Google Scholar] [CrossRef]
- Sun, L.; Wang, S.; Gong, X.; Zhao, M.; Fu, X.; Wang, L. Isolation, Purification and Characteristics of R-Phycoerythrin from a Marine Macroalga Heterosiphonia japonica. Protein Expr. Purif. 2009, 64, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Hsieh-Lo, M.; Castillo, G.; Ochoa-Becerra, M.A.; Mojica, L. Phycocyanin and Phycoerythrin: Strategies to Improve Production Yield and Chemical Stability. Algal Res. 2019, 42, 101600. [Google Scholar] [CrossRef]
- Benavides, J.; Ritopalomares, M. Simplified Two-Stage Method to B-Phycoerythrin Recovery from Porphyridium cruentum. J. Chromatogr. B 2006, 844, 39–44. [Google Scholar] [CrossRef]
- Jubeau, S.; Marchal, L.; Pruvost, J.; Jaouen, P.; Legrand, J.; Fleurence, J. High Pressure Disruption: A Two-Step Treatment for Selective Extraction of Intracellular Components from the Microalga Porphyridium cruentum. J. Appl. Phycol. 2013, 25, 983–989. [Google Scholar] [CrossRef]
- Arulselvan, P.; Fard, M.T.; Tan, W.S.; Gothai, S.; Fakurazi, S.; Norhaizan, M.E.; Kumar, S.S. Role of Antioxidants and Natural Products in Inflammation. Oxidative Med. Cell. Longev. 2016, 2016, 5276130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirata, T.; Tanaka, M.; Ooike, M.; Tsunomura, T.; Sakaguchi, M. Antioxidant Activities of Phycocyanobilin Prepared from Spirulina platensis. J. Appl. Phycol. 2000, 12, 435–439. [Google Scholar] [CrossRef]
- Li, Y. The Bioactivities of Phycocyanobilin from Spirulina. J. Immunol. Res. 2022, 2022, 4008991. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Pliego, E.; Franco-Colin, M.; Rojas-Franco, P.; Blas-Valdivia, V.; Serrano-Contreras, J.I.; Pentón-Rol, G.; Cano-Europa, E. Phycocyanobilin Is the Molecule Responsible for the Nephroprotective Action of Phycocyanin in Acute Kidney Injury Caused by Mercury. Food Funct. J. 2021, 12, 2985–2994. [Google Scholar] [CrossRef]
- Liu, Q.; Li, W.; Qin, S. Therapeutic Effect of Phycocyanin on Acute Liver Oxidative Damage Caused by X-Ray. Biomed. Pharmacother. 2020, 130, 110553. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, S.; Benvenuti, F.; Scoglio, S.; Canestrari, F. Oxygen Radical Absorbance Capacity of Phycocyanin and Phycocyanobilin from the Food Supplement Aphanizomenon flos-aquae. J. Med. Food 2010, 13, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.-P.; Liu, L.-N.; Chen, X.-L.; Wang, J.-X.; Chen, M.; Zhang, Y.-Z.; Zhou, B.-C. Factors that effect antioxidant activity of c-phycocyanins from Spirulina platensis. J. Food Biochem. 2005, 29, 313–322. [Google Scholar] [CrossRef]
- Huang, Z.; Guo, B.J.; Wong, R.N.S.; Jiang, Y. Characterization and Antioxidant Activity of Selenium-Containing Phycocyanin Isolated from Spirulina platensis. Food Chem. 2007, 100, 1137–1143. [Google Scholar] [CrossRef]
- Gdara, N.B.; Belgacem, A.; Khemiri, I.; Mannai, S.; Bitri, L. Protective Effects of Phycocyanin on Ischemia/Reperfusion Liver Injuries. Biomed. Pharmacother. 2018, 102, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Praneel, D. The Wonder Molecule Called Phycocyanin. United States Office 2011. Available online: https://silo.tips/download/the-wonder-molecule-called-phycocyanin (accessed on 10 November 2022).
- Cervantes-Llanos, M.; Lagumersindez-Denis, N.; Marín-Prida, J.; Pavón-Fuentes, N.; Falcon-Cama, V.; Piniella-Matamoros, B.; Camacho-Rodríguez, H.; Fernández-Massó, J.R.; Valenzuela-Silva, C.; Raíces-Cruz, I.; et al. Beneficial Effects of Oral Administration of C-Phycocyanin and Phycocyanobilin in Rodent Models of Experimental Autoimmune Encephalomyelitis. Life Sci. 2018, 194, 130–138. [Google Scholar] [CrossRef]
- Jimeno, J.; Faircloth, G.; Sousa-Faro, J.; Scheuer, P.; Rinehart, K. New Marine Derived Anticancer Therapeutics—A Journey from the Sea to Clinical Trials. Mar. Drugs 2004, 2, 14–29. [Google Scholar] [CrossRef]
- Kuznetsov, G.; Xu, Q.; Rudolph-Owen, L.; TenDyke, K.; Liu, J.; Towle, M.; Zhao, N.; Marsh, J.; Agoulnik, S.; Twine, N.; et al. Potent in Vitro and in Vivo Anticancer Activities of Des-Methyl, Des-Amino Pateamine A, a Synthetic Analogue of Marine Natural Product Pateamine A. Mol. Cancer Ther. 2009, 8, 1250–1260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, L.; Wang, Y.; Yin, Q.; Liu, G.; Liu, H.; Huang, Y.; Li, B. Phycocyanin: A Potential Drug for Cancer Treatment. J. Cancer 2017, 8, 3416–3429. [Google Scholar] [CrossRef] [Green Version]
- Ravi, M.; Tentu, S.; Baskar, G.; Rohan Prasad, S.; Raghavan, S.; Jayaprakash, P.; Jeyakanthan, J.; Rayala, S.K.; Venkatraman, G. Molecular Mechanism of Anti-Cancer Activity of Phycocyanin in Triple-Negative Breast Cancer Cells. BMC Cancer 2015, 15, 768. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Xu, L.; Cheng, N.; Lin, L.; Zhang, C. Inhibitory Effect of Phycocyanin from Spirulina platensis on the Growth of Human Leukemia K562 Cells. J. Appl. Phycol. 2000, 12, 125–130. [Google Scholar] [CrossRef]
- Roy, K.R.; Arunasree, K.M.; Reddy, N.P.; Dheeraj, B.; Reddy, G.V.; Reddanna, P. Alteration of Mitochondrial Membrane Potential by Spirulina platensis C-Phycocyanin Induces Apoptosis in the Doxorubicinresistant Human Hepatocellular-Carcinoma Cell Line HepG2. Biotechnol. Appl. Biochem. 2007, 47, 159. [Google Scholar] [CrossRef] [PubMed]
- Subhashini, J.; Mahipal, S.V.K.; Reddy, M.C.; Mallikarjuna Reddy, M.; Rachamallu, A.; Reddanna, P. Molecular Mechanisms in C-Phycocyanin Induced Apoptosis in Human Chronic Myeloid Leukemia Cell Line-K562. Biochem. Pharmacol. 2004, 68, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Chu, X.; Gao, M.; Li, W. Apoptotic Mechanism of MCF-7 Breast Cells in Vivo and in Vitro Induced by Photodynamic Therapy with C-Phycocyanin. Acta Biochim. Biophys. Sin. 2010, 42, 80–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, B.; Gao, M.-H.; Chu, X.-M.; Teng, L.; Lv, C.-Y.; Yang, P.; Yin, Q.-F. The Synergistic Antitumor Effects of All-Trans Retinoic Acid and C-Phycocyanin on the Lung Cancer A549 Cells in Vitro and in Vivo. Eur. J. Pharmacol. 2015, 749, 107–114. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Y.; Gao, X.; Carter, C.L.; Liu, Z.-R. The Recombinant β Subunit of C-Phycocyanin Inhibits Cell Proliferation and Induces Apoptosis. Cancer Lett. 2007, 247, 150–158. [Google Scholar] [CrossRef]
- Gardeva, E.; Toshkova, R.; Yossifova, L.; Minkova, K.; Ivanova, N.; Gigova, L. Antitumor Activity of C-Phycocyanin from Arthronema africanum (Cyanophyceae). Braz. Arch. Biol. Technol. 2014, 57, 675–684. [Google Scholar] [CrossRef] [Green Version]
- Jiang, L.; Wang, Y.; Zhu, F.; Liu, G.; Liu, H.; Ji, H.; Zheng, S.; Li, B. Molecular Mechanism of Anti-Cancer Activity of the Nano-Drug C-PC/CMC-CD59sp NPs in Cervical Cancer. J. Cancer 2019, 10, 92–104. [Google Scholar] [CrossRef]
- Tan, H.; Gao, S.; Zhuang, Y.; Dong, Y.; Guan, W.; Zhang, K.; Xu, J.; Cui, J. R-Phycoerythrin Induces SGC-7901 Apoptosis by Arresting Cell Cycle at S Phase. Mar. Drugs 2016, 14, 166. [Google Scholar] [CrossRef] [Green Version]
- Hao, S.; Li, S.; Wang, J.; Zhao, L.; Yan, Y.; Wu, T.; Zhang, J.; Wang, C. C-Phycocyanin Suppresses the In Vitro Proliferation and Migration of Non-Small-Cell Lung Cancer Cells through Reduction of RIPK1/NF-ΚB Activity. Mar. Drugs 2019, 17, 362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braithwaite, M.C.; Tyagi, C.; Tomar, L.K.; Kumar, P.; Choonara, Y.E.; Pillay, V. Nutraceutical-Based Therapeutics and Formulation Strategies Augmenting Their Efficiency to Complement Modern Medicine: An Overview. J. Funct. Foods 2014, 6, 82–99. [Google Scholar] [CrossRef]
- Gantar, M.; Dhandayuthapani, S.; Rathinavelu, A. Phycocyanin Induces Apoptosis and Enhances the Effect of Topotecan on Prostate Cell Line LNCaP. J. Med. Food 2012, 15, 1091–1095. [Google Scholar] [CrossRef] [PubMed]
- Saini, M.K.; Vaiphei, K.; Sanyal, S.N. Chemoprevention of DMH-Induced Rat Colon Carcinoma Initiation by Combination Administration of Piroxicam and C-Phycocyanin. Mol. Cell Biochem. 2012, 361, 217–228. [Google Scholar] [CrossRef]
- Wan, D.-H.; Zheng, B.-Y.; Ke, M.-R.; Duan, J.-Y.; Zheng, Y.-Q.; Yeh, C.-K.; Huang, J.-D. C-Phycocyanin as a Tumour-Associated Macrophage-Targeted Photosensitiser and a Vehicle of Phthalocyanine for Enhanced Photodynamic Therapy. Chem. Commun. 2017, 53, 4112–4115. [Google Scholar] [CrossRef] [Green Version]
- Nathan, C. Points of Control in Inflammation. Nature 2002, 420, 846–852. [Google Scholar] [CrossRef]
- Erickson, S.E.; Martin, G.S.; Davis, J.L.; Matthay, M.A.; Eisner, M.D. Recent Trends in Acute Lung Injury Mortality: 1996–2005. Crit. Care Med. 2009, 37, 1574–1579. [Google Scholar] [CrossRef]
- Lu, L.; Li, W.; Sun, C.; Kang, S.; Li, J.; Luo, X.; Su, Q.; Liu, B.; Qin, S. Phycocyanin Ameliorates Radiation-Induced Acute Intestinal Toxicity by Regulating the Effect of the Gut Microbiota on the TLR4/Myd88/NF-κB Pathway. J. Parenter. Enter. Nutr. 2020, 44, 1308–1317. [Google Scholar] [CrossRef]
- Zhu, C.; Ling, Q.; Cai, Z.; Wang, Y.; Zhang, Y.; Hoffmann, P.R.; Zheng, W.; Zhou, T.; Huang, Z. Selenium-Containing Phycocyanin from Se-Enriched Spirulina Platensis Reduces Inflammation in Dextran Sulfate Sodium-Induced Colitis by Inhibiting NF-ΚB Activation. J. Agric. Food Chem. 2016, 64, 5060–5070. [Google Scholar] [CrossRef]
- Xia, D.; Liu, B.; Luan, X.; Sun, J.; Liu, N.; Qin, S.; Du, Z. Protective Effects of C-Phycocyanin on Alcohol-Induced Acute Liver Injury in Mice. Chin. J. Ocean. Limnol. 2016, 34, 399–404. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Zhang, J.; Yu, G.; Yan, Y.; Chen, W.; Chi, M.; Qin, S. Experimental study on the therapeutic effect of C-phycocyanin against pulmonary fibrosis induced by paraquat in rats. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing. Za Zhi 2012, 30, 650–655. [Google Scholar]
- American Diabetes Association Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2009, 32, S62–S67. [CrossRef] [Green Version]
- Ou, Y.; Lin, L.; Yang, X.; Pan, Q.; Cheng, X. Antidiabetic Potential of Phycocyanin: Effects on KKAy Mice. Pharm. Biol. 2013, 51, 539–544. [Google Scholar] [CrossRef]
- Zheng, J.; Inoguchi, T.; Sasaki, S.; Maeda, Y.; McCarty, M.F.; Fujii, M.; Ikeda, N.; Kobayashi, K.; Sonoda, N.; Takayanagi, R. Phycocyanin and Phycocyanobilin from Spirulina Platensis Protect against Diabetic Nephropathy by Inhibiting Oxidative Stress. Am. J. Physiol. -Regul. Integr. Comp. Physiol. 2013, 304, R110–R120. [Google Scholar] [CrossRef] [Green Version]
- Siti Halimatul Munawaroh, H.; Gumilar, G.G.; Nurjanah, F.; Yuliani, G.; Aisyah, S.; Kurnia, D.; Wulandari, A.P.; Kurniawan, I.; Ningrum, A.; Koyande, A.K.; et al. In-Vitro Molecular Docking Analysis of Microalgae Extracted Phycocyanin as an Anti-Diabetic Candidate. Biochem. Eng. J. 2020, 161, 107666. [Google Scholar] [CrossRef]
- Soni, B.; Visavadiya, N.P.; Madamwar, D. Attenuation of Diabetic Complications by C-Phycoerythrin in Rats: Antioxidant Activity of C-Phycoerythrin Including Copper-Induced Lipoprotein and Serum Oxidation. Br. J. Nutr. 2009, 102, 102–109. [Google Scholar] [CrossRef] [Green Version]
- Soni, B.; Visavadiya, N.P.; Madamwar, D. Ameliorative Action of Cyanobacterial Phycoerythrin on CCl4-Induced Toxicity in Rats. Toxicology 2008, 248, 59–65. [Google Scholar] [CrossRef]
- Ou, Y.; Zheng, S.; Lin, L.; Jiang, Q.; Yang, X. Protective Effect of C-Phycocyanin against Carbon Tetrachloride-Induced Hepatocyte Damage in Vitro and in Vivo. Chem. -Biol. Interact. 2010, 185, 94–100. [Google Scholar] [CrossRef]
- Nemoto-Kawamura, C.; Hirahashi, T.; Nagai, T.; Yamada, H.; Katoh, T.; Hayashi, O. Phycocyanin Enhances Secretary IgA Antibody Response and Suppresses Allergic IgE Antibody Response in Mice Immunized with Antigen-Entrapped Biodegradable Microparticles. J. Nutr. Sci. Vitaminol. J. Nutr. Sci. Vitaminol. 2004, 50, 129–136. [Google Scholar] [CrossRef] [Green Version]
- Pentón-Rol, G.; Martínez-Sánchez, G.; Cervantes-Llanos, M.; Lagumersindez-Denis, N.; Acosta-Medina, E.F.; Falcón-Cama, V.; Alonso-Ramírez, R.; Valenzuela-Silva, C.; Rodríguez-Jiménez, E.; Llópiz-Arzuaga, A.; et al. C-Phycocyanin Ameliorates Experimental Autoimmune Encephalomyelitis and Induces Regulatory T Cells. Int. Immunopharmacol. 2011, 11, 29–38. [Google Scholar] [CrossRef]
- Chaubey, M.G.; Patel, S.N.; Rastogi, R.P.; Srivastava, P.L.; Singh, A.K.; Madamwar, D.; Singh, N.K. Therapeutic Potential of Cyanobacterial Pigment Protein Phycoerythrin: In Silico and in Vitro Study of BACE1 Interaction and in Vivo Aβ Reduction. Int. J. Biol. Macromol. 2019, 134, 368–378. [Google Scholar] [CrossRef]
- Sitohu, M.; Osman, A.; Ghany, A.G.A.; Salama, A. Antibacterial Phycocyanin from Anabaena Oryzae SOS13. Int. J. Appl. Res. Nat. Prod. 2015, 8, 27–36. [Google Scholar]
- Murugan, T.; Radhamadhavan. Screening for Antifungal and Antiviral Activity of C-Phycocyanin from Spirulina Platensis. J. Pharm. Res. 2011, 4, 4161–4163. [Google Scholar]
- Han, L.-K.; Li, D.-X.; Xiang, L.; Gong, X.-J.; Kondo, Y.; Suzuki, I.; Okuda, H. Isolation of Pancreatic Lipase Activity-Inhibitory Component of Spirulina Platensis and It Reduce Postprandial Triacylglycerolemia. Yakugaku Zasshi J. Pharm. Soc. Japan 2006, 126, 43–49. [Google Scholar] [CrossRef] [Green Version]
- Ma, P.; Huang, R.; Jiang, J.; Ding, Y.; Li, T.; Ou, Y. Potential Use of C-Phycocyanin in Non-Alcoholic Fatty Liver Disease. Biochem. Biophys. Res. Commun. 2020, 526, 906–912. [Google Scholar] [CrossRef]
- Kavitha, M.D.; Gouda, K.G.M.; Aditya Rao, S.J.; Shilpa, T.S.; Shetty, N.P.; Sarada, R. Atheroprotective Effect of Novel Peptides from Porphyridium Purpureum in RAW 264.7 Macrophage Cell Line and Its Molecular Docking Study. Biotechnol. Lett. 2019, 41, 91–106. [Google Scholar] [CrossRef]
- Dev, A.; Mohanbhai, S.J.; Kushwaha, A.C.; Sood, A.; Sardoiwala, M.N.; Choudhury, S.R.; Karmakar, S. κ-Carrageenan-C-Phycocyanin Based Smart Injectable Hydrogels for Accelerated Wound Recovery and Real-Time Monitoring. Acta Biomater. 2020, 109, 121–131. [Google Scholar] [CrossRef]
- Penna, A.; Durço, B.; Pagani, M.; Pimentel, T.; Mársico, E.; de Oliveira Silva, A.; Esmerino, E. Kefir with Artificial and Natural Dyes: Assessment of Consumer Knowledge, Attitude, and Emotional Profile Using Emojis. J. Sens. Stud. 2022, 37, e12734. [Google Scholar] [CrossRef]
- Galetović, A.; Seura, F.; Gallardo, V.; Graves, R.; Cortés, J.; Valdivia, C.; Núñez, J.; Tapia, C.; Neira, I.; Sanzana, S.; et al. Use of Phycobiliproteins from Atacama Cyanobacteria as Food Colorants in a Dairy Beverage Prototype. Foods 2020, 9, 244. [Google Scholar] [CrossRef] [Green Version]
- García, A.B.; Longo, E.; Murillo, M.C.; Bermejo, R. Using a B-Phycoerythrin Extract as a Natural Colorant: Application in Milk-Based Products. Molecules 2021, 26, 297. [Google Scholar] [CrossRef]
- Stanic-Vucinic, D.; Minic, S.; Nikolic, M.R.; Velickovic, T.C. Spirulina Phycobiliproteins as Food Components and Complements. Microalgal Biotechnol. 2018, 129–149. [Google Scholar]
- Coultate, T.; Blackburn, R.S. Food Colorants: Their Past, Present and Future. Color. Technol. 2018, 134, 165–186. [Google Scholar] [CrossRef]
- Dufossé, L.; Galaup, P.; Yaron, A.; Arad, S.M.; Blanc, P.; Chidambara Murthy, K.N.; Ravishankar, G.A. Microorganisms and Microalgae as Sources of Pigments for Food Use: A Scientific Oddity or an Industrial Reality? Trends Food Sci. Technol. 2005, 16, 389–406. [Google Scholar] [CrossRef]
- Martelli, G.; Folli, C.; Visai, L.; Daglia, M.; Ferrari, D. Thermal Stability Improvement of Blue Colorant C-Phycocyanin from Spirulina Platensis for Food Industry Applications. Process Biochem. 2014, 49, 154–159. [Google Scholar] [CrossRef]
- González-Ramírez, E.; Andújar-Sánchez, M.; Ortiz-Salmerón, E.; Bacarizo, J.; Cuadri, C.; Mazzuca-Sobczuk, T.; Ibáñez, M.J.; Cámara-Artigas, A.; Martínez-Rodríguez, S. Thermal and PH Stability of the B-Phycoerythrin from the Red Algae Porphyridium cruentum. Food Biophys. 2014, 9, 184–192. [Google Scholar] [CrossRef]
- Munier, M.; Jubeau, S.; Wijaya, A.; Morançais, M.; Dumay, J.; Marchal, L.; Jaouen, P.; Fleurence, J. Physicochemical Factors Affecting the Stability of Two Pigments: R-Phycoerythrin of Grateloupia Turuturu and B-Phycoerythrin of Porphyridium Cruentum. Food Chem. 2014, 150, 400–407. [Google Scholar] [CrossRef]
- Chaiklahan, R.; Chirasuwan, N.; Bunnag, B. Stability of Phycocyanin Extracted from Spirulina Sp.: Influence of Temperature, PH and Preservatives. Process Biochem. 2012, 47, 659–664. [Google Scholar] [CrossRef]
- Braga, A.R.; Figueira, F.D.; Silveira, J.T.; Morais, M.G.; Costa, J.A.; Kalil, S.J. Improvement of Thermal Stability of C-Phycocyanin by Nanofiber and Preservative Agents: C-PHYCOCYANIN STABILITY: PRESERVATIVE AND NANOFIBER. J. Food Process. Preserv. 2016, 40, 1264–1269. [Google Scholar] [CrossRef]
- Kannaujiya, V.K.; Sinha, R.P. Thermokinetic Stability of Phycocyanin and Phycoerythrin in Food-Grade Preservatives. J. Appl. Phycol. 2016, 28, 1063–1070. [Google Scholar] [CrossRef]
- Bekasova, O.D.; Borzova, V.A.; Shubin, V.V.; Kovalyov, L.I.; Stein-Margolina, V.A.; Kurganov, B.I. An Increase in the Resistance of R-Phycoerythrin to Thermal Aggregation by Silver Nanoparticles Synthesized in Nanochannels of the Pigment. Appl. Biochem. Microbiol. 2016, 52, 98–104. [Google Scholar] [CrossRef]
- Sun, L.; Wang, S.; Qiao, Z. Chemical Stabilization of the Phycocyanin from Cyanobacterium Spirulina Platensis. J. Biotechnol. 2006, 121, 563–569. [Google Scholar] [CrossRef]
- Selig, M.J.; Malchione, N.M.; Gamaleldin, S.; Padilla-Zakour, O.I.; Abbaspourrad, A. Protection of Blue Color in a Spirulina Derived Phycocyanin Extract from Proteolytic and Thermal Degradation via Complexation with Beet-Pectin. Food Hydrocoll. 2018, 74, 46–52. [Google Scholar] [CrossRef]
- Yan, M.; Liu, B.; Jiao, X.; Qin, S. Preparation of Phycocyanin Microcapsules and Its Properties. Food Bioprod. Process. 2014, 92, 89–97. [Google Scholar] [CrossRef]
- Hadiyanto, H.; Suzery, M.; Setyawan, D.; Majid, D.; Sutanto, H. Encapsulation of Phycocyanin-Alginate for High Stability and Antioxidant Activity. IOP Conf. Ser. Earth Environ. Sci. 2017, 55, 012030. [Google Scholar] [CrossRef]
- Hadiyanto, M.S.; Majid, D.; Setyawan, D. Improvement of Stability and Antioxidant Activities by Using Phycocyanin - Chitosan Encapsulation Technique. IOP Conf. Ser. Earth Environ. Sci. 2017, 55, 012052. [Google Scholar] [CrossRef]
- Purnamayati, L.; Dewi, E.; Kurniasih, R.A. Phycocyanin Stability in Microcapsules Processed by Spray Drying Method Using Different Inlet Temperature. IOP Conf. Ser. Earth Environ. Sci. 2018, 116, 012076. [Google Scholar] [CrossRef]
- Santiago-Santos, M.C.; Ponce-Noyola, T.; Olvera-Ramírez, R.; Ortega-López, J.; Cañizares-Villanueva, R.O. Extraction and Purification of Phycocyanin from Calothrix sp. Process Biochem. 2004, 39, 2047–2052. [Google Scholar] [CrossRef]
- Pigments from Microalgae Handbook; Jacob-Lopes, E.; Queiroz, M.I.; Zepka, L.Q. (Eds.) Springer International Publishing: Cham, Switzerland, 2020; ISBN 978-3-030-50970-5. [Google Scholar]
- Cai, C.; Li, C.; Wu, S.; Wang, Q.; Guo, Z.; He, P. Large Scale Preparation of Phycobiliproteins from <I>Porphyra Yezoensis</I> Using Co-Precipitation with Ammonium Sulfate. NS 2012, 4, 536–543. [Google Scholar] [CrossRef] [Green Version]
- Romay, C.; Gonzalez, R. Phycocyanin Is an Antioxidant Protector of Human Erythrocytes Against Lysis by Peroxyl Radicals. J. Pharm. Pharmacol. 2010, 52, 367–368. [Google Scholar] [CrossRef]
- Romay, C.; Gonzalez, R.; Ledon, N.; Remirez, D.; Rimbau, V. C-Phycocyanin: A Biliprotein with Antioxidant, Anti-Inflammatory and Neuroprotective Effects. Curr. Protein Pept. Sci. 2003, 4, 207–216. [Google Scholar] [CrossRef]
- Allan, A.L.; Vantyghem, S.A.; Tuck, A.B.; Chambers, A.F.; Chin-Yee, I.H.; Keeney, M. Detection and Quantification of Circulating Tumor Cells in Mouse Models of Human Breast Cancer Using Immunomagnetic Enrichment and Multiparameter Flow Cytometry. Cytometry 2005, 65A, 4–14. [Google Scholar] [CrossRef]
- Balboa, E.M.; Conde, E.; Soto, M.L.; Pérez-Armada, L.; Domínguez, H. Cosmetics from Marine Sources; Se-Kwon, K., Ed.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1015–1042. ISBN 978-3-642-53970-1. [Google Scholar]
- Balasubramaniam, V.; Gunasegavan, R.D.-N.; Mustar, S.; Lee, J.C.; Mohd Noh, M.F. Isolation of Industrial Important Bioactive Compounds from Microalgae. Molecules 2021, 26, 943. [Google Scholar] [CrossRef]





| Microalgae Species | Cell Disruption Methods | Yield/Extraction Efficiency PE | Yield/Extraction Efficiency PC | References |
|---|---|---|---|---|
| Porphyridium purpureum | Microwave-assisted extraction | 73.7 ± 2.3 μg/mg | 34.8 ± 6.4 μg/mg | [98] |
| Rhodomonas sp. | Sodium phosphate buffer (0.1 M, pH = 6.0) + repetitive freeze–thaw cycles + sonication for 10 min | 5.36 ± 0.68% | - | [33] |
| Porphyridium cruentum | Acetate buffer (50 mM, pH = 5.5) + five repeated freeze/thawing cycles | 0.27 mg/mL | - | [99] |
| Porphyridium cruentum | Freeze/thawing cycles (−20° C and 20–25° C) | 71 ± 4% | - | [100] |
| Porphyridium cruentum | Freeze–thawing cycles + ultrasound | 69 ± 3% | - | [100] |
| Arthrospira platensis | Freeze–thawing cycles + ultrasound | - | 76 ± 6% | [100] |
| Pseudanabaena amphigranulata | Three cycles of repeated freezing in liquid nitrogen + maceration mortar and pestle | 10.2 ± 3.9 mg/L | 86 ± 14.7 mg/L | [101] |
| Pseudanabaena catenate | Three cycles of repeated freezing in liquid nitrogen + maceration mortar and pestle | 25.5 ± 5.1 mg/L | 28.8 ± 2.8 mg/L | [101] |
| Spirulina maxima | Ultrasonication | 0.8 mg/mL | 11.3 mg/mL | [102] |
| Synechococcus 833 | Incubation of sample for 2 h at 37 °C + nitrogen cavitation cycles (1500 psi, 10 min) | - | - | [103] |
| Limnothrix sp. | Distilled water + activated carbon (1% w/v) and chitosan (0.01 g/L) | - | 18% | [104] |
| Porphyridium cruentum | Homogenization in 1 M acetic acid sodium acetate buffer + sonication (10 min) | 32.7% | - | [105] |
| Phormidium sp. A27DM | Freeze–thaw cycles (−30 °C and 4 °C) in 1 M Tris HCl buffer | 62.6% | - | [106] |
| Arthrospira platensis | Enzymatic extraction (lysozyme) | - | APC: 2.27 mg/g | [107] |
| Nostoc commune | Pulsed electric fields | - | 29.66 ± 0.52 mg/g | [108] |
| Porphyridium marinum | Sodium phosphate buffer (20 mM, pH = 7.2) + freeze–thawing cycles + ultrasound | 57 mg/g | - | [11] |
| Microalgae Species | PBPs | Purification Methods | Yield (%) | Purity | References |
|---|---|---|---|---|---|
| Bangia atropurpurea | PE and PC | Gel filtration with Sephadex G-200 | 91.3 and 68.3 | 4.76 and 2.80 | [113] |
| Rhodella violace | APC | Gradient centrifugation Hydroxylapatite chromatography Preparative PAGE (native) | - | - | [114] |
| Synechochoccus sp. | PC | Hydrophobic interaction chromatography Ion exchange chromatography | - | 4.85 | [115] |
| Galdieria sulphuraria | PC | (NH4)2SO4 fractionation Aqueous two-phase extraction Anion exchange chromatography | 39 | 4.5 | [116] |
| Spirulina platensis | PC | Chitosan adsorption Two-phase aqueous extraction | 66 | 5.1 | [117] |
| Spirulina platensis | PC | Chitosan adsorption Two-phase aqueous extraction Ion exchange chromatography | - | 6.69 | [117] |
| Spirulina platensis | PC | Expanded bed adsorption chromatography Ion exchange chromatography | 8.7 | 3.64 | [118] |
| Spirulina platensis | PC | Repeated two-phase aqueous extraction Ultrafiltration | 85.0 | 4.05 | [119] |
| Pseudanabaena sp. | PE | Precipitation with (NH4)2SO4 Gel filtration chromatography Ion exchange chromatography | 47.0 | 6.86 | [120] |
| Nostoc sp. | PC | Ion exchange chromatography Two-phase aqueous extraction | - | 3.55 | [37] |
| Lyngbya sp. A09DM | PE, PC and APC | Triton X-100 mediated with (NH4)2SO4 precipitation Ion exchange chromatography Gel filtration chromatography | 76.16, 60.23 and 71.91 | 6.75, 5.53 and 5.43 | [121] |
| Nostoc sp. | PE | Ion exchange chromatography Two-phase aqueous extraction | - | - | [37] |
| Porphyridium marinum | PE | Two steps of precipitation with (NH4)2SO4 Dialysis DEAE-cellulose exchange chromatography | 72 | 5 | [11] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tounsi, L.; Ben Hlima, H.; Hentati, F.; Hentati, O.; Derbel, H.; Michaud, P.; Abdelkafi, S. Microalgae: A Promising Source of Bioactive Phycobiliproteins. Mar. Drugs 2023, 21, 440. https://doi.org/10.3390/md21080440
Tounsi L, Ben Hlima H, Hentati F, Hentati O, Derbel H, Michaud P, Abdelkafi S. Microalgae: A Promising Source of Bioactive Phycobiliproteins. Marine Drugs. 2023; 21(8):440. https://doi.org/10.3390/md21080440
Chicago/Turabian StyleTounsi, Latifa, Hajer Ben Hlima, Faiez Hentati, Ons Hentati, Hana Derbel, Philippe Michaud, and Slim Abdelkafi. 2023. "Microalgae: A Promising Source of Bioactive Phycobiliproteins" Marine Drugs 21, no. 8: 440. https://doi.org/10.3390/md21080440
APA StyleTounsi, L., Ben Hlima, H., Hentati, F., Hentati, O., Derbel, H., Michaud, P., & Abdelkafi, S. (2023). Microalgae: A Promising Source of Bioactive Phycobiliproteins. Marine Drugs, 21(8), 440. https://doi.org/10.3390/md21080440